2023.08.02.68
Files > Volume 8 > Vol 8 No 2 2023
The role of Renin angiotensin aldosterone system in women with breast cancer before and after treatment
1 HD in Medical Lab, Kirkuk Health Directorate, Kirkuk, Iraq.
2 Prof Dr. College of Science, Department of Biology/Tikrit University
* Correspondence: [email protected]. [email protected]
Available from: http://dx.doi.org/10.21931/RB/2023.08.02.68
ABSTRACT
Background: Cancer is an abnormal proliferation of cells in a tissue or organ that causes the cells to change their nature, eventually producing a lump or mass and spreading to other body regions in most cases. This study aims to evaluate the serum level of ACE, plasmin, renin, kallikrein, and bradykinin in breast cancer patients and determine their relationship to the proliferation of breast cancer. This study has investigated 90 women (75 patients and 15 controls) aged between (35-76) years. The patients were referred to three main facilities, Kirkuk Oncology Center, consultation of early detection of breast tumors in Azadi Teaching Hospital, and Kirkuk General Hospital from November 2021 to March 2022. The individuals of this study were divided into six groups: The first group was newly diagnosed patients with malignancy breast tumor n (15); the diagnosis was made by the consult of medical staff, which was based on a triple assessment technique (physical breast examination, ultrasonography, with or without mammography and fine-needle aspiration cytology). The second group was patients of surgical interference n (15), who submitted to local surgical removal of the suspected tumor in the breast (lumpectomy) or complete removal of the breast (mastectomy). The third group was who received the first chemotherapy dose n (15). The fourth group was those who received the second chemotherapy dose n (15). The fifth group was those who received the third chemotherapy dose n (15). The sixth group was healthy women with an adverse family history of breast cancer. They were included in this study as a control group, and their ages were comparable to that of the women with breast cancer. This study shows the mean of Angiotensin-converting enzyme (ACE) was observed in breast cancer women (1.16±0.36U/L) as compared with the healthy control group (0.75±0.15U/L). The difference was highly significant at a P. value of 0.0004. The mean and standard deviation of angiotensin-converting enzyme, plasmin, renin, bradykinin, and kallikrein in women with breast cancer (before treatment, before mastectomy, after one dose, after 2 doses, and after doses) in comparison with healthy women. It was concluded that the levels of ACE, Plasmin, Kallikrein, and Bradykinin were significantly elevated in breast cancer women compared with healthy women, while decreased renin level compared with healthy control.
Keywords: ACE; Plasmin; bradykinin, renin, kallikrein.
INTRODUCTION
Cancer is an abnormal growth of cells in tissue or organ that cause the cells to change in nature, eventually forming a lump or mass in most type and then spreading to various parts of the body. 1 Breast cancer is the most common cancer in women worldwide, regardless of whether they live in developing or developed countries. It is still the second leading cause of cancer-related death in women. 2 In Iraq, the Iraqi Ministry of Health registered 63,923 Iraqi patients with various types of newly diagnosed cancer from all Iraqi provinces except three Northern provinces. Of these patients, 37,652 (49.5 percent) were females, and breast cancer alone accounted for 31 percent of all new cancer cases among females, according to the Ministry of Health.3 In recent years, the renin-angiotensin-aldosterone system (RAAS) has become a highly sought-after research topic due to the discovery of a link between the system and the development of several different types of cancer.4 RAS components can be present in both normal and malignant breast tissue, indicating that the RAS is involved in breast cancer development. 