2023.08.04.25
Files > Volume 8 > Vol 8 no 4 2023
Antimicrobial and Antioxidant Activity of Rhamnolipids Biosurfactant is Produced by Pseudomonas aeruginosa
1Department of Ecology, College of Science, University of Basrah. Basrah, Iraq.
2Department of Biology, College of Science, University of Basrah. Basrah, Iraq, [email protected].
3 Department of Biology, College of Science, University of Basrah. Basrah, Iraq, [email protected].
*Corresponding author: [email protected].
Available from. http://dx.doi.org/10.21931/RB/2023.08.04.25
ABSTRACT
Rhamnolipids are the glycolipid biosurfactant produced by different Pseudomonas species; they show antimicrobial activity and antioxidant. The findings of antimicrobial activity showed the rhamnolipid biosurfactant had an antimicrobial effect against the microorganisms at different concentrations, such as toward Bacillus cereus and Klebsiella pneumoniae, while a lower inhibitory effect toward Staphylococcus aureus and Pseudomonas aeruginosa. The rhamnolipid biosurfactant was shown to have a lower inhibitory effect against fungal strains Candida albicans and Aspergillus niger. The lower minimum inhibitory concentration (MIC) values of rhamnolipid biosurfactant toward the investigated microorganisms were 2 mg/ml for E. coli, Klebsiella pneumoniae and Pseudomonas aeruginosa and 3 mg/ml for Staphylococcus aureus, Enterobacter cloacae, Bacillus cereus, Proteus mirabilis, Candida albicans and Aspergillus niger. Rhamnolipid biosurfactant was tested as an antioxidant agent; the results showed 22.7 %, 47.4 %, 79.8 %, 85 % and 91.4 % of antioxidant activity at concentrations 5, 10. 15, 20 and 25 mg/ml, respectively. Cytotoxicity of the rhamnolipid biosurfactant was also examined at different concentrations against human erythrocytes. Hemolysis of the erythrocytes was observed at concentrations 100, 75, 50, 40 and 35 mg/ml, whereas the results exhibited no hemolysis at concentrations 25 and 15 mg/ ml. The study concluded that rhamnolipid biosurfactant showed effective antioxidant activity, no hemolysis at lower concentrations and has a high antimicrobial effect. The rhamnolipid biosurfactant is a suitable and great alternative to be employed as an effective and safe therapeutic agent.
Keywords: Antimicrobial; Antioxidant; Cytotoxicity; Rhamnolipid; Pseudomonas aeruginosa
INTRODUCTION
Antibiotic abuse has led to the developing of multi-drug resistant pathogens to commercially marketed antibiotics. The combatting of resistant infections emergence is required to search for the development of novel antimicrobial drugs having broad spectrum antimicrobial activity1. The problem of the increasing resistance by pathogens to some antimicrobial compounds has paid attention to investing in natural compounds with various mechanisms of action as suitable alternatives to existing antibiotics 2. Several biosurfactant compounds have exhibited antimicrobial activity against different human pathogenic bacteria, making them a suitable substitute for existing antimicrobial agents as potent therapeutic agents 3.
Rhamnolipids are the glycolipid biosurfactant that is produced by different bacterial species such as Pseudomonas aeruginosa, Pseudomonas plantarii, Pseudomonas chlororaphis, Pseudomonas putida, Pseudomonas fluorescens, Burkholderia pseudomallei, Burkholderia thailandensis, Burkholderia plantarii, Burkholderia glumae and Serratia rubidaea SNAU02 4, 5, 6, 7.
Pseudomonas aeruginosa is a significant producer of the rhamnolipid biosurfactant, which is widely distributed in the environment and can be available in various habitats, where it survives in these habitats due to its extraordinary physiological abilities8. Rhamnolipids are sustainable and have excellent physicochemical properties, making them attractive for utilization in cosmetic, food, pharmaceutical, and detergent manufacturing 9. Rhamnolipids are suitable food additives as food preservatives and texturizing agents such as antimicrobials, antioxidants, emulsifiers and stabilizers that can be used in food processing10.
