2016.01.03.5
Files > Volume 1 > Vol 1 No 3 2016 > Investigaciones
INVESTIGACIÓN
Biological activity of a new Growth hormone secretagogue: study in fish and murine cell line
La actividad biológica de un nuevo secretagogo de la hormona de crecimiento: estudio en el pescado y la línea celular murina
Available from: http://dx.doi.org/10.21931/RB/2016.01.03.5
Rebeca Martínez, Katerina Gonzalez, Alain Gonzalez, Kenia Ubieta, Fidel Herrera, Osvaldo Reyes, Hilda Garay, Ayme Oliva, Elsa Rodriguez, Mario Pablo Estrada
ABSTRACT
Growth hormone (GH) has pleiotropic functions in all vertebrates. In addition to its essential role in the regulation of body growth and development, it can also influence reproduction, immunity, osmoregulation, and behavior Immune and neuroendocrine systems have bidirectional communications and it is well document the enhancing action of GH on teleost immune system .It is desirable controlled GH administration to allow growth and stimulation of the innate immune system of fish. In this study, we have characterized a chemical peptide compound, A228, designed by molecular modeling, which is able to perform the function of a GH peptide secretagogue. In pituitary cell culture, the peptide A228 induces GH secretion. It is able to increase superoxide production in tilapia peripheral blood leukocytes cultures and in a macrophage cell line J774 from mice, therefore using this molecule, innate immune system stimulation is obtained in vitro both in fish and in mammals cell cultures. In this paper is also shown the biological action in vivo of the molecule, to assess growth stimulation in tilapia larvae.
Keywords : Biological activity, Growth hormone secretagogue, murine cell line.
RESUMEN
La hormona del crecimiento (GH) tiene funciones pleiotrópicas en todos los vertebrados y juega un papel esencial en la regulación del crecimiento y el desarrollo del cuerpo. También puede influir en la reproducción, la inmunidad, la osmorregulación, y el comportamiento inmunológico. Los sistemas neuroendocrinos tienen comunicaciones bidireccionales y esta bien documentada la acción de la GH sobre el sistema inmune de teleósteo. La administración controlada de GH permitir el crecimiento y la estimulación del sistema inmune innato de los peces. En este estudio, hemos caracterizado un compuesto peptídico químico, A228, diseñado por modelado molecular, que es capaz de realizar la función de un péptido secretagogo de GH. En el cultivo de células pituitaria, el péptido A228 induce la secreción de GH y es capaz de aumentar la producción de superóxido en tilapia. En este documento también se muestra la acción biológica in vivo de la molécula, para evaluar la estimulación del crecimiento en larvas de tilapia.
Palabras clave: actividad biológica, scretagogo, hormona del crecimiento, línea celular murina.
INTRODUCTION
Growth hormone (GH) is a pluripotent hormone with essential role in the regulation of body growth and development; it can also influence reproduction, immunity, osmoregulation, and behavior (1).It is produced by the pituitary gland in teleosts as in other vertebrates .GH gene is also expressed in other tissues of fish, especially in lymphoid organs and cells (2). The expression of GH is tightly regulated by several factors.
There are have been demonstrated the interactions among elements of the endocrine and immune systems in fish. And it is desirable controlled GH administration to allow growth and stimulation of the innate immune system of fish.
The innate response is the basis of the immune defence of invertebrates and lower vertebrates. In fish, the innate immune response has been considered the essential component in combating pathogen invasions due to the limits placed on their adaptive immune response.(3) The macrophage cell lineage represents an important group of cells which play a central role in the initiation and coordination of the immune response
Among the different peptides involved in the regulation of expression of GH are the GHS. The synthetic GH secretagogues (GHSs) consist of a family of ligands, first described by Momany et al. 1981(4). Multiple efforts have been directed toward finding the active molecules, yielding the discovery of new GHSs (5). GHS bind to a receptor inducing calcium mobilization, named GHS receptor (GHSR) first identified in pigs and humans. but currently GHS receptor (GHSR) has been identified in teleosts and birds (6,7,8,9).The ligand endogenous of this receptor correspond to the ghrelin hormone , which elicits a diverse biological action besides increasing GH secretion from the anterior pituitary gland, enhancing anabolic effects such as increasing appetite, adiposity and blood glucose, enhancing gastric function and increasing cardiac output via GHS-R. GHS-R is expressed predominantly in the brain and pituitary, but it is also expressed in many peripheral organs such as the heart, lungs, liver, kidneys, pancreas, stomach, small and large intestines , adipose tissue and immune cells
The moleculeA228 was described by the exhaustive molecular modeling of the human GRLN receptor using bioinformatics tools, after which a virtual library was built and a massive docking experiment was performed against the receptor model (10).