5 Cell growth, proliferation, differentiation, migration, and apoptosis, as well as ECM remodeling and inflammation, are all regulated by the RAS.6 The reduced intravascular volume impairs renal blood flow and activates systemic RAS through a complex cascade, in which angiotensin I converts to angiotensin II by the angiotensin-converting enzyme(ACE).7 Ang II increases blood pressure, stimulates water intake, and increases sodium retention in kidney tubules to restore blood volume.8 The purpose of this study is to evaluate the serum level of ACE, plasmin, renin, kallikrein, and bradykinin in breast cancer patients and to determine their relationship to the proliferation of breast cancer
MATERIALS AND METHODS
This study has investigated 90 women (75 patients and 15 controls) aged between (35-76) years. The patients were referred to three main facilities, Kirkuk Oncology Center, consultation of early detection of breast tumors in Azady hospital, and Kirkuk General Hospital from November 2021 to March 2022. The individuals of this study were divided into six groups: The first group was newly diagnosed patients with malignancy breast tumor n (15); the diagnosis was made by the consult of medical staff, which was based on a triple assessment technique (physical breast examination, ultrasonography, with or without mammography and fine-needle aspiration cytology). The second group was patients of surgical interference n (15), who submitted to local surgical removal of the suspected tumor in the breast (lumpectomy) or complete removal of the breast (mastectomy). The third group was who received the first chemotherapy dose n (15). The fourth group was those who received the second chemotherapy dose n (15). The fifth group received the third chemotherapy dose n (15). Clinical history data, information on age, weight, height, marital status, menopausal status, family history of breast cancer, chronic disease, and course of treatment were collected in a short questionnaire form (Appendix I). The sixth group was healthy female volunteers with no prior history of breast cancer n (15). About 5 ml of venous blood was collected from each case using a sterile disposable syringe, then unloaded into gel tubes and allowed to clot at room temperature for 20 minutes. All samples were centrifuged at 3000 rpm for 15 minutes; sera removed and divided into three Eppendorf tubes, 500 µl for each sample, then stored at - 30 C until used to the time of biochemical assay, which included parameters: ACE, Plasmin, renin, kallikrein, and Bradykinin. The kit was an enzyme-linked immunosorbent assay (ELISA) for all parameters, which worked manually and then measured by the Mindry device. The work included measuring the weight and height of each woman in this study, and BMI was calculated using the following formula: weight in kilograms divided by height in squared meters (Atoum, 2020). It quantified obesity by the BMI classification of WHO and the International Obesity Task Force.
RESULTS
Table 1 explains the mean and standard deviation of angiotensin-converting enzyme, plasmin, renin, kallikrein, and bradykinin among 75 breast cancer women and 15 controls.
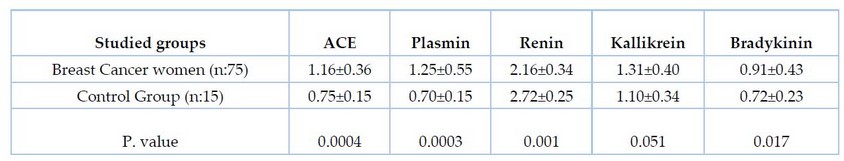
Table 1. Comparison between breast cancer and healthy women regarding the mean of angiotensin-converting enzyme, plasmin, renin, kallikrein, and bradykinin.
Table 2 explains the mean and standard deviation of angiotensin-converting enzyme and plasmin in women with breast cancer (before treatment, before mastectomy, after one dose, after 2 doses, and after three doses) compared to healthy women.
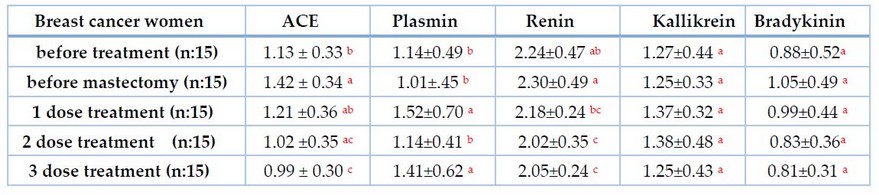
Table 2. Relation of ACE and plasmin with the stage of disease breast cancer women
The same letters mean no differences between them at p.value 0.05, (Mean±SD)
Table 3 explains the mean and standard deviation of ACE, renin, plasmin, kallikrein, and bradykinin among 75 breast cancer women; 27 had hypertension,9 were diabetes type II, and 38 had no other diseases.
