CS 2019.02.01.22
Files > Conference Series > 2019 > Humboldt Kolleg 2019
Bionatura Conference Series Vol 2. No 1. 2019
“Breaking Paradigms: Towards a Multi-, Inter- and Transdisciplinary Science” In commemoration of the 250th Anniversary of Alexander von Humboldt
Genetically Engineered Probiotics and Therapies Applications
Maldonado C. Stephanie and Jijón V. Santiago
available in: http://dx.doi.org/10.21931/RB/CS/2019.02.01.22
ABSTRACT
The idea of using probiotics for health benefits in the human body is still biased since there is skepticism and since it is quite a new field of research. However, recent experiments are trying to debate that given the naturality of their consumption and the successful results from in vitro tests in combination with other therapies. Food scientists are eager to take advantage of the known beneficial properties of probiotics by using engineering technologies in order to enhance them. Using CRISPR systems present in lactobacilli aids in strain identification, while offering information on phylogeny and ecological interactions. Also, the use of genetic engineering tools could also allow the use of plasmid vaccines to prevent antibiotic resistance and the development of synthetic probiotics as microbial treatments.
Keywords: Probiotics, engineering technologies, CRISPR,
INTRODUCTION
The discovery of microorganisms that have beneficial effects on humans began in the mid-seventeenth century 1. Afterward, the human microbiome project found the purpose of such microbes that live inside the human body’s guts which used to be considered as a region of the body with only wastes and pathogenic bacteria 1.
Probiotics are quite a new field of research for scientists since their potential has been recently recognized. They are defined as microorganisms that produce a benefit in health when administered in an adequate dose on a host 2. The typical organisms are lactic acid bacteria, bifidobacteria, and genera like Bacillus and Saccharomyces 2.
Probiotics are usually presented in a compressed format like pills, tablets or powder but a method to define which of these is best has not been fully developed 2. Then they are added to foods like dairy products like yogurt, milk, and approaches to include them in nondairy foods. This is done to acquire probiotics in the most natural way possible. It is not necessary to use an oral way of administration. Now, lotions, mouthwashes, and vagitories are classified as medical devices which could carry the probiotics 2.
In 2016 there was skepticism towards the idea of using probiotics regarding their lack of reproducibility, health issues, and difficulty at standardization. Still, new research is proving these statements wrong since the results from animal trials seem promising 3. On the other hand, phages interactions with mammals research have been completely studied although they show their interactions could induce immune responses and proinflammatory attributes 3.
This review presents the uses of genetical engineering techniques on probiotics in order to enhance their existing health benefits, as well as the potential breakthrough of using synthetic probiotics as new antimicrobial therapies.
Microbiota and Phages future in medicine
A host is dependent on its microbiota because it affects the metabolism of the organism. It helps to fight against pathogens, stimulates the immune system and keeps the homeostatic state of the host. Scientists aim to find opportunities to modulate it to produce even more positive effects from it. Fecal transplantation therapy, for example, consists of extracting a sample of the microbiota from the feces of an organism and passing it to another one 4. An experiment using this therapy consisted of passing the microbiota from obese mice into germ-free mice and resulted in weight gain of the new host 4. Trials on humans have also been done to stop infections of antibiotic-resistant bacteria.
Just as probiotics, bacteriophages are part of living beings. In the case of humans, intestinal microbiota has shown to have the most interactions between phages and bacteria. With the potential to genetically engineer phages, there are many investigations that show how using phages in medicine and agriculture could be the solution to reduce humanity’s dependency on antibiotics 4.
Phage therapy has two purposes: equilibrate the microbiota or for acute antibacterial objectives. They need to be consistently conditioned by training them with samples of local bacteria or by locating those phages that already have the experience of working with these organisms. Genetic engineering of these organisms can be reached by using the CRISPR-Cas9 method to add bacterial resistance to those bacteria that are beneficial to human health or to target virulent genes inside a bacteria to kill them 4. Other ideas propose that they could help during metabolic disorders given their ability to promote the differential energy harvest 3. However, these ideas are still at the early stages, manipulating a population that lives inside an organism could result in negative consequences if not properly studied.
When the gut enters a state where its ecosystem has been disturbed and its protection functions are reduced is called dysbiosis so diseases can appear like inflammatory bowel disease 3. This information sets the initiative on how to modulate the microbiota but there are some methods to achieve this:
- By doing T cell modulation which was proven through studies in germ-free mice and their CD4 T-cell induction. T-regulatory cells are essential to control the presence of pathogenic bacteria which can be targeted to prevent illnesses 3.
- By doing dendritic cell modulation although their studies are not pretty deep 3.
- By controlling the intestinal barrier function through alterations of its permeability or inducing secretion of antimicrobial peptides and mucins with the aid of the microbiota 3.