Remarkable inhibitory activity of rhamnolipids biosurfactants against Gram-positive bacteria and two fungi species Chaetonium globosum and Penicillium funiculosum, while no inhibition was shown effects against Gram-negative bacteria and two fungi species Aureobasidium pullulans and Penicillium chrysogeum 11. The antimicrobial activity of rhamnolipid biosurfactants produced by P. aeruginosa ST5 against a variety of opportunistic and pathogenic microorganisms, including antibiotic-resistant Staphylococcus aureus and Escherichia coli and the fungal pathogens including Candida albicans and Cryptococcus neoformans12.
The exact mechanism action of rhamnolipid biosurfactant against microbes is not entirely understood and unknown. Still, it is supposed that the plasma membrane of the cell is the target, as the rhamnolipid has an amphipathic nature that allows it to interact with the phospholipids of the plasma membrane13. The current study aimed to assess the antimicrobial and antioxidant activity of rhamnolipids biosurfactants formed by local Pseudomonas aeruginosa isolate.
MATERIALS AND METHODS
The rhamnolipid biosurfactant Production
The rhamnolipid biosurfactant in the current study was produced from Pseudomonas aeruginosa in a previous study from hydrocarbon-contaminated soil 14. The rhamnolipid biosurfactant was extracted, purified, and characterized in a previous study15.
Biological activity
Antimicrobial activity of the rhamnolipid biosurfactant
The antimicrobial effect of the rhamnolipid biosurfactant was carried out according to Nanda and Saravanan16. The antimicrobial effect was measured using the agar well diffusion method. The bacterial isolates (Bacillus cereus, Escherichia coli, Enterobacter cloacae, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa and Staphylococcus aureus) and two fungal strains include Candida albicans and Aspergillus niger were activated individually on Mollar Hinton agar. The rhamnolipid biosurfactant was dissolved in DMSO to prepare the following concentrations (5, 10,15, 20 and 25 mg/ ml) using the serial rhamnolipid dilution technique. The antimicrobial effect of the rhamnolipid biosurfactant was determined by measuring the inhibition zone diameter against each strain.
Minimal Inhibition Concentration (MIC)
The rhamnolipid biosurfactant's minimal inhibition concentration (MIC) value was determined according to TC et al.17. MIC was determined by a serial dilution technique using the agar diffusion method. A 10 mg/ml stock solution of the rhamnolipid biosurfactant in DMSO was diluted to concentrations 1, 2, 3 and 4 mg/ml. The minimal inhibition concentration (MIC) value was determined against a panel of pathogenic isolates provided by the Department of Biology, College of Science, University of Basrah.
Antioxidant activity of rhamnolipid biosurfactant
According to Barros et al.18, the method was carried out with some modifications in the present study. One ml of sample at concentrations 5 and 10. 15, 20 and 25 mg/ml of Dimethylsulfoxide (DMSO) were added to 1 ml of 0.2 mM methanolic 2,2-diphenyl-1-picrylhydrazyl (DPPH) of freshly prepared DPPH solution. The reduction of DPPH radicals was measured by spectrophotometer (PG, UK) at 517 nm after incubation in the dark for 30 min. Ascorbic acid was used as the positive control with the same concentration of the samples. The percentage of scavenged DPPH radical was calculated using the following formula:
DPPH radical scavenging % = [(A0 – A1)/A0] x 100
Where A0 is the absorbance of the DPPH solution, and A1 is the absorbance of the sample.
Cytotoxicity assay of rhamnolipid biosurfactant
The toxicity of rhamnolipid was tested against human erythrocytes according to a method of He et al.19. The blood suspension was prepared by adding 1 ml of blood into 20 ml of physiological saline. The rhamnolipid concentrations (15, 25, 35, 50, 75 and 100 mg/ml) were prepared in DMSO. The test was performed by adding 100 µl of varying concentrations to 2 ml of blood suspension. Then, the tubes were incubated at 37 °C, and the turbidity of the solution was monitored after (3-24 h). The positive result was turning the blood solution into turbid, while the negative result was turning the blood solution into clear. Blood suspension with tap water was used as a positive control, and blood suspension with normal saline was used as the negative control in addition to the DMSO as a control. The concentrations that gave a turbid solution due to lysing of erythrocytes as an indication of the toxicity degree of rhamnolipid.