Previous results have demonstrated the biological activity of another GHS , the peptide A233 , that induces GH secretion and it is also able to increase superoxide production in tilapia head-kidney leukocyte cultures. This effect is blocked by preincubation with the GHS receptor antagonist [D-Lys3]- GHRP6.and GH immunonetralization experiments propose a GH-mediated mechanism for the action of A233. The in vivo biological action of the decapeptide was also demonstrated for growth stimulation in fish larvae and the enhancement of some parameters of innate immune system in the treated larvae.
The aim of this study was to assess the biological activity of another GHS, the synthetic peptide A228 as a stimulator of growth and the innate immune system of teleosts fish, through studies performed in vitro and in vivo. It is also interesting to know if in a murine macrophage cell line J774 , the A 228 could stimulate the superoxide anion production .
METHODS
Fish
Tilapia (Oreochromis sp.) juvenile and larvae were obtained from the Center for Aquaculture of Mamposto´n
(CPAM). Fish were kept alive in aerated freshwater under a 12 h light:12 h darkness photoperiod. They were fed commercial dry diet for fish (CENPALAB, Habana, Cuba).Water temperature was maintained at 26 and 28 8C. All animal experiments were previously approved by the Ethics Committee of the Center for Genetic Engineering and Biotechnology, Havana, Cuba.
GH secretagogues
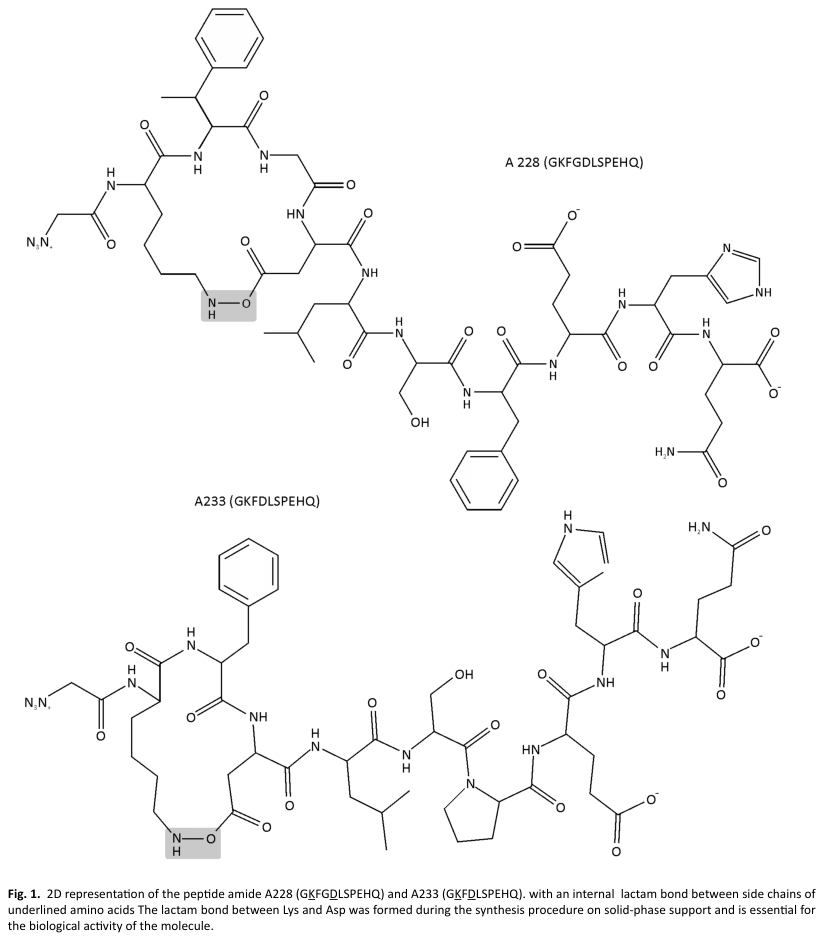
The decapeptide A228 (GKFGDLSPEHQ) y A233 (GKFDLSPEHQ) with an internal lactam bond between side chains of underlined amino acids) was manually synthesized on a solid-phase support. Crude peptide was purified by reverse-phase, high-performance, liquid chromatography up to 95% on a C-18 preparative column with an acetonitrile/water linear gradient. Trifluoracetic acid was used in both solvents for counter-ion pair formation. The correct sequence of the purified peptide was confirmed by electrospray mass spectrometry (Micromass, Manchester, UK). The positive control used was GHRP6 (Lipotec, Barcelona, Spain) lyophilized peptides were reconstituted in PBS.
