CS 2019.02.01.16
Files > Conference Series > 2019 > Humboldt Kolleg 2019
Bionatura Conference Series Vol 2. No 1. 2019
“Breaking Paradigms: Towards a Multi-, Inter- and Transdisciplinary Science” In commemoration of the 250th Anniversary of Alexander von Humboldt
A Hemophilia Disorder Review: Gene Therapy for Hemophilia B Treatment using rAAV vectors
Abad Gallardo Claudia Sofía and Merchán Muñoz Brian David
available in: http://dx.doi.org/10.21931/RB/CS/2019.02.01.16
ABSTRACT
Hemophilia is an X-linked recessive disorder characterized by the deficiency in one protein essential for blood coagulation. There are two main types of variants of this disease; hemophilia A (HA) which is related with blood clotting factor VIII (FVIII) deficiency and hemophilia B (HB) which is related with factor IX (FIX) deficiency. Nowadays, there are several options to treat this disorder, however, the most efficient is gene therapy since it has a long-term effect, and contrasts with traditional methods. This review is focused on hemophilia B treatment because FIX is a smaller protein than FVIII (<1kb), and thereby is easier to study. Within gene therapy, methods which use recombinant adeno-associated virus (rAAV) vectors are the best alternative to treat HB since they are safe and reliable. Moreover, rAAV vectors have the advantage of having a low inflammatory potential, a non-pathogenic status, plus the potential for long-term expression of the transferred gene. However, some patients showed an immune response to the capsids of the vectors before treatment. Hence, possible solutions were needed; one of them being the use of anti-antibodies. Finally, clinical trials results showed that under the use of the optimized codon hFIXco and serotype 8 the levels of expression were persistent, demonstrating the potential of gene therapy for hemophilia B treatment.
Keywords: Hemophilia B, treatment, gene therapy, rAAV vectors, blood clotting factors.
INTRODUCTION
According to Nienhuis et al.1 hemophilia is an X-linked bleeding disorder consequence of mutations in blood clotting factor, FVIII for hemophilia A or FIX for hemophilia B. These are proteins produced by the liver to be secreted in the blood system. On one hand, FIX is an important intermediary in the cascade of reactions responsible for coagulation. On the other hand, its cofactor FVII is another intermediary that has an important role in the enzymatic activity of FIX. Therefore, any mutation in one of these proteins will interrupt the cascade of responses for coagulation resulting in a total lack of it or frequent bleed. That is why the major innovation that gene therapy aims for is to correct these mutations in such a way that the treatment is applied only once and not periodically as traditional treatments do.
Since this discovery, the recombinant adeno-associated virus became popular due to its advantages over other vectors. Berns and Srivastava2 state that Adeno-associated virus (AAV) is a small single-stranded DNA with the nonpathogenic human virus inside, which has been proved to be an efficient and safe vector for gene transfer. Thus, AAV as a vector has been already used in a lot of clinical trials and, promisingly, has not shown adverse effects. Furthermore, in the last years at least 12 AAV serotype vectors were developed and specifically the AAV serotype 8 (AAV8) has shown to be suitable and efficient for gene therapy for hemophilia B treatment. However, there are still some challenges to solve in order to develop the full potential of the vector, one of this is to find the optimal dose to evade the host immune response at the same time of getting the best expression levels during the treatment. This paper focuses on the current research regarding gene therapy for hemophilia B that is the production of rAAV, its underlying mechanisms and the results of its application in some clinical trials.
Hemophilia Types, Treatments and Clinical Trials
Genetic differences between Hemophilia A and B
Hemophilia disease results from the deficiency in one protein fundamental for blood coagulation. Hemophilia A (HA) is associated with a deficiency of blood clotting factor VIII (FVIII) while Hemophilia B (HB) is the deficiency of factor IX (FIX) 3. Hemophilia A is the most widespread form of this disease. It affects 1 of 5000 live male births, and it is caused by the lack of FVIII protein due to a mutation in the gene coding for this factor. In the other hand, patients with hemophilia B have a lack of FIX. Both factors are normally synthesized in the liver. Liver sinusoidal cells (LSEC) produce FVIII while hepatocytes originate FIX4, 5.