A case study using phages and probiotics against E. coli
Enterohemorrhagic E. coli is a pathogen that produces a cytotoxic effect in the renal endothelium and in the intestinal epithelial cells 5. It is not recommended to fight this infection using antibiotics because it produces secondary effects and is not efficient 5. Because of the latter statement, there is a need to find an alternative treatment to this bacterium which is why research in 2016 used phage and probiotics to test in vitro their interactions with the bacteria. The reason to pick these two organisms is that in the past probiotics have worked to control E. coli infections by maintaining epithelial integrity, increasing mucin production and goblet cell secretion whereas phage therapy uses a specific and self-replicating therapeutic agent to stop the infection of E. coli 5.
After three assays to define the best combination of these therapies, it was concluded that the best one is applying first the probiotic treatment and then the phages in vitro. This is because if phages are applied first, they will not interfere with probiotic activity and will counterpoise the lysis effect produced from killing the pathogenic bacteria which releases toxins 5. Furthermore, in an experiment in vivo, it is probable that the host’s defenses shall get rid of the phage-resistant bacteria which survived in the in vitro experiment 5.
CRISPR-Cas engineering of food-grade probiotics
Currently, the food industry is aiming to take advantage of the popular media surrounding probiotic benefits which include gastrointestinal health and increased longevity 6. Therefore, several studies on the use of genetic engineering tools to enhance probiotic characteristics such as extended survival in the gastrointestinal tract by catabolism of non-digestible oligosaccharides, acid and bile resistance and improved host colonization 7; as well as, the use of CRISPR-Cas technology for strain-typing, plasmid vaccination, genome editing, and as potential antimicrobials 6 have been made.
Regarding immunomodulation, teichoic acids (TAs) present in lactobacilli cell walls, activate immunological responses in the host by stimulating dendritic cells and affect the adhesion of bacteria to host cells (Fig. 1A)(8). Genetic engineering of lactobacilli could alter these cell wall components suggesting better survival times in the host by improving adhesion. In addition, another study on lactobacilli TAs focused on modifying proinflammatory responses by integrating a knockout vector in the gene responsible for D-alanylation of TAs in order to acquire anti-inflammatory responses 8. Such discovery could be useful in colon cancer prevention which occurs due to chronic inflammation in the colon (Fig.1B) 8.
In silico studies discovered the presence of CRISPR Cas9 type II systems in most species of Lactobacillus 9. Food scientists have tried to exploit this particularity by applying CRISPR-Cas technology to probiotics in the following ways:
- CRISPR-Cas reacts to outside threats by adding novel spacers to the repeat-spacer array; giving an idea of the strain evolution and microbial ecology 6.
- CRISPR array genotyping is faster, more affordable, and offers higher resolution than PCR-based genotyping 9. Therefore, CRISPR-Cas genotyping has proven to be an effective method for identifying beneficial microbial strains while learning about their ecology and evolution 6.
- Plasmid vaccination implies the use of CRISPR-Cas systems to prevent the uptake of undesirable elements like antibiotic resistance genes in the host DNA (Fig.1C)

Figure 1: Genetic engineering of food-grade probiotics. (A)Removal of teichoic acids (TA) in the probiotic cell wall results in improved adhesion. (B)Engineered probiotics have anti-inflammatory responses in the colon. (C)Plasmid vaccination removes antibiotic resistance genes in plasmids before DNA is uptaken by the host.
Synthetic probiotics
Synthetic probiotics can be used in antimicrobial therapies by providing localized treatments with controlled release of therapeutics (Fig. 1A)10. Genetic engineering could allow the use of probiotics as treatment of infectious diseases by inducing a host immune response (Fig. 1B), or by mimicking toxin receptors and acting as competitive inhibitors 10). In addition, synthetic probiotics could be used as prophylactics which are engineered to express localizing peptides in order to colonize desired niches outcompeting pathogens and preventing toxin release 10.
Furthermore, the scientist is working on the development of autonomous synthetic probiotics that are able to detect pathogens and respond by expressing targeted antimicrobial agents (Fig. 1C) 10.