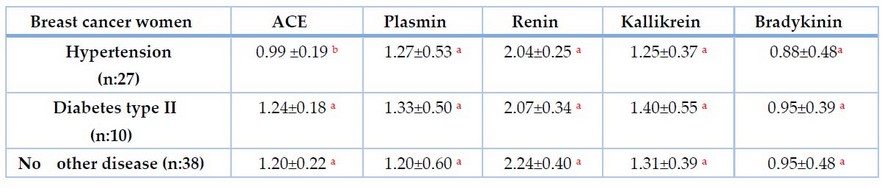
Table 3. Relation of ACE, renin, plasmin, kallikrein, and bradykinin with hypertension and diabetes in breast cancer.
DISCUSSION
The mean of Angiotensin-converting enzyme (ACE) was observed in breast cancer women (1.16±0.36U/L) as compared with the healthy control group (0.75±0.15U/L). The difference was highly significant at a P. value of 0.0004. The result has shown elevated serum ACE in breast cancer women compared with the control group, and this result, in agreement with Lubrano et al., which showed a higher level of ACE in women with metastatic breast cancer, suggested that this occurred based on the stage and grade of breast cancer.9 The highest circulating ACE levels in plasma carcinogenesis are suggested due to smoking, diet, and environment.10 Although the role of ACE in breast cancer pathophysiology is unknown, its hypothesized RAS components (ACE/AT1/Ang II) have been found in breast tissue. They may play a role in disease progression by increasing cell proliferation and promoting VEGF-mediated angiogenesis in breast cancer by directly affecting tumor and stromal cells and indirectly modulating vascular cell growth during angiogenesis.11 Our results show an increase in ACE levels when compared to the control group; this may occur as a result of hormonal imbalance in patients with breast cancer, which may result in abnormal expression of hormones dependent on genes such as ACE, which is usually affected by hormonal such as steroid hormone.12 The anti-angiogenic properties of ACE were investigated by other researchers, who were prompted to do so by their observation that patients with breast cancer had a higher risk of developing ACE.13 Our result disagrees with Varela& Saez, which found a decreased level of ACE in the serum of breast cancer compared with control and suggested a related stage of cancer with a level of ACE.14
A high level of plasmin produced by plasminogen in breast cancer patients occurs by binding of tissue-type plasminogen activator (tPA) to its receptor annexin II, promotes neoangiogenesis, cancer invasion, and metastasis, which predicts poor survival and is associated with a high rate of relapse for patients with breast cancer. In contrast, a lack of annexin II and tpA leads to decreased plasmin levels.15 Plasmin is a protein that plays an important role in the dissolution of a fibrin clot that is involved in the regulation of breast cancer invasion and metastasis, which may occur due to an increase in the production of plasmin. As the amount of plasminogen activator (uPA) in the bloodstream rises, the conversion of inactive plasminogen to active plasmin takes place, which results in the degradation and regeneration of the basement membrane and extracellular matrix (ECM), which ultimately leads to metastasis (spreading of cancer cells).16 Several growth factors and other proteolytic systems appear to be controlled by the PA/plasmin system, which is essential in this regard.17 Our findings indicate a decreased level of renin in the blood, which could be due to a decrease in the stimulation of juxtaglomerular cells in the renal nephron to produce renin or damage to the cells that synthesize and secrete hormones, among other possibilities. Our findings are consistent with.18 Low renin levels and high plasma aldosterone levels have been observed, which may be related to increased angiotensin receptor-1 (ATR1) levels and augmented angiotensin II action.19 Other studies have discovered that the renin levels in breast cancer patients are unaffected.20 The mean level of kallikrein was found to be elevated in breast cancer patients, which agrees with the findings.21, 22 The levels of kallikrein in the serum of breast cancer patients increased before the operation and after treatment.23 Breast cancer cells secrete kininogenases and kininogen substrates, which activate the bradykinin receptor (B1R), in which B1R agonist causes an estrogen-sensitive breast cancer cell to secrete more kallikreins that increase its secretion and the presence of kininogens with B1R in these cells may contribute to breast cancer proliferation and invasion.24 Also, kallikrein level was slightly higher (although not statistically significant) in the patients' estrogen receptor-negative and progesterone receptor-negative subgroups. Kallikrein is up-regulated by androgens in breast cancer patients.25 The mean bradykinin level was elevated in breast cancer patients, consistent with previous findings.26 Breast cancer patients have higher levels of bradykinin and desArg-bradykinin in their sera, whereas bladder cancer patients have lower levels.27 BK can be found in stomach, pituitary, uterus, and breast cancer tumors, among other places.26 The presence of kinin cascade proteins on functional cells such as neutrophils, fibroblasts, and endothelial cells in the breast tumor microenvironment further increases the likelihood of the formation of kinins in the breast tumor microenvironment, supporting their potential role as modulators of breast cell carcinogenesis.28