RESULTS
The antimicrobial activity
The results of antimicrobial activity showed that the rhamnolipid biosurfactant had an antimicrobial effect against all the tested pathogenic isolates at all concentrations (5, 10, 15, 20 and 25 mg/ml); the antimicrobial effect was proportional to the rhamnolipid biosurfactant concentrations as shown in (figure 1 and table 1). The rhamnolipid biosurfactant showed a higher inhibitory effect against Bacillus cereus (21/5, 23/10, 24/15, 25/20 and 25/25 mm/mg) and Klebsiella pneumoniae (19/5, 20/10, 22/15, 23/20 and 24/25 mm/mg), while lower inhibitory effect against Staphylococcus aureus (15/5, 19/10, 20/15, 20/20 and 20/25 mm/mg) and Pseudomonas aeruginosa (16/5, 19/10, 20/15, 20/20 and 20/25 mm/mg). The rhamnolipid biosurfactant was shown to lower inhibitory effect against fungal strains Candida albicans (15/5,18/10, 19/15, 19/20 and 19/25 mm/mg) and Aspergillus niger (14/5, 15/10, 15/15, 15/20 and 16/25 mm/mg)
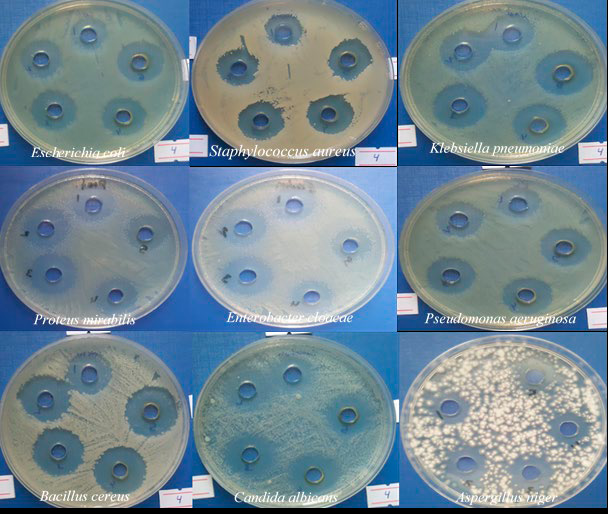
Figure 1. The antimicrobial activity of rhamnolipid biosurfactant at different concentrations (5, 10,15, 20 and 25 mg/ ml) against nine pathogenic isolates, including seven bacteria and two fungi
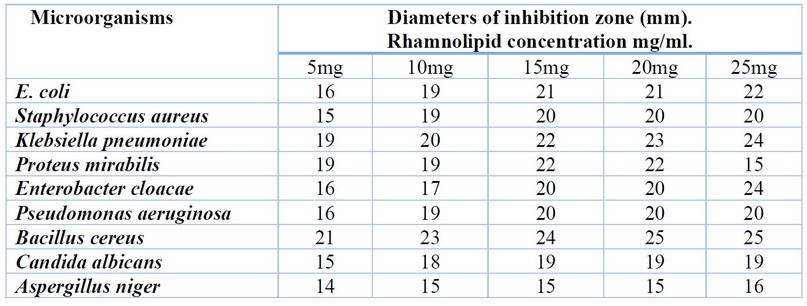
Table 1. The antimicrobial activity of rhamnolipid biosurfactant against seven pathogenic bacteria and two pathogenic fungi
Minimal Inhibition Concentration
The MIC values of rhamnolipid biosurfactant against pathogenic isolates are shown in Figure 2. The rhamnolipid biosurfactant showed the lowest minimum inhibitory concentration (MIC) values against the pathogenic isolates; it was 2 mg/ml for E. coli, Klebsiella pneumoniae and Pseudomonas aeruginosa and 3 mg/ml for Staphylococcus aureus, Enterobacter cloacae, Bacillus cereus, Proteus mirabilis, Candida albicans and Aspergillus niger as shown in table 2.
