2023.08.02.71
Files > Volume 8 > Vol 8 No 2 2023
Extraction and purification of lectin from rice grains and using it as a novel prebiotic and inhibitor toward some gastrointestinal organisms
Sahira Nsayef Muslim1, Wafaa Hassan Muslem1,*, Raghad J. Fayyad1, Alaa Naseer Mohammed Ali1, Mohamed Faraj Edbeib2
1 Biology Dept. College of Science. Mustansiriyah University, Iraq.
2 Medical Lab. Dept. Faculty of Medical Technology, Bani Walid University, Libya.
* Correspondence: wafaahassan2017@uomustansiriyah. Phone number: 009647736187364
Available from: http://dx.doi.org/10.21931/RB/2023.08.02.71
ABSTRACT
Aims: Lectins are carbohydrates with structure usually binding with proteins. The isolation of lectins from local and inexpensive sources, such as rice, considered one of the chief cereal crops, is necessary due to its broad application. Methods: Lectin was extracted from a novel source, Oryza sativa grains, with solvent (hexane) of ratios (1:5 w/v) for 15 minutes. The extract solution was fractionated with ammonium sulfate at 25-65% saturation concentrations and then applied to a DEAE-cellulose column followed by a Sephadex G-100 column. SDS-PAGE has been done to vitrify from lectin purity. An enhancement and inhibition activities were calculated to detect the effect of lectin on lactic acid bacteria and pathogenic bacterial growth. The extracted lectin from three types of Oryza sativa grains showed various levels of erythrocyte agglutination from 8 to 32U/ml. Then, the specimen was loaded into a DEAE-cellulose column followed by gel using Sephadex G-100 column with a final specific activity of 246.15U/mg, 24.15 fold of purification and 70% yield of lectin. Findings: Lectin SDS-PAGE result revealed a single protein band with 43 k Da. The purified lectin exhibited a substantial prebiotic property of lactic acid bacteria growth enhancement while exhibiting apparent growth inhibition against tested pathogenic bacteria. Typically, prebiotic properties should inhibit the growth of pathogens and enhance the growth of beneficial and desirable bacteria like Lactobacillus reuteri. Conclusion: The lectin may be used in animal diet to improve digestibility and support gastrointestinal tract health.
Keywords: Inhibitor agent; lectin; prebiotic; purification; Phyto hemagglutinins; rice grains.
INTRODUCTION
Lectins are carbohydrates of non-immune origin with multivalent or divalent structures usually binding with proteins; lectin has with its structures in at least one domain with non-catalytic properties, which exhibit the reversible ability to bind with specific mono or oligosaccharides that can cause agglutination of erythrocytes (human and animal) by combining to the carbohydrate molecules on the surface of the erythrocyte 1. Lectins were initially called phyto hemagglutinins because they were first discovered in plants.
Numerous parts of the plant contain lectin with high levels like fruits, tubers, legumes and seeds such as cereals 2. Rice (Oryza sativa) is one of the chief cereal crops that is considered the major diet source consumed by up to half of the human population around the world 3. In a study by 4, authors defined and sequenced the genes responsible for lectin production in rise; the previous study also mentioned that lectin contributes to rise resistance and tolerance against biotic and abiotic stress. Probiotics, defined as diet supplements, consist of live microbes that possess health benefits and are usually used as a dietary method to treat the influenced composition of intestinal tract flora 5. On the other hand, prebiotics is non-digestible food constituents and benefit by selectively promoting the activity and growth of beneficial bacteria in the colon 6. Probiotics and prebiotics are the new suggestions to modulate the target microflora balance in the gastrointestinal gut 7.
Lectins have various applications in many research fields like molecular biology, cell biology, genetic engineering, immunology and pharmacology 8. In addition, it acts as a potential cancer diagnostic tool 9. Moreover, diagnostic tools such as lectin microarrays 10. Since many plant-derived lectins are comparatively stable against denaturation by heat and proteolytic digestion, the digestive gut is regularly exposed to the biologically active lectins through feeding 11. Therefore, this investigation aimed to extract and purify lectin from local non-expensive Oryzae sativa grains and use purified lectin as a prebiotic agent by determining its efficacy as a promoter of probiotic lactic acid bacteria growth and as an antibacterial against several pathogenic bacteria isolated from the intestinal gut.