The main purpose of hemophilia A and B treatment is to prevent bleeding episodes and, hence, reduce bleeding into vital organs and joints. This prevention will have the effect of prolonging the patients' lives and improving their lifestyle as well6. Between the two types of hemophilia disorder, hemophilia B is easier to study since FIX is a smaller protein than FVIII (<1kb). Moreover, it only requires the correct post-translational carboxylation and glycosylation, a process that most cell types can perform7. Functional plasma levels of FIX which are less than 1% of the normal value in patients with hemophilia B represent a severe spectrum of the disease. This is usually accompanied by frequents bleeding episodes that occur in a spontaneous manner and that can lead to a chronic joint bleeding disease called hemarthropathy and, sometimes, can result in the patient's death. One option to treat this condition is to increase the plasma FIX levels above 1%. One way to achieve this is by injecting the protein to prevent and control the bleeding episodes in patients with hemophilia B; this technique refers to a replacement method which is the main traditional method to treat this blood disorder. However, this may not be enough to treat it on a long-term basis. Therefore, gene therapy is an excellent alternative to treat hemophilia B8.
Traditional treatments for Hemophilia
There are two main types of hemophilia, hemophilia A and hemophilia B. Different types of hemophilia are associated with different types of clotting factors9. The main treatment for stern hemophilia is to replace the missing clotting factors in blood according to the variety of the disease. This treatment is administrated by placing a tube with the specific clotting factor in a patient's vein so it will allow the correct clotting of the blood10. Some of the advantages of this treatment are that it can battle a bleeding episode while is occurring and that it can be administrated regularly at home in order to prevent new episodes.
According to Mayo Clinic9 and Centers for Disease Control and Prevention10 other therapies for hemophilia are the following
Treatment Medications
Clotting Factor Products are part of this category. The two main types are:
Plasma-derived Factor Concentrates: Plasma is the liquid part of the blood that contains proteins; between them are antibodies, albumin, and clotting factors. In this treatment, plasma is collected from many different people and separated into components to obtain the clotting factors. These are processed and purified to create a product free of any virus that only then will be ready to be sold.
Recombinant Factor Concentrates: These are factor replacement products used to be done from human plasma until 1992. However, nowadays, there is a recombinant factor concentrate approved by the U.S. Food and Drug Administration (FDA) which has a different origin. A risk for health that presents the clotting factor concentrates is that some patients can develop an inhibitor which could be really dangerous since it will make harder to stop a bleeding episode by interfering with the treatment.
Other Treatment Products
· Hemlibra (ACE 910 or emicizumab): This medicine works by refunding the function of factor VIII instead of directly substituting it. It helps to prevent or reduce bleeding episodes in patients with type A hemophilia. The main administration route is subcutaneous.
· Desmopressin Acetate (DDAVP or Stimate): It is a hormone that stimulates the releasing of the needed clotting factor in the body. The administration can be done by intravenous or nasal routes.
· Epsilon Amino Caproic Acid (Amicar): It is a medication that can be administrated by oral or intravenous routes. It prevents the breaking down of clots, making them more resistant.
· Cryoprecipitate: It is a substance that is originated by defrosting fresh frozen plasma. Due to its high factor VIII content, it was generally used to control severe bleeding. Nevertheless, since there is no method to avoid HIV and hepatitis contamination, it is no longer used in the United States.
Other Treatment Options
· Clot-preserving medication (anti-fibrinolytic): It is a drug that helps to prevent the breakdown of clots.
· Fibrin sealants: They are medications that promote clotting and healing. They can be employed directly in wounds and are very helpful in dental therapy.
· Physical therapy: This serves to treat the damage and symptoms caused by internal bleeding. However, in the damage is severe, surgery may be required.
· First aid for minor cuts: For small wounds or minor injuries, using pressure and a bandage will usually stop the bleeding. For small areas of subcutaneous bleeding, using a block of ice back should be enough.