Our findings in Table 2 revealed that serum ACE levels were elevated in breast cancer patients due to various stages and chronic diseases, as well as sex hormonal disturbances such as androgen, which cause an increase in ACE levels.29 Breast cancer is associated with both androgen and estrogen risk factors; 30 women30 Women with low plasma estrogen levels are at a higher risk of breast cancer. On the other hand, estrogen insufficiency has been linked to a rise in ACE production.31 Furthermore, most patients have hypertension that may or may not be controlled with antihypertensive medications, and diabetic patients are exposed to elevated ACE levels.32 Several studies have demonstrated that angiotensin-converting enzyme levels are elevated in cancer, particularly breast cancer.33 In addition, we observed a decrease in the level of ACE after systemic treatment, which is consistent with. 9 The mean in the first dose increased from before treatment. This may be caused by the use of tamoxifen chemotherapy that develops resistance in some breast cancer women, which increases the RAS component, which suggests a combination of angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor-1 (AT1R) blockers with tamoxifen can be a new strategy to prevent tamoxifen resistance in ER-a positive breast cancer.34 The mean 3 doses observed decreased from 2 and 1. This may be due to using an antihypertensive drug such as Capital, which is an effective drug for breast cancer.35 Chemotherapy as bradykinin-potentiating peptide paclitaxel(BPP-PTX) targets the angiotensin-converting enzyme in breast cancer.36 Also, paclitaxel injection inhibited ACE activity and increased the expressions of B1 and B2 receptors and bradykinin-related peptide levels in peripheral tissue.37 The results of plasmin in women with breast cancer before mastectomy groups were, (1.01 ±0.45) ng/ml, (1.14 ±0.497) ng/ml in women with breast cancer before treatment, (1.52 ±0.70) ng/ml after one dose chemotherapy, (1.14 ±0.41) ng/ml, (1.41 ±0.62) in 2 doses and 3 doses of chemotherapy respectively. The study showed that serum plasmin levels elevated the mean of plasmin after chemotherapy doses. This result agrees with Samaneva et al .23 Systemic fibrinolytic occurs when there is a deficiency in plasmin inhibitor a2PI; the levels in our patients gradually increase after treatment with therapy. 38
ACE inhibitor increases fibrinolytic action by increasing tissue plasminogen activation TPA and decreasing TPA-1 inhibitor.39Elevated urokinase-type plasminogen activator (uPA) and its receptor in women with breast cancer that responsible for the conversion of plasminogen into plasmin which is the main enzyme involved in the degradation of the vascular basement membranes facilitates migration and the adhesion of cancer cells to the surrounding tissues also elevated plasmin activation of metalloproteinase, which is the only enzyme involved in the processing of type IV collagen, is responsible for the formation of the skeleton of the vascular basement membranes in the cancer cell.40 Our result observed an increase in plasmin level after dose administration. This may be due to inflammation and viral infection that elevated plasminogen activation to plasmin.41 In addition, Asprin used by breast cancer women increases plasmin activity.42 Other results suggested that patients with increased concentrations of plasmin fail to respond to hormone therapy in advanced disease.16 Our results show a decreased level of renin gradually in women before the operation, before treatment, and after 3 doses of chemotherapy; this may be due to breast cancer effect on renal cells such as juxtaglomerular cells or a decrease in nerve stimulation that ultimately lead to reduced in renin secretion. Renin levels, aldosterone, and sodium levels decreased in breast cancer women before trial therapy, and no elevated renin levels when aldosterone levels were subnormal.43 Estradiol has a cardiovascular protective impact on its antihypertensive activity, and estrogens can