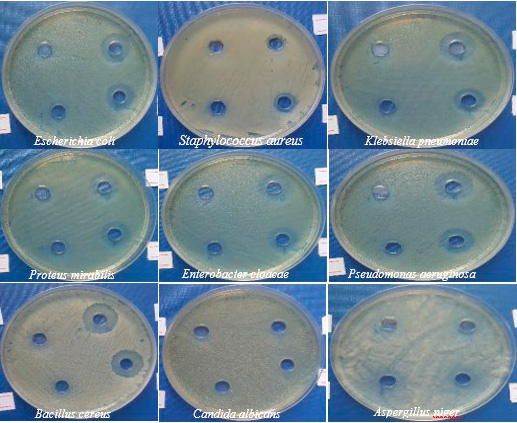
Figure 2. Minimal inhibitory concentration of rhamnolipid biosurfactant using concentrations 1, 2, 3 and 4 mg/ml against nine pathogenic isolates, including seven bacteria and two fungi
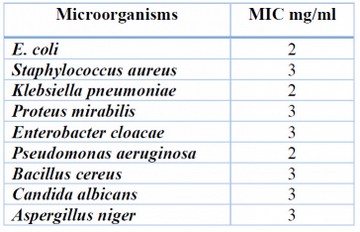
Table 2. The minimal inhibitory concentration of rhamnolipid biosurfactant against seven pathogenic bacteria and two pathogenic fungi
Antioxidant activity
The antioxidant activity results of rhamnolipid biosurfactant showed 22.7 %, 47.4 %, 79.8 %, 85 % and 91.4 % at the concentrations (5, 10. 15, 20 and 25 mg/ml) respectively, as in Figure (3). The rhamnolipid biosurfactant revealed effective antioxidant activity toward DPPH in a concentration-dependent manner. In fact, at 25 mg/ ml, the rhamnolipid biosurfactant showed a potential scavenging effect of 91.4 %, four times higher than that obtained at 5 mg/ ml (22.7 %).
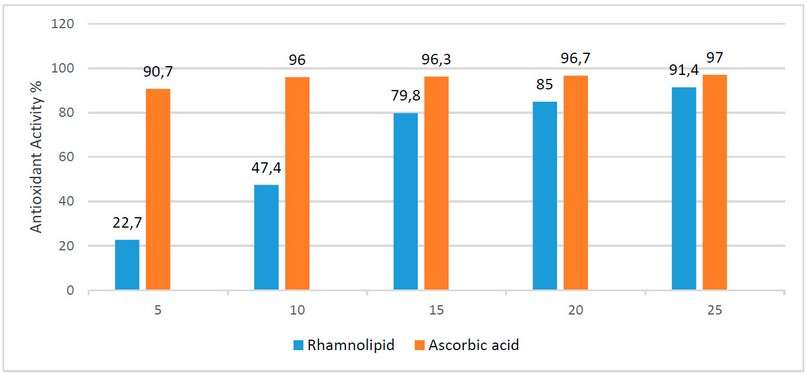
Figure 3. Antioxidant activity of different concentrations of rhamnolipid biosurfactant compared to the standard antioxidant ascorbic acid
The solution color was changed from violet to yellow; it turned into a non-radical form after saturation of the electronic layer, leading to a loss of violet color due to lack of absorption in terms of time at a wavelength of 517 nm, as shown in figure 4.

Figure 4. The antioxidant activity of different concentrations (mg/ml) of rhamnolipid biosurfactant using the DPPH method
The antioxidant activity of rhamnolipid biosurfactant was assessed by calculating the IC50 value to determine the concentration of rhamnolipid biosurfactant required to inhibit 50% of free radical DPPH present in the mixture. High-IC50 values refer to low antioxidant activity. The IC50 value of rhamnolipid biosurfactant showed 10.6 mg/ml, as in Figure 5. Thus, the low IC50 value refers to high antioxidant activity.