MATERIAL AND METHODS
Bacterial isolates collection
In this study, diagnosed bacterial isolates have been obtained from the microbiology laboratory for higher graduates at Mustansiriyah University. These include probiotic bacteria ( 3 Lactobacillus reuteri isolates as well as 3 isolates of Pediococcus sp) and pathogenic bacteria (3 isolates of Salmonella sp., 3 isolates of E. coli, 3isolates of Enterobacter aerogens, 2 isolates of Eromonas hydrophila and two isolates of Serrata marcescence). These isolates have been used to estimate the effect of purified lectin on probiotic and intestinal pathogenic bacteria.
Extraction of lectin from rice grains (Oryza sativa)
Three samples of rice grains have been purchased from local markets. Grain powder was obtained by grinding with mortar and then defatted with solvent (hexane) of ratios (1:5 w/v) for 15 minutes. This procedure was repeated twice. Fatherly 0.02 M phosphate buffer saline (pH 7.2) was added to the sample at a 1:5 (v/w) ratio and stirred continuously using a magnetic stirrer for sixteen hours, followed by immediate filtration. The yielded solution was exposed to centrifugation (10000 rpm/15 min), the residual precipitation was neglected, and the resultant solution was kept by freezing for further analysis 12.
Hem agglutination assay in microtiter plate
Rice extract solution (50 μl) was diluted with (0.02M) Phosphate Buffer Saline (PBS) with (pH 7.2) in microtiter U-plate, then mixed with an equal volume of (3%) human erythrocytes suspension. After 2 hours of incubation at 37°C, The activity was expressed as hem agglutination units (HU). As reported in 13, One H. U. was defined as the inverse of the highest dilution still capable of agglutinating erythrocyte. Then, the lectin was purified by the method reported by 1.
Determination of protein content
The protein makeup of lectin was estimated using the Bradford dye method with BSA as a standard 14. The absorbance at wavelength 280 nm was used to calculate the protein concentration in column elutes.
Electrophoresis
After the final purification step, the fractions that exhibited the hem agglutination activity were displayed to electrophoresis by using sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) according to the method of 15.
Effect of lectin on lactic acid bacteria growth
The prebiotic properties of lectin were determined by its effect on the growth of 6 bacterial isolates (lactic acid bacteria), including 3 Lactobacillus reuteri and 3 isolates of Pediococcus sp. These isolates were grown in MRS broth( volume 50 ml) at30ºC. After 24 hr. incubation, 1% of the growth suspension, which was adjusted to the absorbance of 0.5 at wavelength 600 nm, was transferred into tubes with MRS broth (5 ml) with or without 1 % of the purified lectin and transferred to the incubator at 30ºC. After 4 hr., the number of bacterial cells was calculated by plating on MRS agar and incubating overnight at 30ºC. Then, the ratio of enhanced activity (%) was calculated using the following formula (1) according to 16:
Enhancement activity (%) = (SB−CB)/CB×100 (1)
SB: the number of cells in agar with purified lectin (CFU/ml), CB: the number of cells without purified lectin (CFU/ml).
Effect of lectin on pathogenic bacteria growth
This experiment was achieved according to a method described in the previous paragraph, except by using the nutrient broth for tested pathogenic bacteria cultivation and using shaking at 150 rpm at 37ºC during bacterial incubation. The percentage of activity inhibition was also calculated as formula (2) according to 16:
Inhibition activity (%) = (CB−SB)/CB×100 (2)
SB: number of cells in nutrient broth with purified lectin (cfu/ml), CB: the number of cells in nutrient broth without purified lectin (cfu/ml).
RESULTS
Lectin extraction and purification
After extraction of three types of Oryzae sativa grains collected from local markets, the amount of extracted lectin showed various levels of erythrocyte agglutination ranging from 8 to 32U/ml, 32U/ml was the best hem agglutination activity given by extract Oryzae sativa grain.
After extraction of lectin by organic solvent (hexane). This produced crude extract supplied to precipitation by ammonium sulfate and found that 40% saturation raised the specific activity to 34.59 U/mg, with 3.39 purification folds and 80% yield. After this step, the dialysis removed the access salts from the purified solution. After the dialysis, the specimen was loaded into a DEAE-cellulose column, followed by an elution step with gradient concentrations of NaCl (25 m M - 3 M). According to the obtained results, three protein peaks appeared, and the hem agglutination activity located in the third peak of protein, as reported in Figure 1. In this chromatographic step, the specific activity increased to 246.15U/mg with 24.15 fold of purification and 70% yield of lectin.