Primary culture of pituitary cells
The in vitro effects of A233 GHS were examined using cells dispersed from whole pituitary. Mature tilapias of both sexes weighing 300-500 g were used after anesthesia in tricaine methanesulfonate (MS-222, Sigma). Pituitaries were collected aseptically in isotonic medium (Krebs bicarbonate- Ringer solution, 330 m Osmolal, pH 7.4) supplemented with penicillin (100 IU/ml), streptomycin (0.1 mg/ml), and nystatin (250 IU/ml, all from Sigma). The pituitaries were diced with a sterile razor blade and treated with collagenase for 1 h at room temperature in 2.5 ml trypsin-EDTA solution (0.25% trypsinC0.02% EDTA in PBS, pH 7.4). Tissues were
aspirated repeatedly through a pipette during enzymatic treatment to promote dissociation of cells. The process was terminated by the addition of 0.5 ml (20%) fetal bovine serum (Sigma). Cells were counted on a hemocytometer under a light microscope and viability determined by trypan blue exclusion. Viability of the cells was always O95%. Cells were then plated at a density of 4.0!105 cells/well into a 24-well plate (Falcon, Primaria 24, Becton Dickinson, Franklin Lakes, NJ, USA) at a volume of 300 ml/well of isotonic medium supplemented with 10% fetal bovine serum. The cells were preincubated for 4 days at 26-28 8C under a humidified atmosphere of 95% O2 and 5% CO2, with one change of culture medium at 48 h post-plating. Before each experiment, cells were washed once with serum-free medium. A final 300 ml serum-free medium was added containing A233, GHRP6 (Lipotec), or control medium without hormones. The medium was replaced at 4 h.Incubations were terminated at 8 h, and hormone release was quantified for the 0-4 and 4-8 h intervals. GH release was expressed as secretion per unit volume of medium (ng/ml).
GH assays
The GH secreted in vitro was measured using a noncompetitive ELISA as described by Lugo et al. 2008 (11). The 96-well MaxiSorp plates (Nalge Nunc International, Roskilde, Denmark) were coated (3 h at 37 8C) with anti-tilapia GH monoclonal antibody 1 (tiGH1 mAb; CIGB, Santi Spiritus, Cuba) at 10 mg/ml in 0.05 M carbonate buffer (pH 9.6, 100 ml/well). The plates were washed two times with PBS-T (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4 .7H2O, and 0.05% Tween 20, pH 7.3) and blocked with 3% skim milk (Oxoid, Cambridge, UK) in PBS 1! (200 ml/well) for 1 h at 37 8C. A standard tiGH curve in the range of 35-0.136 ng/ml was obtained by twofold dilutions in 0.5% skim milk in PBS 1!, dispensed by duplicate in the same plate. Test samples were diluted at a ratio of 1:2 as described earlier and were incubated on the plates overnight at 4 8C. After washing the plates four times with PBS-T, 100 ml HRP - tiGH2 mAb conjugate (CIGB), diluted at a ratio of 1:15 000 in PBS 1!containing 0.5% skim milk, were added to each well. The plates were incubated for 1 h at 37 8C and then washed eight times with PBS-T. Then, the substrate buffer (0.2 M Na2HPO4, 0.1 M citric acid (pH 5.0) containing 0.5 mg/ml ortho-phenylenediamine and 5 ml 30% H2O2) was added (100 ml/well). The reaction was stopped 15 min later by adding 50 ml of 2.5 M sulfuric acid per well. The absorbance was measured at 492 nm using the Titertek Multiskan Plus spectrophotometer. The accepted upper limit of the assay background was 0.094. The lower detection limit of the assay was 0.1 ng/ml. The degree of variation and intra- and interassay coefficients of variation are 3.90 and 13.45% respectively.
Isolation and culture of leukocytes
Peripheral blood leukocytes were isolated as described by Acosta et al, 2010(12) .Tilapias were anesthetized in tricaine methanesulfonate (MS222), and blood was collected from the caudal vessels using a heparinized syringe. Blood was diluted 1:2 in RPMI-1640 medium, containing 4 μg/mL of gentamicin. The mixture was placed on Ficoll-Paque™ Research Grade (Amersham Pharmacia Biotech, Sweden) and centrifuged at 400 g for 25 min. The leukocyte band was harvested, washed with phosphate-buffered saline (PBS, pH 7.6), and suspended in RPMI-1640 medium with 0.5% tilapia serum, and cultured at 25 _C, in 5% CO2 for 24 h. Leukocyte viability was assessed by trypan blue exclusion (viability N95%).