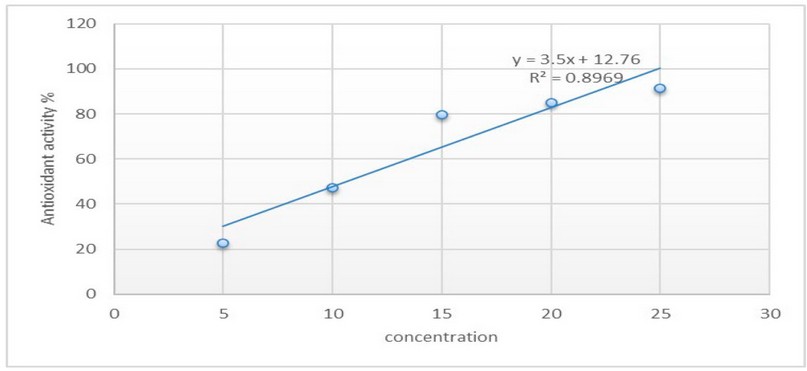
Figure 5. The curve of IC50 value for rhamnolipid biosurfactant
Cytotoxicity assay
Cytotoxicity of the rhamnolipid biosurfactant was examined at different concentrations (15, 25, 35, 50, 75 and 100 mg/ml) against human erythrocytes. Hemolysis of the erythrocytes was observed at concentrations (100, 75, 50, 40 and 35 mg/ml) to a similar degree as in a positive control of tap water. In contrast, the results exhibited no hemolysis at concentrations (25 and 15 mg/ ml) to a similar degree as in a negative control of DMSO exhibited no hemolysis as in figure 6.
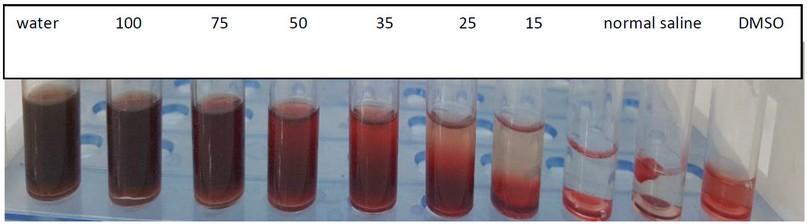
Figure 6. Cytotoxicity activity of rhamnolipid biosurfactant at different concentrations (15, 25, 35, 50, 75 and 100 mg/ml) against human erythrocytes
DISCUSSION
The antimicrobial activity
The current study's findings showed that the rhamnolipid biosurfactant had an antimicrobial effect against all the tested microorganisms (bacteria and fungi) at all concentrations used. The inhibition zones against the tested microorganisms increased with increasing rhamnolipid biosurfactant concentrations. The antimicrobial mechanism action of rhamnolipid biosurfactant against microorganisms is not entirely understood, but, it is suggested that the cellular plasma membrane is the target, as the rhamnolipid has an amphipathic nature that allows it to interact with phospholipids of plasma membrane. Another suggestion about the mechanical action of the rhamnolipid was increasing the membrane permeability of microbial cells with consequent alteration of this membrane, causing cell damage13. Lotfabad et al.11 reported results that exhibit the remarkable inhibitory effects of two rhamnolipids biosurfactants against Gram-positive bacteria. At the same time, none of the two rhamnolipids biosurfactants exhibited inhibition effects on Gram-negative bacteria. The two rhamnolipids biosurfactants showed a high inhibitory effect against Chaetonium globosum and Penicillium funiculosum.
In contrast, none showed inhibition effects on Aureobasidium pullulans and Penicillium chrysogeum, and two biosurfactants revealed different inhibitory behaviors against Aspergillus niger. Another study conducted by De Freitas Ferreira et al.20 examined the antimicrobial activity of rhamnolipid with different pH values (from 5.0 to 9.0) against food pathogens. The antimicrobial rhamnolipid activity against the Gram-positive bacterial pathogens such as Staphylococcus aureus, Listeria monocytogenes and Bacillus cereus was pH-dependent and favored at more acidic conditions. In contrast, the Gram-negative bacterial pathogens such as Salmonella enterica and Escherichia coli (EHEC) revealed resistance at all pH levels studied. The rhamnolipids are anionic biosurfactants when at pH conditions of neutral or alkaline while, at acidic conditions, they behave as nonionic. The antimicrobial rhamnolipid activity can be increased in acid food, favoring the control of the Gram-positive bacteria in acidic products.