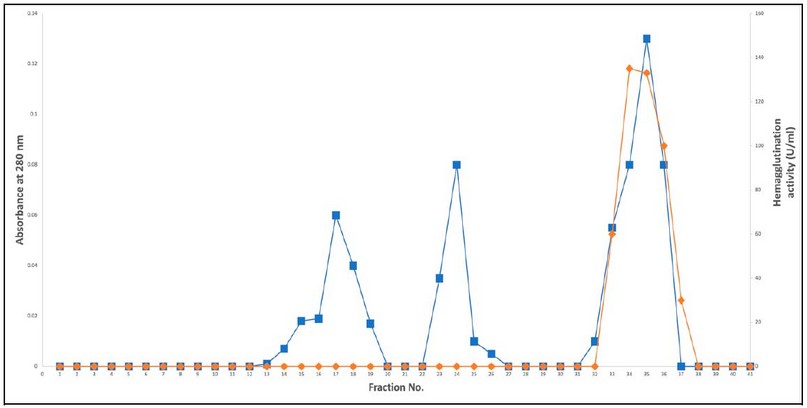
Figure 1. Lectin purification from Oryza sativa grains by using ion exchange chromatography on DEAE –cellulose column.
The active fraction was loaded on Sephadex G-100 in the last step of lectin purification. After the elution, two protein peaks appeared, with the hem agglutination activity located in the first protein peak, as shown in Figure 2.
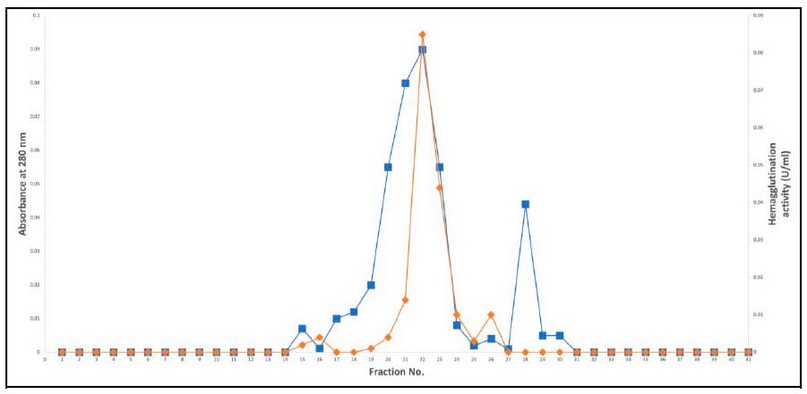
Figure 2. Lectin purification from Oryzae sativa grains by using gel filtration chromatography on Sephadex G-100column
In this final step, the specific activity of lectin reached 825.80U/mg with 81.04 fold of purification and a yield of 70% (Table 1).
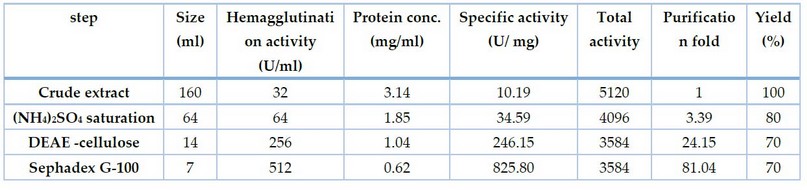
Table1. Purification steps for lectin from Oryzae sativa grains
In the crude extract, the target proteins are purified from other materials in the culture medium by using the ammonium sulfate salts by a process called salting-out, where water molecules are absorbed by ammonium sulfate and magnesium sulfate and decrease the amount of the molecules around the protein (lowering the solubility) and lead to coagulate and precipitate the protein of interest (Scopes 2013). The presence of ammonium sulfate from the settled solution causes false positive agglutination; therefore, removing these salts after the precipitation process 1 is essential.
Electrophoresis
In using dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), when the purified protein was analyzed, the results demonstrated that there was a single protein band, and this suggested that the lectin could be a monomeric protein. This single protein band, whose molecular weight was 43kDa, is shown in Figure 3.