Growth conditions for macrophage cell line J774
Murine macrophage cell lines J774 was obtained from the American Type Culture Collection (ATCC ). The cells were cultured as monolayers in Dulbecco's Modified Essential Medium (DMEM) F12 supplemented with 20% fetal bovine serum,100 mg/mL penicillin/streptomycin, and 2mM L-glutamine (Mediatech, Inc., Herndon, VA) at 37 1C in a humidified atmosphere of 5% CO2. The cultured cells were grown to 70-80% confluence in 75 cm2 flasks for preparation of samples that were used in further analysis.
Superoxide production in cell culture
To analyze the effect of GHSs on the immune system, the superoxide anion was measured in tilapia PBLs and macrophage cell line J774 as the reduction of NBT (Sigma) as described by Yada et al. 2006 (13)
Growth performance experiment
The growth promoting activity of A233 was evaluated in tilapia larvae (Oreochromis sp.) of 0.007G0.001 g mean weight and (n=200). The effect was compared with GHRP6 as positive control. The fish were acclimated at
28 8C in 120 l tanks with running fresh water for 1 week before the experiment. They were fed with a basal diet to satiation twice a day. Before treatment, the tanks were cleaned. The fish were immersed into each treatment for 60 min without water recirculation. The immersion procedure was performed three times a week, for a period
of 1 month. Three experimental groups were handled with the following treatments: groups 1 and 2 received the peptides A228 and GHRP6 (positive control) at a dose of 0.1 mg/l, respectively, and a no treated group in the third one. Growth-promoting effects were evaluated by measuring the body weight increase.
Statistical analysis
Results were evaluated using GraphPad Prism version 4.0 for Windows, GraphPad Software, San Diego, CA, USA. All results are expressed as the mean ±S.D. Statistical analysis was performed using one-way ANOVA by Newman-Keuls's or Dunnett's method for data with normal distribution and equal variances: GH assays, superoxide production, growth performance experiment of tilapia (Oreochromis sp.) larvae. Data with unequal variances were analyzed by the Kruskal-Wallis test followed by Dunn's multiple comparisons post-test. Treatments were considered to be significantly different if P<0.05.
RESULTS
The molecules A 228 and A233 (Fig. 1) were selected from the virtual libraries described by Rodriguez et al. 2007(10), and, in order to determine in vitro and in vivo whether this novels mimetic molecules function as GHSs, the following assays were conducted on the growth and immune system.
GH in vitro assays using pituitary cell culture
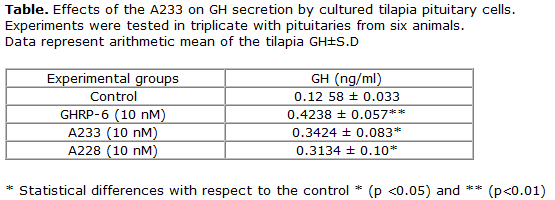
To evaluate the effect of A228 and A233 peptide on GH secretion, we performed an in vitro culture of cells in the pituitary gland of tilapia (Oreochromis sp.). the peptides at a concentration of 10 nM stimulated GH secretion at 8 h by cells in the anterior pituitary gland (Table). The stimulatory effect of GRLN and GHSs on the release of GH in vitro by the pituitary gland has been reported in mammals, birds, and different species of fish (14,15,16 17, 18).
Determination and characterization of superoxide anion induction in PBLs and macrophage cell line J774
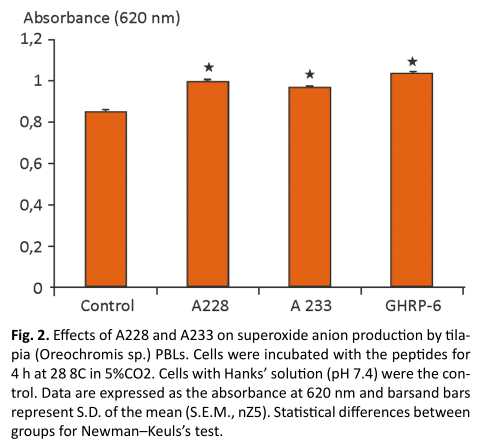
Superoxide anion produced by cultured tilapia (Oreochromis sp.) PBLs were measured by the addition of A 228 as positive control GHRP-6 was included and also A233, which in previous results was capable of enhance superoxide anion production in leukocytes from tilapia head kidney. The peptides induced significant increases in superoxide production. The concentration of peptide used was 10 n M taking in consideration previous results ( 19) , The increase of superoxide anion production was statistically higher in cells stimulated with the peptides, to compare with no treated cells, among the different treatments there were not statistical differences, in both cultures, PBLs ( Fig. 2 ) this effect is similar to that reported by Acosta et al. 2010,(12) who stimulated phagocytic peripheral blood cells of tilapia (Oreochromis sp.) with recombinant tiGH (Oreochromis hornorum).