Minimal Inhibition Concentration (MIC)
The minimal inhibition concentrations (MICs) are the lowest antimicrobial compound concentrations, inhibiting the microorganism's growth after incubation. MIC depends on the types of microorganisms and the antimicrobial compound itself. The effectiveness of antimicrobial compounds by inhibiting microbial growth will increase with the increasing concentrations of antimicrobial compounds used in the experiment 21. The antimicrobial activity of rhamnolipid biosurfactants is attributed to their effect on plasma membrane permeability. The antimicrobial activity of the crude rhamnolipids biosurfactants produced by Pseudomonas fluorescens, Pseudomonas poae and Pseudomonas libanensis, examined against two species of Gram-negative bacteria including (E. coli, Serratia marcescens) and two species of Gram-positive bacteria including (B. cereus, S. aureus) by the conventional MIC. The rhamnolipid biosurfactant positively correlated with increasing concentrations and the inhibition zone toward test microorganisms. The rhamnolipids from Pseudomonas libanensis had the lowest value of MIC among the other types of rhamnolipids, which indicates its ability against the tested bacteria22.
Antioxidant activity
The antioxidant activity of the rhamnolipid biosurfactant was examined with the DPPH scavenging test. This test is based on the ability of rhamnolipid to act as a DPPH free radical scavenger, a stable free radical with an unpaired valence electron at one nitrogen atom bridge23. It was shown in Figure 2 that the rhamnolipid biosurfactant revealed the practical activity of antioxidants against DPPH free radicals in a concentration-dependent manner. At 25 mg/ ml, the rhamnolipid biosurfactant showed a potential scavenging effect of 91.4 %, which is four times higher than that obtained at 5 mg/ ml (22.7 %). When the DPPH free radical encounters a substance of hydrogen-donor, the free radical is scavenged and the absorbance is reduced due to changing its color from purple to yellow, the rhamnolipid antioxidant activity was due to the free radical neutralization by transferring electrons24. The potent DPPH scavenging activity of rhamnolipid biosurfactant could be attributed to the content of unsaturated fatty acids, and the reducing power of rhamnolipid biosurfactant could be increased with increasing unsaturated fatty acids content23. The maximum antioxidant activity of rhamnolipid produced by Marinobacter litoralis was reported 72.6% at 5 mg/ml25. Both surfactin and rhamnolipids biosurfactants had antioxidant activity, but surfactin revealed higher antioxidant activity than rhamnolipids23. The low antioxidant activity of rhamnolipid biosurfactant produced from P. aeruginosa MN1 may be attributed to a lower content of unsaturated fatty acids. Antioxidant compounds are considered essential additives that are used for the preservation of different products in pharmaceutical, cosmetic and food industries by hindering oxidative rancidity of lipids and retarding their spoilage26.
Cytotoxicity assay
Cytotoxicity of the rhamnolipid biosurfactant was examined against human erythrocytes. The results exhibited that rhamnolipid biosurfactant had no hemolysis effect at concentrations 25 and 15 mg/ ml, where the erythrocytes were not precipitated. The lower sediment layer appeared in red, representing the human blood, while the upper layer represented the rhamnolipid and the physiological solution; if the compound were toxic, it would result in the degradation of the red blood cells. However, in the present study, the rhamnolipid obtained from P. aeruginosa showed no toxic effect at concentrations 25 and 15 mg/ ml on the erythrocytes and is considered a non-cytotoxic biosurfactant that could be used as a possible biological material in various clinical aspects. The current result agrees with Al-waely's 27 studies that revealed all the concentrations he used of rhamnolipid did not show any hemolysis and then did not cause any cytotoxicity towards the erythrocytes. The rhamnolipid biosurfactant is a suitable and great alternative to be employed as an effective and safe therapeutic agent.
CONCLUSIONS
The rhamnolipid biosurfactant exhibited a significant antimicrobial effect toward gram-negative bacteria, gram-positive bacteria and fungi, and it has an antioxidant effect toward DPPH in a concentration-dependent manner, with no hemolysis at lower concentrations. Based on these results, the isolated rhamnolipid biosurfactant from Pseudomonas aeruginosa could be utilized for various medical and pharmaceutical purposes. The importance of the subject lies in obtaining local bacterial isolates with the ability to produce valuable substances that are an alternative to antibiotics and to use them in various industrial and therapeutic fields.
Acknowledgments
The authors thank the Biology Department and Ecology Department, University of Basrah, Iraq, for providing facilities.