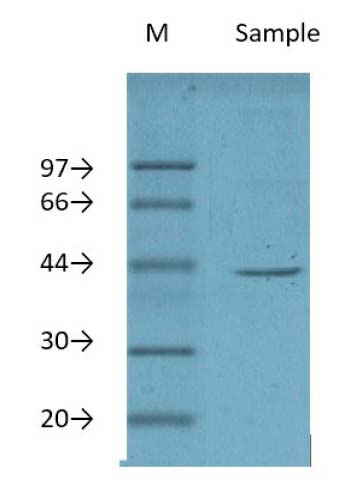
Figure 3. Lectin purification from Oryzae sativa grains by using gel filtration chromatography on Sephadex G-100column
Evaluation of prebiotic properties
Prebiotic properties assay of purified lectin was carried out using lactic acid bacteria such as Pediococcus sp. and Lactobacillus reuteri. According to the resulting data, the lectin caused stimulation of Pediococcus sp. growth with 47-82%, followed by Lactobacillus reuteri with 55-77%. As described in Figure 4.
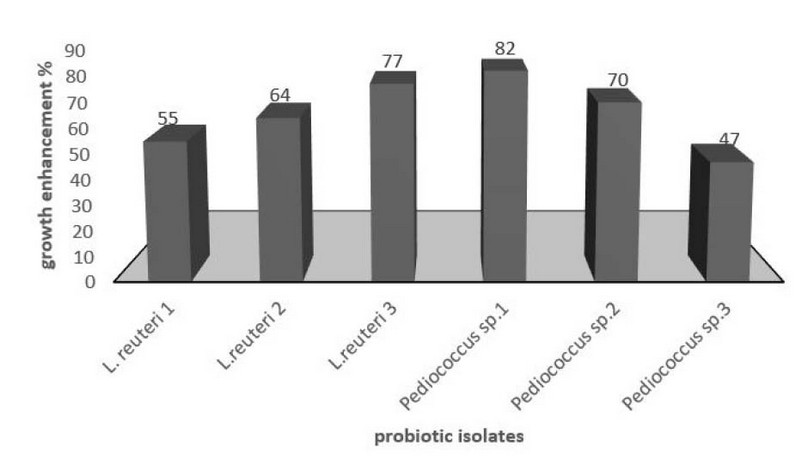
Figure 4. Enhancement of probiotic bacteria by lectin purified from Oryza sativa grains.
When mixed with pathogenic bacteria, the purified lectin revealed an inhibition activity with different as shown in Figure 5.
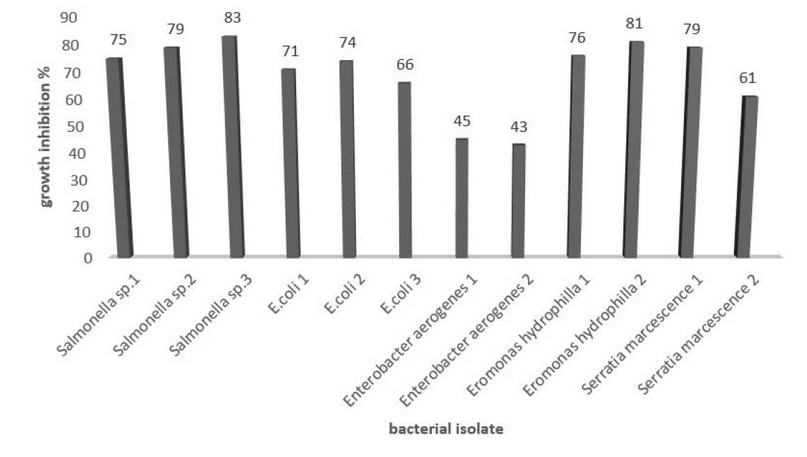
Figure 5. Inhibition percentage of pathogenic bacteria by lectin purified from Oryza sativa grains.
Many authors studied the antibacterial activity of lectin derived from different plant seeds. The resulting data indicate that lectin has a strong prebiotic property of enhancing probiotic bacterial growth in contrast to its inhibitory property for pathogenic bacteria growth. Principally, prebiotic properties should not only eliminate the growth of pathogens but also promote the growth of required and advantageous bacteria, such as Lactobacillus. This is the first study dealing with the purification of lectin from Oryza sativa grain and elucidating its prebiotic properties and the possibility of using lectin as a prebiotic agent.