· Vaccinations: To prevent infections by the continuous use of blood products, receiving vaccines is recommendable. The most necessary ones are hepatitis A and B vaccines.
Gene therapy for Hemophilia treatment
Hemophilia is an X-linked recessive bleeding disorder that has many associated treatments which are still in clinical development5, 11. Gene therapy is a promising treatment for hemophilia because it proposed a long term and lasting cure with only one drug administration4. Gene therapy refers to the transference of genetic material as a form of disease treatment. The main objective of gene therapy is a long-term manifestation or expression of the gene that has been transferred to treat a certain genetic disease. The gene must be transferred at high enough levels to be therapeutic. Commonly, this gene is a normal copy of a mutated gene. Another option within gene therapy as a treatment for hemophilia disease is to repress a specific genes’ expression by using RNA interference or techniques such as genome editing12.
rAAV vector as gene therapy for the treatment of Hemophilia B
Adeno-associated virus is a single-stranded DNA parvovirus which can be used as a versatile vector technology that can be genetically modified to have specific functions for gene therapy applications13, 14. Recombinant adeno-associated virus (rAAV) vectors have gain popularity for gene therapy applications because they are safe and effective both in preclinical and clinical trials14. The main advantages of rAAV vectors are their low inflammatory potential, non-pathogenic status, and the potential for long-term expression of the transferred gene. Another important feature to consider when choosing AAV for hemophilia B treatment is that it lacks an envelope and can be engineered to deliver DNA to target cells14. Therefore, these characteristics make rAAVs secure, reliable and proficient therapeutic alternatives for treating HB15.
It is important to acknowledge the biology of AAV. They contain three different genes within their single-stranded genome: Rep (Replication), Cap (Capsid), and aap (Assembly). These genes originate at least nine gene products by using three promoters, different translation start sites, and differential splicing. These sequences are side by inverted terminal repeats (ITRs) which will help in the replication and packaging processes. There is a great part of wild-type AAV biology that is not well understood yet, nonetheless, that information is not fundamental for gene therapy applications. Recombinant AAV is a virus of interest since it lacks viral DNA, so it is mainly a protein-based nanoparticle that is modified to break through the cell membrane in order to deliver the DNA that is carrying into the cell nucleus. Adeno-associated viruses enter cells by interacting with carbohydrates at located on the surface of target cells, in the first stage. Normally sialic acid, galactose and heparin sulfate. Then, in the second stage, viral transduction continue through interactions with secondary receptors14.
According to Naso et al.14 the production of the recombinant adeno-associated virus vector consists of four principal steps:
· Cell Platform: A suitable cell system is grown on 150 mm tissue culture-treated dish, hyperflasks, or shake flasks. Then, cells are infected with free nucleic acid, and the ones that have integrated the expression of it will be infected with adenovirus and grown to scale.
· Scale-up: Here cells are grown to scale.
· Purification/Polishing: In this step, the culture will be separated from any contaminant. Benzonase/DNAse treatment is needed for extraviral DNA removal.
· Quality Control (QC) and Release: Finally, several representation techniques are applied to visualize the purified particles, as well as other assays to evaluate the presence of residual host-cell protein contamination. After QC, rAAV vectors can be released to be used.
Finally, among the important criteria to be considered concerning rAAV vectors are vector design, capsid selection, the type of tissue and the selected target cell, and administration route of the vector. Choosing these parameters correctly will allow the right treatment development for hemophilia B and will result in future health improvements.
Clinical trials on Hemophilia B
It is important to take into account some considerations such as bleeding is correlated with the residual circulating factor level, thus depending on the residual factor level, there are three categories. First, mild hemophilia when the residual factor level is 5% of normal or greater, patients inside this range experience bleeding only after significant trauma. Second, moderate hemophilia if the residual factor level is less than 5% but greater than 1%, in this case, patients in this range seldom have any bleeding or bleeding after minor trauma. The last, severe hemophilia when the factor levels are less than 1% of normal, almost 50% of the patients are inside this range and in this case, individuals experience bleeding with the frequency with additional spontaneous musculoskeletal and soft tissue bleeding16.