downregulate components of the renin-angiotensin system (RAS) and reduce the expression and activity of angiotensin I-converting.44 Surgical trauma and postoperative pain can produce stress responses. Cortisol is an essential indicator of the body's stress response when the body produces pain excitement under the hypothalamus-pituitary-adrenal cortical axis regulation, secret glucocorticoids to blood and make cortisol concentration increase while releasing a large number of stress hormones, including angiotensin, renin, aldosterone. The results of this study showed that the levels of angiotensin, renin, cortisol, and aldosterone first increased and then decreased; levels at 1 d and 3 d after the operation were significantly higher than those before an operation. Moreover, the levels of angiotensinⅡ, renin, cortisol, and aldosterone were significantly lower than those in the control group at the same time point.18 Our results show kallikrein level is not affected by the chemotherapy dose. This may be due to kallikrein resistance to chemotherapy. This result agrees with.23 Kallikreins are expressed in several human tissues, mainly the hormone-producing or hormone-dependent ones such as the breast, ovary, prostate, and testis. In cancer cell lines, all kallikreins are under sex steroid hormone regulation.45
The highest mean of ACE in Table 3 was recorded in breast cancer women with diabetes (1.24 ± 0.18 U/L), followed by women with no diseases (1.20 ± 0.22 U/L), and the low means were in women with hypertension (0.99 ± 0.19 U/L), the difference was highly significant at P. value: 0.007. Our results showed that serum ACE levels were increased. There were no significant differences in the mean of angiotensin-converting enzyme (ACE); the mean in hypertension decreased due to the use of an antihypertensive drug such as ACE inhibitor that decreases ACE and increases plasmin inhibitor such as tissue plasminogen inhibitor TPA-1 increases fibrinolytic activity.46 Although hypertensive drugs such as spironolactone caused a significant increase in plasmin by increasing tissue plasminogen activation levels, this incompatibility might be due to the number of patients included and the short duration of our study.47 Tamoxifen chemotherapy develops resistance in some breast cancer women, which increases the RAS component and increases ACE level.34 increases ACE level moderately in controlled diabetic patients and also a significant increase in uncontrolled diabetic patients. This is due to irregular carbohydrate, lipid, and protein metabolism, 48, and breast cancer women at high risk of hypertension. Several mechanisms have been proposed for the relationship between hypertension and breast cancer risk. First, breast cancer and hypertension may share a common pathophysiological pathway mediated by adipose tissue, which could cause chronic inflammation and further increase the risk of both breast cancer and hypertension.49
CONCLUSIONS
It was concluded that ACE, plasmin, kallikrein, and bradykinin levels were significantly elevated in breast cancer women compared to healthy women but decreased renin levels. The mean of plasmin in breast cancer women with hypertension was 1.27 ± 0.53ng/ml, followed by women with diabetes (1.33 ± 0.50 ng/ml), and the means were in women with no disease (1.20 ± 0.60 ng/ml). There were no significant differences in the mean of renin, plasmin, kallikrein, and bradykinin in the breast cancer women group who suffered from hypertension, the group who suffered from diabetes, and the group with no disease.
REFERENCES
1. Denton, M.. Breast Cancer: Risks, Detection, and Treatment. Greenhaven Publishing LLC, 2017, pp. 233-277
2. Power EJ, Chin ML, Haq MM. Breast cancer incidence and risk reduction in the Hispanic population. Cureus. 2018 Feb 26;10(2).
3. Al-obesity E.H and Al-obesity S.M.Serum prostate-specific antigen level in women with breast cancer and its relationship with some reproductive hormones in Kirkuk city, MSC thesis, collage of science, Tikrit University,2019.
4. Nakamura K, Yaguchi T, Ohmura G, Kobayashi A, Kawamura N, Iwata T, Kiniwa Y, Okuyama R, Kawakami Y. Involvement of the local renin‐angiotensin system in immunosuppression of tumor microenvironment. Cancer science. 2018 Jan;109(1):54-64.
5. de Miranda FS, Guimarães JP, Menikdiwela KR, Mabry B, Dhakal R, layeequr Rahman R, Moussa H, Moustaid-Moussa N. Breast cancer and the renin-angiotensin system (RAS): Therapeutic approaches and related metabolic diseases. Molecular and Cellular Endocrinology. 2021 May 15;528:111245.