DISCUSSION
According to 17, the extract of the grains of peanut and black gram seeds (Arachi hypogaea and Vigna mungo) contained a detectable amount of lectin—another result reported by 18. The lectin extracted from mature horseradish seeds with PBS at pH 7.2 exhibited a clear agglutination to human erythrocytes. Also, 19 mentioned that a lectin extracted from the seeds of Diocleawilsonii as metalloprotein exhibited strong agglutinating activity against rabbit and ABO erythrocytes. The lectin was extracted and purified by ammonium sulfate precipitation and gel filtration using Sephadix G-75 20. In 21, lectin was purified from Cissuspopulnea through sequential steps: centrifugation, salting out, ultrafiltration, dialysis and anion exchange affinity chromatography. The health benefit prebiotic agents to the host initially target the modulation of intestinal tract microbiota, including the indigenous gut microflora that contributes to different functions that advance host health 22. Probiotics also produce many compounds with various pharmaceutical properties that improve gut health, such as antibiotics and anti-carcinogens 23. Prebiotics are usually chemical, like fructans, inulin, galacto-oligosaccharides, lactulose, carbohydrates 24. Dietary fibers and their hydrolysis products are becoming an evolving source of new constituents with vigorous prebiotic activity 25. A combination of a prebiotic agent and a probiotic organism yielded a symbiotic which targets the existence and the establishment of the probiotic in the gastrointestinal tract to promote groups of beneficial bacteria 26. In a study of 27, the results showed a significant decrease in total biomass for gram-negative and positive pathogenic bacteria treated with lectin purified from Phaseolus vulgaris seeds. The previous researcher suggested that the antibacterial effect of lectins yielded from its binding with components of the bacterial surface cell wall peptidoglycans like N-acetyl glucosamine, N-acetyl muramic acid and muramyl dipeptides. These results also strongly agreed with lectin purified from seeds of Indigofera heterantha legume as well as those extracted from Chenopodium quinoa seeds 28. Another researcher mentioned that lectins play an essential role in the plant's defense against bacteria by preventing microorganisms from reaching the plant cell cytoplasm—an indirection mechanism based on lectin interactions with bacterial cell wall carbohydrates or extracellular glycan 29.
CONCLUSION
Authors suggest that purified lectin from rice grain may be used as an animal food additive to improve digestibility and for gastrointestinal health provision. Further investigations are needed to examine the ability of its combination with a suitable probiotic, which may lead to using this combination product in symbiotic applications.
REFERENCES
1 Muslim SN. Improving antibacterial activity for antibiotics by purified and characterized lectin from Acinetobacter baumannii. Iraqi Journal of Biotechnology. 2015;14(1).
2 Nakata H, Lin CY, Abolhassani M, Ogawa T, Tateno H, Hirabayashi J, Muramoto K. Isolation of rice bran lectins and characterizing their unique behavior in caco-2 cells. International journal of molecular sciences. 2017 May;18(5):1052.
3 Phuc, T. H., Van Manh, N., Ky, H. Genetic diversity of local rice varieties (Oryza sativa L.) in Vietnam's Mekong Delta based on SSR markers and morphological characteristics. Indonesian Journal of Biotechnology, 2021, 26: 76-81
4 Tsaneva M, De Schutter K, Verstraeten B, Van Damme EJ. Lectin sequence distribution in QTLs from rice (Oryza sativa) suggests a role in morphological traits and stress responses. International journal of molecular sciences. 2019 Jan;20(2):437.
5 Khaleel MM, Thaer AA. Using probiotics and inulin to prolong fermented dairy products shelf life. Iraqi Journal of Agricultural Sciences. 2017 Jan 1;48(2):608-17.
6 Getahun A, Tesfaye A, Muleta D. Investigation of the potential benefits and risks of probiotics and prebiotics and their synergy in fermented foods. Singap J Chem Biol. 2016;6(1):1-6.
7 Bandyopadhyay B, Mandal NC. Probiotics, prebiotics and synbiotics-in health improvement by modulating gut microbiota: The concept revisited. International Journal of Current Microbiology and Applied Sciences. 2014;3(3):410-20.
8 Ali AN. Cytotoxic effect of the purified lectin from locally isolated Acinetobacter baumannii on Hep-2 tumor cell line. J Biolo Agricul Health. 2014;4(26):168-73.
9 Mazalovska M, Kouokam JC. Plant-derived lectins as potential cancer therapeutics and diagnostic tools. BioMed research is international. 2020 May 15, 2020.
10 Yu H, Shu J, Li Z. Lectin microarrays for glycoproteomics: an overview of their use and potential. Expert Review of Proteomics. 2020 Jan 2;17(1):27-39.
11 Pusztai A, Bardocz S. Biological effects of plant lectins on the gastrointestinal tract metabolic consequences and applications. Trends in glycoscience and glycotechnology. 1996 May 2;8(41):149-65.