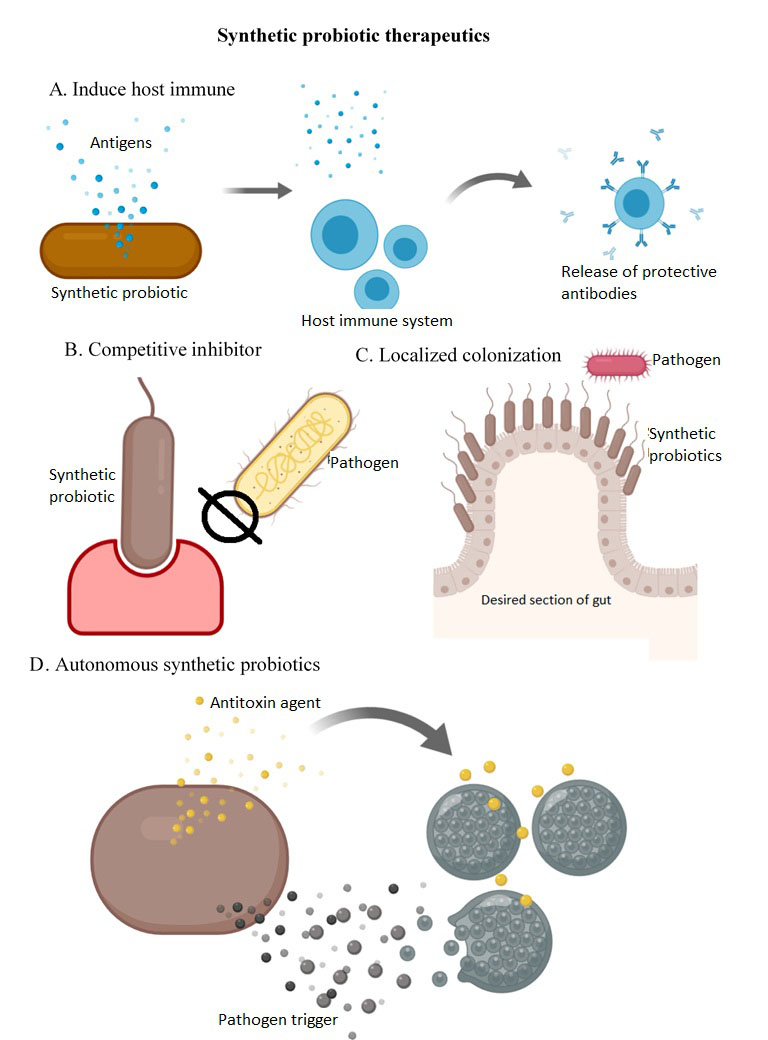
Figure 2: Synthetic probiotics as living therapeutics. (A) Probiotics can respond to pathogens by inducing the host immune response. (B)Probiotics mimic toxin receptors acting as competitive inhibitors. (C)Probiotics outcompete pathogens by colonizing desired locations. (D)Autonomous synthetic probiotics are able to sense and respond to pathogens by the release of targeted antimicrobial agents.
Synthetic probiotics could also be used as living diagnostics, which would monitor the microbiome constantly for pathogen detection responding with reporter expression 10. Diagnostic probiotics would develop a long-term synthetic memory making them able to remember the event even after the trigger disappears, lasting for multiple cellular generations 10. In order to utilize synthetic probiotics as clinical diagnostic agents, further studies are necessary to improve probiotic sensing capabilities; since probiotics have been found to respond better to exogenous inducers than to pathogen presence 10.
CONCLUSIONS
Probiotics are a promising field of study. Their attribute of being natural and the methods to introduce them into the human body have been gaining popularity. Furthermore, tests using combined therapies like phages have successful results in vitro and the in vivo expectancies are also ambitious. In food science, probiotics have also undergone numerous studies in which the use of genetic engineering tools like CRISPR promise to enhance the beneficial properties of probiotics while also developing new tools to aid in antibiotic resistance. Synthetic probiotics would act as novel treatments to microbial diseases by preventing pathogen colonization, inducing host immune responses, or by acting as competitive inhibitors.
Further studies are necessary in order to incorporate these techniques in the medical and food industries, in addition to seeking regulatory and consumer approvals.
REFERENCES
1. Vitetta L, Vitetta G, Hall S. Immunological Tolerance and Function : Associations Between Intestinal Bacteria , Probiotics ,. 2018;9(October):1–15.
2. Fenster K, Freeburg B, Hollard C, Wong C, Laursen RR, Ouwehand AC. The Production and Delivery of Probiotics : A Review of a Practical Approach. 2019;1–17.
3. Mccarville JL, Caminero A, Verdu EF. Novel perspectives on therapeutic modulation of the gut microbiota. 2016;580–93.
4. Paule A, Frezza D, Edeas M. medical sciences Microbiota and Phage Therapy : Future Challenges in Medicine. 2018;1–10.
5. Dini C, Bolla PA, Urraza PJ De. Treatment of in vitro enterohemorrhagic Escherichia coli infection using phage and probiotics. 2016;
6. Stout E, Klaenhammer T, Barrangou R. CRISPR-Cas Technologies and Applications in Food Bacteria. Annu Rev Food Sci Technol. 2017;8(1):413–37.
7. Hidalgo-Cantabrana C, O’Flaherty S, Barrangou R. CRISPR-based engineering of next-generation lactic acid bacteria. Curr Opin Microbiol [Internet]. 2017;37:79–87. Available from: http://dx.doi.org/10.1016/j.mib.2017.05.015
8. Van Pijkeren J-P, Barrangou R. Genome Editing of Food-Grade Lactobacilli To Develop Therapeutic Probiotics. Microbiol Sprectrum. 2017;5(3):1–16.
9. Selle K, Barrangou R. CRISPR-Based Technologies and the Future of Food Science. J Food Sci. 2015;80(11):R2367–72.
10. Braff D, Shis D, Collins JJ. Synthetic biology platform technologies for antimicrobial applications. Adv Drug Deliv Rev [Internet]. 2016;105:35–43. Available from: http://dx.doi.org/10.1016/j.addr.2016.04.006
Received: 17 April 2019
Accepted: 23 May 2019
Maldonado C. Stephanie* and Jijón V. Santiago*
*School of Biological Sciences and Engineering, YachayTech, Urcuquí. Ecuador.
Corresponding author: [email protected]