Summarizing the steps and discovers during the trials (table 1), in the beginning, the initial efforts of gene therapy were focused on monogenic diseases in which the target protein was altered to become non-functional or was missing. In the case of hemophilia B, the first clinical trials tried to solve the non-function of the protein replacing the FIX due to the facility of placing the coding region and regulatory sequences in the AAV vector. Thus, the initial phase I studied the intramuscular injection of an AAV-2 vector in 7 subjects16, however, no transgenic FIX was detected. Later researchers determined that the reason of the lack of expression was that they were no targeting the normal site of synthesis, the liver, which was then reached via the hepatic artery17.
Thereby, taking into account the last observation, the vector was administered to patients using increasing amounts from 0.08 to 2 x 1012 vector genomes/kg. In one hand, detectable transgenic FIX was observed at 10% of normal level in 1 subject of 2 that were applied with the highest dose (2x 1012 vg/kg) from 4 to 9 weeks. In contrast, the other subject showed high levels of neutralizing antibodies, therefore researchers also found out that that the AAV capsid induce a cytotoxic T lymphocytes responses. The hypothesis is that at the highest dose the degradation products of the capsid were enough displayed on the surface of the hepatocytes to activate CTL response17. In the end, the possible solutions to this problem were to induce tolerance to the AAV capsid fragments or developing more efficient vectors.
In this basis, the next approach was using an optimized version of the codon for the human FIX (hFIXco) gene, and as a vector the AAV with serotype 8 capsid18 thus the administration could be done via peripheral circulation. Another trial with 6 subjects with severe hemophilia B were treated with low (2x 1011 vg/kg), intermediate (6x 1011 vg/kg) and high doses (2x 1012 vg/kg). All of them show FIX expression levels at 1% to 6% and 4 could stop the prophylaxis without suffering spontaneous hemorrhage and the other 2 increased the interval between prophylaxes. Although all showed enough expression levels, as previous studies, the subjects of the high doses showed an immune response between 7 and 10 weeks after the vector application that according to Nathwani et al.18 were treated without major complications with prednisolone and finally the subjects were stabilized and maintained expression levels in a range of 2% to 4%. Up to date, ongoing trials are looking for the optimum dose to determine if the immune response can be void while maintaining a favorable FIX level.
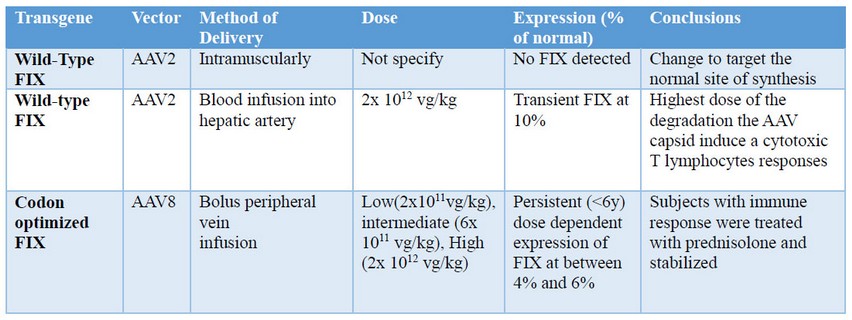
Table 1. Summary of Gene Therapy Trials for Hemophilia B
CONCLUSIONS
Finally, as well as the studies mentioned there are many other studies that have evaluated the levels of expression of FIX using the rAAV as a vector to treat Hemophilia B and almost all have demonstrated its effectiveness18. Although exceptions were presented, these were due to other factors such as, for example, that some of the patients had an immune response to the capsids of the vectors before treatment, however, solutions such as the use of anti-antibodies were found. In the same way, there are other methods for the treatment of hemophilia B, but the greatest advantage of gene therapy over classic treatments is that the first one is applied only once while the others are performed periodically. On the other hand, the results of the trials showed, under the use of the optimized codon hFIXco and serotype 8, that the levels of expression were persistent observing levels of expression from 1% to 12%, with constancy for more than 2 years, thus demonstrating the potential of gene therapy in the treatment of hemophilia B.