12 Reddy, M. V. B., Banik, N. L., Sasikala, P. Extraction and purification of lectin from plant seeds an empirical study on artocarpus sp. International Journal of Research in Ayurveda and Pharmacy, 2019; 7, 236-240
13 Muslim SN, Al-Kadmy IM, Auda IG, Mohammed Ali AN, Al-Jubori SS. A novel genetic determination of a lectin gene in Iraqi Acinetobacter baumannii isolates and use of purified lectin as an antibiofilm agent. Journal of AOAC International. 2018 Sep 1;101(5):1623-30.
14 Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical biochemistry. 1976 May 7;72(1-2):248-54.
15 LAEMMLI UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. nature. 1970 Aug;227(5259):680-5.
16 Phothichitto K, Nitisinprasert S, Keawsompong S. Isolation, screening and identification of mannanase producing microorganisms. Agriculture and Natural Resources. 2006 Dec 30;40(6 (Suppl.)):26-38.
17 Sun J, Yang QL, Zhang CS, Yu LN, Zhu F. Purification and Identification of a Natural Lectin from the Seed of Peanut Arachis hypogaea. The Open Materials Science Journal. 2011 May 2;5(1).
18 Suseelan KN, Bhatia AR, Mitra R. Purification and characterization of two major lectins from Vigna mungo (black gram). Journal of Biosciences. 1997 Sep;22(4):439-55.
19 Rangel TB, Assreuy AM, Pires AD, Carvalho AU, Benevides RG, Simoes RD, Silva HC, Bezerra MJ, Nascimento AS, Nascimento KS, Nagano CS. Crystallization and characterization of an inflammatory lectin purified from the seeds of Dioclea wilsonii. Molecules. 2011 Jun;16(6):5087-103.
20 De Mesa MD, Mojica ER, Merca FE. Purification of lectin from mature seeds of malunggay (Moringa pterygosperma). Philippine Journal of Crop Science. 2004 Dec;29(3):13-24.
21 Awoyinka OA, Dada OO. Partial Purification and Characterization of Lectin from the Seeds of Cissus poplunea. European Journal of Medicinal Plants. 2011 Oct 1;1(4):130.
22 Meng C, Bai C, Brown TD, Hood LE, Tian Q. Human gut microbiota and gastrointestinal cancer. Genomics, proteomics & bioinformatics. 2018 Feb 1;16(1):33-49.
23 Bautista-Gallego J, Ferrocino I, Botta C, Ercolini D, Cocolin L, Rantsiou K. Probiotic potential of a Lactobacillus rhamnosus cheese isolate and its effect on the fecal microbiota of healthy volunteers. Food Research International. 2019 May 1;119:305-14.
24 Markowitz, P., Śliżewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients, 2017; 9, 1021
25 Ruiz-Moreno, M. J., Muñoz-Redondo, J. M., Cuevas, F. J., Marrufo-Curtido, A., León, J. M., Ramírez, P., Moreno-Rojas, J. M. The influence of pre-fermentative maceration and ageing factors on ester profile and marker determination of Pedro Ximenez sparkling wines. Food Chemistry, 2017; 230, 697-704
26 Kolida, S., Gibson, G. R. Synbiotics in health and disease. Annual Review of Food Science and Technology, 2011; 2, 373-393
27 Hamed ES, Ibrahim EA, Mounir SM. Antimicrobial Activities of Lectins Extracted from Some Cultivars of Phaseolus vulgaris Seeds. J Microb Biochem Technol. 2017;9(3):109-16.
28 Naito, Y., Uchiyama, K., Takagi, T. A next-generation beneficial microbe: Akkermansia muciniphila. Journal of Clinical Biochemistry and Nutrition, 2018; 63, 33-35
29 Hivrale AU, Ingale AG. Plant as a plenteous reserve of lectin. Plant signaling & behavior. 2013 Dec 1;8(12):e26595.
Received: May 15, 2023/ Accepted: June 10, 2023 / Published: June 15, 2023
Citation: Muslim, SN; Muslim, WH; Fayyad, RJ; Ali, A.N.M.; Edbeib, M.F. Extraction and purification of lectin from rice grains and using it as a novel prebiotic and inhibitor toward some gastrointestinal organisms. Revis Bionatura 2023;8 (2) 71. http://dx.doi.org/10.21931/RB/2023.08.02.71