6. Rasha F, Ramalingam L, Menikdiwela K, Hernandez A, Moussa H, Gollahon L, Rahman RL, Moustaid-Moussa N. Renin angiotensin system inhibition attenuates adipocyte-breast cancer cell interactions. Experimental Cell Research. 2020 Sep 1;394(1):112114.
7. Yan Y, Zhou A, Carrell RW, Read RJ. Structural basis for the specificity of renin-mediated angiotensinogen cleavage. Journal of Biological Chemistry. 2019 Feb 15;294(7):2353-64.
8. Qureshi Hj, Hamid N. Review Article Renin–Angiotensin Aldosterone System (Raas): Hamid Javaid Qureshi1, Naila Hamid2. Journal Of Akhtar Saeed Medical & Dental College. 2020 Oct 1;2(4):205-8.
9. Lubrano J, Roman H, Tarrab S, Resch B, Marpeau L, Scotté M. Liver resection for breast cancer metastasis: does it improve survival?. Surgery today. 2008 Apr;38(4):293-9.
10. Arima H, Kiyohara Y, Tanizaki Y, Nakabeppu Y, Kubo M, Katoa I, Sueishi K, Tsuneyoshi M, Fujishima M, Iida M. Angiotensin I-converting enzyme gene polymorphism modifies the smoking-cancer association: the Hisayama Study. European Journal of Cancer Prevention. 2006 Jun 1:196-201.
11. Bujak-Gizycka B, Madej J, Bystrowska B, Toton-Zuranska J, Kus K, Kolton-Wroz M, Jawien J, Olszanecki R. Angiotensin 1-7 formation in breast tissue is attenuated in breast cancer-a study on the metabolism of angiotensinogen in breast cancer cell lines. J Physiol Pharmacol. 2019 Aug 1;70(4):503-14.
12. Ghosh Roy A, Purkait P, Raha O. Association Between the polymorphism of the angiotensin-converting enzyme gene and breast cancer risk among the Bengalee Caste Hindu Females of West Bengal, India. Int J Forensic Sci Pathol. 2015 Feb 25;3(2):85-8.
13. Chang JS, Shin J, Park EC, Kim YB. Risk of cardiac disease after adjuvant radiation therapy among breast cancer survivors. The Breast. 2019 Feb 1;43:48-54.
14. Varela S, Saez BL. Utility of serum activity of angiotensin-converting enzyme as a tumor marker. Oncology. 1993;50(6):430-5.
15. Sharma M, Ownbey RT, Sharma MC. Breast cancer cell surface annexin II induces cell migration and neoangiogenesis via tPA-dependent plasmin generation. Experimental and molecular pathology. 2010 Apr 1;88(2):278-86.
16. Tang L, Han X. The urokinase plasminogen activator system in breast cancer invasion and metastasis. Biomedicine & Pharmacotherapy. 2013 Mar 1;67(2):179-82.
17. Chappuis PO, Dieterich B, Sciretta V, Lohse C, Bonnefoi H, Remadi S, Sappino AP. Functional evaluation of plasmin formation in primary breast cancer. Journal of Clinical Oncology. 2001 May 15;19(10):2731-8.
18. Lu JM, Kang C, Yan JJ, Zhang YY, Gong H. Effects of dezocine on postoperative stress response and immune function in patients with breast cancer. Journal of Hainan Medical University. 2017;23(21):110-3.
19. Moreno‐Muñoz D, de la Haba‐Rodríguez JR, Conde F, López‐Sánchez LM, Valverde A, Hernández V, Martínez A, Villar C, Gómez‐España A, Porras I, Rodríguez‐Ariza A. Genetic variants in the renin–angiotensin system predict response to bevacizumab in cancer patients. European Journal of Clinical Investigation. 2015 Dec;45(12):1325-32.
20. Hashida H, Honda T, Morimoto H, SASAKI T, Aibara Y, Yamanaka M. Breast cancer presenting with the syndrome of inappropriate secretion of antidiuretic hormone after simple mastectomy. Internal medicine. 2001;40(9):911-4.