REFERENCES
1. Terreni, M., Taccani, M. & Pregnolato, M. New Antibiotics for Multidrug-Resistant Bacterial Strains: Latest Research Developments and Future Perspectives. Molecules, 2021, 26: 2671. https://doi.org/ 10.3390/molecules26092671.
2. Álvarez-Martínez, F.J., Barrajón-Catalán, E. & Micol, V. Tackling Antibiotic Resistance with Compounds of Natural Origin: A Comprehensive Review. Biomedicines, 2020, 8: 405. doi:10.3390/biomedicines8100405.
3. De Giani, A., Zampolli, J. & Di Gennaro, P. Recent trends on biosurfactants with antimicrobial activity produced by bacteria associated with human health: different perspectives on their properties, challenges, and potential applications. Frontiers in Microbiology, 2021, 12:655150. doi: 10.3389/fmicb.2021.655150.
4. Costa, SGVAO, Déziel, E & Lépine, F. Characterization of rhamnolipid production by Burkholderia glumae. Letters in Applied Microbiology, 2011, 53:620– 627. doi:10.1111/j.1472 765X.2011.03154.x.
5. Nalini, S. & Parthasarathi, R. Biosurfactant production by Serratia rubidaea SNAU02 isolated from hydrocarbon contaminated soil and its physicochemical characterization. Bioresource Technology, 2013, 147: 619-622.
6. Irorere, V.U., Tripathi, L., Marchant, R., McClean, S. & Banat, IM Microbial rhamnolipid production: a critical re-evaluation of published data and suggested future publication criteria. Applied Microbiology and Biotechnology, 2017, doi: 10.1007/s00253-017-8262-0.
7. Tan, Y.N. & Li, Q. Microbial production of rhamnolipids using sugars as carbon sources. Microbial Cell Factories, 2018, 17(1). doi:10.1186/s12934-018-0938-3.
8. Moradali, M.F., Ghods, S. & Rehm, B.H.A. Pseudomonas aeruginosa Lifestyle: A Paradigm for Adaptation, Survival, and Persistence. Frontiers in Cellular and Infection Microbiology, 2017, 7:39. doi: 10.3389/fcimb.2017.00039.
9. Sekhon Randhawa, K.K. & Rahman, PKSM Rhamnolipid biosurfactants—past, present, and future scenario of global market. Frontier in Microbiology, 2014, 5:454. doi: 10.3389/fmicb.2014.00454.
10. Nitsche, M. and Silva, S.S. Recent Food Applications of Microbial Surfactants. Critical Reviews in Food Science and Nutrition, 2018, doi: 10.1080/10408398.2016.1208635.
11. Lotfabad, T.B., Shahcheraghi, F. & Shooraj, F. Assessment of antibacterial capability of rhamnolipids produced by two indigenous Pseudomonas aeruginosa strains. Jundishapur Journal of Microbiology, 2013, 6(1): 29-35. DOI: 10.5812/jjm.2662.
12. Ndlovu, T., Rautenbach, M., Vosloo, J.A., Khan, S. & and Khan, W. Characterization and antimicrobial activity of biosurfactant extracts produced by Bacillus amyloliquefaciens and Pseudomonas aeruginosa isolated from a wastewater treatment plant. AMB Express, 2017, 7:108. doi: 10.1186/s13568-017-0363-8.
13. Magalhaes, L. & Nitschke, M. Antimicrobial activity of rhamnolipids against Listeria monocytogenes and their synergistic interaction with nisin. Food Control, 2013, 29:138–142.
14. Alyousif, N.A., Al-Luaibi, Y.Y.Y. & and Hussein, W. Distribution and molecular characterization of biosurfactant-producing bacteria. Biodiversitas, 2020, 21: 4034-4040. DOI: 10.13057/biodiv/d210914.
15. Alyousif, N.A., Al-Tamimi, W.H. & Al-Luaibi, Y.Y.Y. Screening, enhance production and characterization of biosurfactant produced by Pseudomonas aeruginosa isolated from hydrocarbon contaminated soil. Eurasia Journal of Bioscience, 2020, 14: 4377-4391.