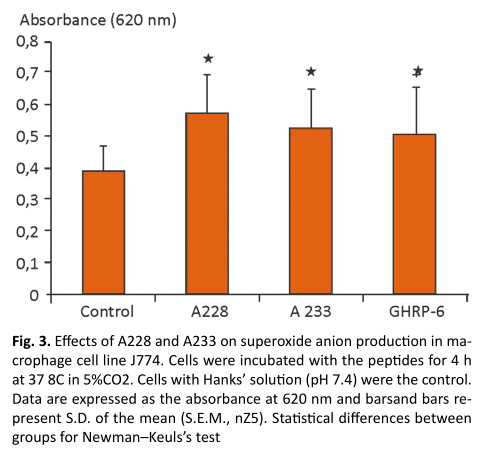
The results obtained in the production of superoxide anion with the macrophage cell line J774.after the stimulation with the peptides, were very similar, there were obtained statistically differences in the superoxide anion production of cells treated with the peptides , and compare with negative control. (Fig. 3)
In vivo biological activity assays
Experiments were performed to evaluate the biological effect of the peptide on the growth rate of tilapia larvae. The effect of these peptides on fish growth was determined by measuring the increase in body weight. The tilapia larvae treated with A228 showed a significant increase in body weight compared with the controls as well as the larvae treated with GHRP -6 , just 9 days after treatment.
DISCUSSION
In this study, we demonstrate the effect of another peptide molecule having internal cycles and composed solely of L-amino acids that are capable of exerting, due to their chemical structure, similar functions to those attributed to GRLN, des-acyl GRLN, and other peptidic GHS. They are a group of peptide compounds and peptides capable of stimulating production and GH secretion in vitro and in vivo.
There were not differences between both peptides A228 and A233, the structural difference do not interfere on GH secretagogue function at least in this kind of primary culture of pituitary cells experiment.
Our results are similar to those obtained in tilapia (Oreochromis mossambicus), where the effect of GRLN on GH secretion in vitro was dependent on the concentration of the endogenous secretagogue used (15). Other researchers have reported the stimulatory effect of the synthetic peptides GHRP-6, PACAP, and PACAP-related peptide from Clarias gariepinus on the release of GH in vitro by the pituitary gland of tilapia (Oreochromis niloticus); (11,19).There should be conducted an experiment using the A228 peptide to study the GH releasing in vivo after the administration of GHS.
It has been reported that the immune system of both fish and mammals possesses both non-specific and specific immune responses with cellular and humoral components. However, fish depends more heavily on non-specific defence mechanisms. The use of immunostimulants given as dietary supplement can improve the innate defense of aquatic animals principally fish and thus providing resistance to pathogens during period of stress, such as grading, sea transfer and vaccination.
Neutrophils and macrophages are known as the phagocytic leucocytes in fish peripheral blood (20) . Macrophages play a key role in the host immune system. They are in the first line of defence, participating in detection and identification of potential pathogens. As part of mechanisms involved in the innate system they respond to stimuli activating phagocytosis and releasing reactive oxygen and nitrogen species which destroy microbes. They also initiate the inflammatory response through cytokine production, and furthermore, macrophage acts a link between the innate and adaptive immune responses acting as antigen-presenting cells to prime T cells. ( 21)
The existence of the binding sites of GH has been shown in both types of leucocytes in the gilthead sea bream Sparus aurata .It is known also that the administration in vivo and in vitro GH stimulates the production of superoxide anion after phagocytosis as a mechanism for elimination of pathogens (2)
In our result we could state the biological action of or peptide in fish and mammals system, There are controversial reports about the effects of ghrelin on phagocytotic activity of leukocytes. In fish (rainbow trout), the administration of ghrelin increased phagocytosis and superoxide production in zymosan-stimulated leukocytes, which was abolished by pretreatment of leukocytes with a GHS-R antagonist (2). It was also shown that ghrelin increased mRNA levels of superoxide dismutase and GH in leukocytes, suggesting that the effects of ghrelin was mediated, at least in part, by stimulating GH secretion from leukocytes. On the other hand, ghrelin administration reportedly reduced the elevated phagocytic activity of peritoneal macrophages induced by acute cold-restraint stress in rats ( 22 ) . In our study we have found an increase of superoxide anion in fish PBL and cell line J774 from murine macrophages after A228 stimulation even has been reported that ghrelin modulates phagocytosis directly or indirectly via GH, but in a different way in different species it should be considered the treatment conditions to evaluate this biological activity.