21. Paliouras M, Borgono C, Diamandis EP. Human tissue kallikreins: the cancer biomarker family. Cancer letters. 2007 Apr 28;249(1):61-79.
22. Emami N, Diamandis EP. New insights into the functional mechanisms and clinical applications of the kallikrein-related peptidase family. Molecular oncology. 2007 Dec 1;1(3):269-87.
23. Samaneva NY, Vladimirova LY, Frantsiyants EM, Storozhakova AE, Kalabanova EA, Kabanov SN, Tishina AV, Svetitskaya YV, Shatova IS, Vashchenko LN. Plasma kallikrein-kinin system as markers of locally advanced breast cancer prognosis.
24. Ehrenfeld P, Manso L, Pavicic MF, Matus CE, Borquez C, Lizama A, Sarmiento J, Poblete MT, Bhoola KD, Naran A, Figueroa CD. Bioregulation of kallikrein-related peptidases 6, 10 and 11 by the kinin B1 receptor in breast cancer cells. Anticancer Research. 2014 Dec 1;34(12):6925-38.
25. Yousef GM, Scorilas A, Magklara A, Memari N, Ponzone R, Sismondi P, Biglia N, Abd Ellatif M, Diamandis EP. The androgen-regulated gene human kallikrein 15 (KLK15) is an independent and favorable prognostic marker for breast cancer. British Journal of Cancer. 2002 Nov;87(11):1294-300.
26. Van Winden AW, van den Broek I, Gast MC, Engwegen JY, Sparidans RW, van Dulken EJ, Depla AC, Cats A, Schellens JH, Peeters PH, Beijnen JH. Serum degradome markers for the detection of breast cancer. Journal of proteome research. 2010 Aug 6;9(8):3781-8.
27. Villanueva J, Shaffer DR, Philip J, Chaparro CA, Erdjument-Bromage H, Olshen AB, Fleisher M, Lilja H, Brogi E, Boyd J, Sanchez-Carbayo M. Differential exoprotease activities confer tumor-specific serum peptidome patterns—the Journal of Clinical Investigation. 2006 Jan 4;116(1):271-84.
28. Farhan, S. M., Abdulateef, S. M., Al-Enzy, A. F. M., Mohammed, Th. T., Saeid, Z. J. M., Al-Khalani, F. M. H. & Abdulateef, F. M. Effect of heat stress on blood alkalinity of broiler chicks and its reflection in improving the productive performance. Indian Journal of Ecology, 2020; 47: 107-109
29. Komukai K, Mochizuki S, Yoshimura M. Gender and the renin–angiotensin–aldosterone system. Fundamental & clinical pharmacology. 2010 Dec;24(6):687-98.
30. Giovannelli P, Di Donato M, Galasso G, Di Zazzo E, Bilancio A, Migliaccio A. The androgen receptor in breast cancer. Frontiers in endocrinology. 2018 Aug 28;9:492.
31. Martínez-Martos JM, del Pilar Carrera-González M, Dueñas B, Mayas MD, García MJ, Ramírez-Expósito MJ. Renin angiotensin system-regulating aminopeptidase activities in serum of pre-and postmenopausal women with breast cancer. The Breast. 2011 Oct 1;20(5):444-7.
32. . Wofford MR, Hall JE. Pathophysiology and treatment of obesity hypertension. Current pharmaceutical design. 2004 Nov 1;10(29):3621-37.
33. Beyazit F, Ayhan S, Celik HT, Gungor T. Assessment of serum angiotensin-converting enzyme in patients with epithelial ovarian cancer. Archives of Gynecology and Obstetrics. 2015 Aug;292(2):415-20.
34. Namazi S, Ardeshir-Rouhani-Fard S, Abedtash H. The effect of renin angiotensin system on tamoxifen resistance. Medical Hypotheses. 2011 Jul 1;77(1):152-5.
35. Chae YK, Valsecchi ME, Kim J, Bianchi AL, Khemasuwan D, Desai A, Tester W. Reduced risk of breast cancer recurrence in patients using ACE inhibitors, ARBs, and/or statins. Cancer investigation. 2011 Oct 24;29(9):585-93.