16. Nanda, A. & Saravanan, M. Biosynthesis of silver nanoparticles from Staphylococcus aureus and its antimicrobial activity against MRSA and MRSE. Nanomedicine, 2009, 5:452–6. doi: 10.1016/j.nano.2009.01.012.
17. TC,V., Srirama, K., Mikkili, I., Md, N.B., Dulla, J.B., Alugunulla, V.N., Sweety, D. & Karlapudi, A.P. Estimation of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Antimicrobial peptides of Saccharomyces boulardii against Selected Pathogenic Strains. Karbala International Journal of Modern Science, 2019, 5: (4). doi.org/10.33640/2405-609X.1219.
18. Barros, L., Baptista, P. & Ferreira, ICFR Effect of Lactarius piperatus fruiting body maturity stage on antioxidant activity measured by several biochemical assays. Food and Chemical Toxicology, 2007, 45: 1731-1737.
19. He, X.-G., Moceka, U., Floss, H.G., Caceres, A., Giron, L., Buckley, H, Cooney, G., Manns, J. & Wilsond, B.W. An antifungal compound from Solanum nigrescens. Journal of Ethnopharmacology, 1994, 43(3): 173–177. doi:10.1016/0378- 8741(94)90039-6.
20. De Freitas Ferreira, J., Vieira, E.A. & Nitschke, M. The antibacterial activity of rhamnolipid biosurfactant is pH dependent. Food Research International, 2018, doi:10.1016 /jfoodres.2018.09.005.
21. Andrews, J.M. Determination of minimum inhibitory concentrations. Journal of Antimicrobial Chemotherapy, 2001, 48(suppl_1), 5–16. doi: 10.1093/jac/48.suppl_1.5.
22. Mendoza, A.R.R., Patalinghug, J.M.R., Canonigo, G.O. & Yee, J.C. Screening of rhamnolipids from Pseudomonas spp. and evaluation of its antimicrobial and antioxidant potential. Bacterial Empire, 2020, 3:(3) 46-51. doi: 10.36547/be.2020.3.3.46-51.
23. Abdollahia, S., Tofighib, Z., Babaeea, T., Shamsia, M., Rahimzadehc, G., Rezvanifara, H., Saeidia, E., Amiria, M.M., Ashtianid, Y.S. & Nasrin Samadi, N. Evaluation of Antioxidant and Anti-biofilm Activities of Biogenic Surfactants Derived from Bacillus amyloliquefaciens and Pseudomonas aeruginosa. Iranian journal pharmaceutical Research, 2020, 19 (2): 115-126. doi: 10.22037/IJPR.2020.1101033.
24. Jemil, N., Ayed, HB, Manresa, A., Nasri, M. & Hmidet, N. Antioxidant properties, antimicrobial and anti-adhesive activities of DCS1 lipopeptides from Bacillus methylotrophicus DCS1. BMC Microbiology, 2017, 17:144 doi: 10.1186/s12866-017-1050-2.
25. Haque, E., Kayalvizhi, K. & Saqib Hassan, S. Biocompatibility, Antioxidant and Anti-Infective Effect of Biosurfactant Produced by Marinobacter litoralis MB15. International Journal of Pharmaceutical Investigation, 2020, 10(2):173-178.
26. Ohadi, M., Forootanfar, H., Rahimi, H.R., Jafari, E., Shakibaie, M., Eslaminejad, T. & Dehghannoudeh, G. Antioxidant potential and wound healing activity of biosurfactant produced by acinetobacter junii B6. Current Pharmaceutical Biotechnology, 2017, 18: 900-8.
27. Al-waely, W.A.S. Production, purification and characterization of rhamnolipid from a local isolate of Pseudomonas aeroginosa. Ph.D. Thesis, College of Agriculture, University of Basrah (in Arabic), 2013.
Received: 28 September 2023/ Accepted: 15 November 2023 / Published:15 December 2023
Citation. Abdullah Alyousif N, Al-tamimi W H., Al-luaibi Y Y Y. Antimicrobial and Antioxidant Activity of Rhamnolipids Biosurfactant is Produced by Pseudomonas aeruginosa. Revis Bionatura 2023;8 (4) 25. http://dx.doi.org/10.21931/RB/2023.08.04.25