GHSs are useful molecules as growth enhancement molecules These synthetic molecules are effective in stimulating production and release of endogenous hormone as a physiological response, with no side effects on the pituitary or toxicity potential; besides their low molecular weight, it makes a better entrance to the organism.
In our laboratory, we have successfully employed the immersion bath technique to study the effects of nutritional supplements and growth factors on growth control and the immune system in fish (12). The immersion bath method used for our studies requires little manipulation and causes minimum stress to fish during treatment. There is evidence suggesting that the gill pillar cells are a possible entry site for some molecules when fish are treated by immersion bath (6).
This study evaluated the biological function of synthetic peptide A228 on weight gain of tilapia (Oreochromis sp). Tilapia larvae showed a significant increase in growth after 9 days of treatment with peptide A228 (0.1 mg/l). All animals received the same commercial diet, so the increase in weight and height is due to the administration of peptide A228. The positive control group represented by the fish treated with the peptide GHRP6 significantly increased their growth. Similar results were obtained in a former work to administrate A233 peptide, another GHS, to fish larvae enhancing growth and some. humoral innate immune system parameters (Fig. 4)(19).
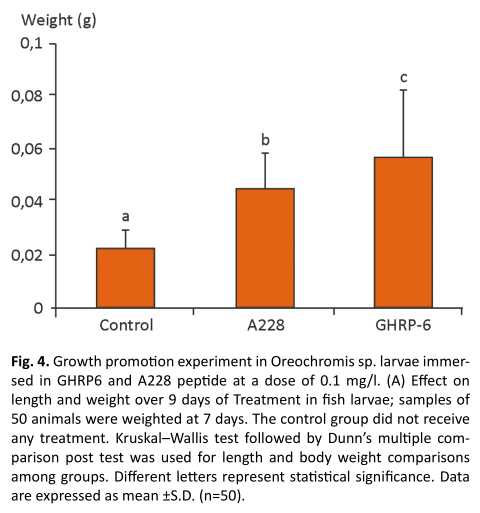
These results are similar to those found in mice where there was an increase in body weight after dosing by s.c. injection of the synthetic peptide GHRP2 (23). In adult rats, GHRP6 also increased body weight (24) as well as other GHSR agonists, like SM-130686 administered orally (25) and BIM-28131 administered by s.c. injection (26). It has been reported that GHSR1a agonist, a pentapeptide with D-amino acids, promotes weight gain in rats, by i.p. administration during 7 days (27). Moreover, the administration, by the same method, of GH tilapia (O. hornorum) secreted into the culture supernatant of yeast Pichia pastori (28) to larvae of tilapia (Oreochromis sp.), significantly increased growth of these animals. Administration of tilapia recombinant neuropeptide Y (Oreochromis sp.) to larvae of African catfish (C. gariepinus) also produced an increase in animal weight (29).
In addition, treatment with PACAP and PACAP-related peptide from C. gariepinus larvae of African catfish (C. gariepinus), tilapia (O. niloticus), and common carp (Cyprinus carpio) increased body weight and length in three fish species (11).
The application of the peptide during the early stages of fish development should be very important, The peptide designed by modeling bioinformatics from human ghrelin receptor and obtained by chemical synthesis is recognized by the secretagogue receptor GHS-R1a, stimulating the secretion of growth hormone due to the action of growth hormone results in further development of cells and tissues of the larvae. It promotes accelerated growth with increased weight concurrently is recognized by immune system cells and stimulates the innate immune response, the main defense mechanism of fish during the early stages of life as a result you can get higher quality larvae
To summarize, the action of this GHS has been assessed employing in vitro and in vivo methods, we have demonstrated the effect of a new growth hormone secretagogue , A228, as a growth hormone secretagogue, that elicits GH in tilapia pituitary cell culture and also has stimulatory effect on the superoxide anion production on tilapia PBL and a cell line J774 from mice macrophages .To administrate the molecule using immersion baths, the weight gain is enhanced in tilapia larvae . These results support the interrelation between endocrine and immune system.