REFERENCES
1. Nienhuis AW, Nathwani AC, Davidoff AM. (2017). Gene Therapy for Hemophilia. 2017; 25(5):1163–1167.
2. Berns KI. Next Generation of Adeno- Associated Virus Vectors for Gene Therapy for Human Liver Diseases. 2019.
3. Bloem E, Karpf DM, Johansen PB, Loftager M, Petersen HH, Blouse E, et al. No Title.
4. Perrin GQ, Herzog RW, Markusic DM, Herzog RW, Markusic DM. Update on Clinical Gene Therapy for Hemophilia Word count : manuscript : 4222 ; abstract : 236 Figures : 1 Tables : 1. 2018.
5. Doncarli A, Demiguel V, Guseva I, Goulet V, Bayart S. FranceCoag : a 22 ‑ year prospective follow ‑ up of the national French cohort of patients with inherited bleeding disorders. 2018; (0123456789).
6. Iorio A, Edginton AN, Blanchette V, Blatny J, Boban A, Cnossen M, et al. Performing and interpreting individual pharmacokinetic profiles in patients with Hemophilia A or B : Rationale and general considerations. 2018; (February):1–14.
7. Ward P, Walsh CE. Expert Review of Hematology Current and Future Prospects for Hemophilia Gene Therapy Current and Future Prospects for Hemophilia Gene Therapy. 2016; 4086(May).
8. Gao J, Bergmann T, Zhang W, Schiwon M, Ehrke-schulz E, Ehrhardt A. Viral Vector-Based Delivery of CRISPR / Cas9 and Donor DNA for Homology-Directed Repair in an In Vitro Model for Canine Hemophilia B. Mol Ther Nucleic Acid. 2019; 14(March):364–76.
9. Hemophilia [Internet]. Mayo Clinic. Mayo Foundation for Medical Education and Research; 2018 [cited 2019May14]. Available from: https://www.mayoclinic.org/diseases-conditions/hemophilia/diagnosis-treatment/drc-20373333
10. Treatment of Hemophilia | CDC [Internet]. Centers for Disease Control and Prevention. Centers for Disease Control and Prevention; [cited 2019May14]. Available from: https://www.cdc.gov/ncbddd/hemophilia/treatment.html
11. Herzog RW, Kuteyeva V, Saboungi R, Terhorst C. Reprogrammed CD4 + T Cells That Express FoxP3 + Control Inhibitory Antibody Formation in Hemophilia A Mice. 2019; 10(February):1–12.
12. Anguela XM, High KA. Entering the Modern Era of Gene Therapy. 2019; (October 2018):1–16.
13. Paul D, Qazilbash MH, Song K, Xu H, Sinha BK, Liu J, et al. (rAAV) vector expressing murine interleukin-12 (IL-12). 2000; 7(2):308–15.
14. Naso MF. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs. 2017; 31(4):315–32.
15. Aponte-Ubillus JJ, Barajas D, Peltier J, Bardliving C, Shamlou P, Gold D, et al. Molecular design for recombinant adeno-associated virus (rAAV) vector production. 2017.
16. Nathwani AC, Davidoff AM. Gene Therapy for Hemophilia. 2017; 31:853–68.
17. Daya S, Berns KI, Daya S, Berns KI. Gene Therapy Using Adeno-Associated Virus Vectors Gene Therapy Using Adeno-Associated Virus Vectors. 2008; 21(4).
18. Nathwani AC, Davidoff AM, Tuddenham EGD, Haemophilia KD, Centre T, Free R, et al. Advances in Gene Therapy for Haemophilia. 44(0).
Received: 17 April, 2019
Accepted: 23 May 2019
Abad Gallardo Claudia Sofía and Merchán Muñoz Brian David
School of Biological Sciences and Engineering, Yachay Tech University. Ecuador
Corresponding author: [email protected]