REFERENCES
1. Devlin R, Sakhrania D, TymchukaW, RiseM& Goha B. Domestication and growth hormone transgenesis cause similar changes in gene expression in coho salmon (Oncorhynchus kisutch). PNAS 2009;106: 3047-3052. (doi:10.1073/pnas.0809798106)
2. Yada T . Growth hormone and fish immune system. General and Comparative Endocrinology 2007; 152 : 353-358. (doi:10.1016/j.ygcen.2007. 01.045)
3. Magnadottir B (2006) Innate immunity of fish (overview). Fish and Shellfish Immunol. 20: 137-151.
4. Momany FA, Bowers CY, Reynolds GA, Chang D, Hong A & Newlander K Design, synthesis and biological activity of peptides which release growth hormone, in vitro. Endocrinology 1981;108 :31-39. (doi:10.1210/endo-108-1-31)
5. Moulin A, Ryan J, Martinez J & Fehrentz JA Recent developments in ghrelin receptor ligands. ChemMedChem 2007; 2: 1242-1259. (doi:10.1002/cmdc. 200700015)
6. Palyha OC, Feighner SD, Tan CP, McKee KK, Hreniuk DL & Gao YD Ligand activation domain of human orphan growth hormone (GH) secretagogue receptor (GHS-R) conserved from Pufferfish to humans. Molecular Endocrinology 2000; 14: 160-169. (doi:10.1210/me.14.1.160)
7. Kaiya H, Kodama S, Ishiguro K, Matsuda K, Uchiyama M, Miyazato M, Uchiyama M, Miyazato M & Kangawa K Ghrelin-like peptide with fatty acid modification and O-glycosylation in the red stingray, Dasyatis akajei. BMC Biochemistry 2009a ;10: 30. (doi:10.1186/1471-2091-10-30)
8. Kaiya H, Mori T, Miyazato M & Kangawa K 2009b Ghrelin receptor (GHS-R)-like receptor and its genomic organisation in rainbow trout, Oncorhynchus mykiss. Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology 153 438-450. (doi:10.1016/j.cbpa.2009. 04.612)
9. Kaiya H, Riley LG, Janzen W, Hirano T, Grau EG & Miyazato M . Identification and genomic sequence of a ghrelin receptor (GHS-R)-like receptor in the Mozambique tilapia, Oreochromis mossambicus. Zoological Science 2009c ; 26: 330-337. (doi:10.2108/zsj.26.330)
10. Rodriguez R, De la Nuez A, Estrada MP, Martinez R, Chinea G, Reyes O, Fernandez R, Garcia D, Berlanga A & Musacchio A Compounds analogous to growth hormone peptide secretagogues and preparations containing them. 2007 (Patent publication No.: WO 2007/098716 A1).
11. Lugo JM, Rodriguez A, Helguera Y, Morales R, Gonzalez O, Acosta J, Besada V, Sanchez A & Estrada MP Recombinant novel pituitary adenylate cyclase activating polypeptide (PACAP) from African catfish (Clarias gariepinus) authenticates its biological function as a growth promoting factor in low vertebrates. Journal of Endocrinology 2008;197 :583-597. (doi:10.1677/JOE-07-0555)
12. Acosta J, Carpio Y, Morales R, Aguila JC, Acanda Y, Herrera F & Estrada MP New insights into the biological activity and secretion properties of a polypeptide derived from tilapia somatotropin. Comparative Biochemistry and Physiology. Part B, Biochemistry & Molecular Biology 2010; 156: 264-272. (doi:10.1016/j.cbpb.2010.04.001)
13. Yada T, Kaiya H, Mutoh K, Azuma T, Hyodo S, y Kangawa K (2006) Ghrelin stimulates phagocytosis and superoxide production in fish leukocytes. J. Endocrinol. 189: 57-65.
14. Kaiya H, Kojima M, Hosoda H, Riley LG, Hirano T, Grau EG & Kangawa K Amidated fish ghrelin: purification, cDNA cloning in the Japanese eel and its biological activity. Journal of Endocrinology 2003 a ; 176: 415-423. (doi:10.1677/joe.0.1760415)
15. Kaiya H, Kojima M, Hosoda H, Riley LG, Hirano T & Grau EG. Identification of tilapia ghrelin and its effects on growth hormone and prolactin release in the tilapia, Oreochromis mossambicus. Comparative Biochemistry and Physiology. Part B, Biochemistry & Molecular Biology 2003b; 135: 421-429. (doi:10.1016/S1096-4959(03)00109-X)
16. Unniappan S & Peter RE In vitro and in vivo effects of ghrelin on luteinizing hormone and growth hormone release in goldfish. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 2004; 286: R1093-R1101. (doi:10.1152/ajpregu.00669.2003)
17. Fox BK, Riley LG, Dorough C, Kaiya H, Hirano T & Grau EG Effects of homologous ghrelins on the growth hormone/insulin-like growth factor-I axis in the tilapia, Oreochromis mossambicus. Zoological Science 2007;24: 391-400. (doi:10.2108/zsj.24.391)
18. Picha ME, Strom CN, Riley LG, Walker AA, Won ET, Jhnstone WM & Borski RJ Plasma ghrelin and growth hormone regulation in response to metabolic state in hybrid striped bass: effects of feeding, ghrelin and insulin-like growth factor-I on in vivo and in vitro GH secretion. General and Comparative Endocrinology 2009;161: 365-372. (doi:10.1016/j.ygcen. 2009.01.026)
19. Martinez R., Ubieta K, Herrera , F, Forellat, A , Morales R , de la Nuez A, Rodriguez, R, Reyes,O, Oliva , A, Estrada , MP. A novel GH secretagogue, A233, exhibits enhanced growth activity and innate immune system stimulation in teleosts fish Journal ofEndocrinology 2012; 214 : 409- 419, (doi : 10.1530/JOE-11-0373)
20. Secombes, C.J., The nonspecific immune system: cellular defenses. In: Iwama, G., Nakanishi, T. Eds. The Fish Immune System: Organism, Pathogen, and Environment. 1996 Academic Press, New York, pp. 63_103. Siwicki, A.K., Anderson, D.P., Rumsey.
21. Janeway CA, Jr Medzhitov R : Innate immune recognition. Ann Rew Immunol 2002 ; 20: 197- 216.
22. Hattori N. Expression, regulation and biological actions of growth hormone (GH) and ghrelin in the immune system. Growth Hormone & IGF Research 2009;19 :187-197. (doi:10.1016/j.ghir.2008.12.001)
23. Tscho¨p M, Statnick MA, Suter TM & Heiman ML GH-releasing peptide-2 increases fat mass in mice lacking NPY: indication for a crucial mediating role of hypothalamic agouti-related protein. Endocrinology 2002;143: 558-568. (doi:10.1210/en.143.2.558)
24. Svensson SJ, Lall S, Dickson L, Bengtssonl B-A , Romer J, Ahnfelt-Ronne I, Ohlsson C & Jansson J-O The GH secretagogues ipamorelin and GH-releasing peptide-6 increase bone mineral content in adult female rats. Journal of Endocrinology2000; 165: 569-577. (doi:10.1677/joe.0.1650569)
25. Nagamine J, Nagata R, Seki H, Nomura-Akimaru N, Ueki Y, Kumagai K, Taiji M & Noguchi H Pharmacological profile of a new orally active growth hormone secretagogue, SM-130686. Journal of Endocrinology2001; 171: 481-489. (doi:10.1677/joe.0.1710481)
26. Strassburg S, Anker SD, Castaneda TD, Burget L, Perez-Tilve D, Pfluger PT, Nogueiras R, Halem H, Dong J, Culler M et al. Long-term effects of ghrelin and ghrelin receptor agonists on energy balance in rats. American Journal of Physiology. Endocrinology and Metabolism 2008;295:E78-E84. (doi:10.1152/ajpendo.00040.2008)
27. Dong JZ, Zhang J, Taylor JE, Halem H, Datta R, Culler M & Eynon J .GHS-1a agonists that effectively stimulate food intake and body weight gain. Peptides for Youth: the Proceedings of the 20th American Peptide Symposium, Advances in Experimental Medicine and Biology 2009; 611: 487-488. (doi:10.1007/978-0-387-73657-0_210)
28. Acosta J, Carpio Y, Besada V, Farnos O, Morales R, Sánchez A, Curbelo Y, Ayala J, y Estrada MP (2008) Recombinant truncated tilapia growth hormone enhances growth and innate immunity in tilapia fry (Oreochromis sp.). Gen Comp Endocrinol. 157:49-57
29. Carpio Y, Leon K, Acosta J, Morales R & Estrada MP Recombinant tilapia neuropeptide Y promotes growth and antioxidant defenses in African catfish (Clarias gariepinus) fry. Aquaculture 2007; 272 :649-655. (doi:10.1016/j. aquaculture.2007.08.024)
Recibido: 14 de marzo de 2016.
Aprobado: 20 de mayo de 2016.
Aprobado: 20 de mayo de 2016.
Rebeca Martínez1, Katerina Gonzalez2, Alain Gonzalez1, Kenia Ubieta1, Fidel Herrera1, Osvaldo Reyes3, Hilda Garay 3, Ayme Oliva 1, Elsa Rodriguez1, Mario Pablo Estrada1
1 Biotechnology Animal Division, Aquatic Biotechnology Department, CIGB, PO Box 6162, Havana 10600, Cuba.
2 Escuela Cs. Ambientales, Laboratorio Fisiología de Peces. Universidad Católica de Temuco. Chile.
3 Physico-Chemistry Division, Center for Genetic Engineering and Biotechnology, PO Box 6162, Havana 10600, Cuba.
Correspondence : Rebeca Martinez. Email: [email protected]