36. Guo XM, Yadav MB, Khan M, Hao CW, Lin CY, Huang T, Wu J, Fan BM, Bian ZX. Bradykinin-Potentiating Peptide-Paclitaxel Conjugate Directed at Ectopically Expressed Angiotensin-Converting Enzyme in Triple-Negative Breast Cancer. Journal of Medicinal Chemistry. 2021 Oct 26;64(23):17051-62.
37. Brusco I, Silva CR, Trevisan G, de Campos Velho Gewehr C, Rigo FK, La Rocca Tamiozzo L, Rossato MF, Tonello R, Dalmolin GD, de Almeida Cabrini D, Gomez MV. Potentiation of paclitaxel-induced pain syndrome in mice by angiotensin I converting enzyme inhibition and involvement of Kinins. Molecular Neurobiology. 2017 Dec;54(10):7824-37.
38. Jänicke F, Prechtl A, Thomssen C, Harbeck N, Meisner C, Untch M, Sweep CF, Selbmann HK, Graeff H, Schmitt M. Randomized adjuvant chemotherapy trial in high-risk, lymph node-negative breast cancer patients identified by urokinase-type plasminogen activator and plasminogen activator inhibitor type 1. Journal of the National Cancer Institute. 2001 Jun 20;93(12):913-20.
39. Fogari R, Zoppi A. Antihypertensive drugs and fibrinolytic function: Impact of dual calcium channel and renin-angiotensin system blockade. American Journal of Hypertension. 2006 Dec 1;19(12):1293-9.
40. Thielemann A, Baszczuk A, Kopczyński P, Kopczyński Z. High concentration of urokinase-type plasminogen activator receptor in the serum of women with primary breast cancer. Contemporary Oncology/Współczesna Onkologia. 2013 Nov 14;17(5):440-5.
41. Mojcik CF, Levy JH. Aprotinin and the systemic inflammatory response after cardiopulmonary bypass. The Annals of Thoracic Surgery. 2001 Feb 1;71(2):745-54.
42. Milwidsky A, Finci-Yeheskel Z, Mayer M. Stimulation of plasmin activity by aspirin. Thrombosis and haemostasis. 1991;65(04):389-93.
43. Buzdar AU, Smith R, Vogel C, Bonomi P, Keller AM, Favis G, Mulagha M, Cooper J. Fadrozole HCL (CGS‐16949A) versus megestrol acetate treatment of postmenopausal patients with metastatic breast carcinoma: Results of two randomized double-blind controlled multi-institutional trials. Cancer: Interdisciplinary International Journal of the American Cancer Society. 1996 Jun 15;77(12):2503-13.
44. Suba Z. Circulatory estrogen level protects against breast cancer in obese women. Recent patents on anti-cancer drug discovery. 2013 May 1;8(2):154-67.
45. Diamandis EP, Yousef GM. Human tissue kallikreins: a family of new cancer biomarkers. Clinical Chemistry. 2002 Aug 1;48(8):1198-205.
46. Tiryaki O, Usalan C, Buyukhatipoglu H. Effect of combined angiotensin‐converting enzyme and aldosterone inhibition on plasma plasminogen activator inhibitor type 1 levels in chronic hypertensive patients. Nephrology. 2010 Mar;15(2):211-5.
47. Yalcin AU, Dincer M, Aslan V, Gulbas Z. Effect of spironolactone on impaired fibrinolysis of hypertensive patients. Kidney and Blood Pressure Research. 2002;25(4):260-4.
48. Üstündağ B, Canatan H, Çi̇nkilinç N, Hali̇feoğlu İ, Bahçeci̇oğlu İH. Angiotensin-converting enzyme (ACE) activity levels in insulin‐independent diabetes mellitus and effect of ACE levels on diabetic patients with nephropathy. Cell Biochemistry and Function: Cellular biochemistry and its modulation by active agents or disease. 2000 Jan;18(1):23-8.
49. Han H, Guo W, Shi W, Yu Y, Zhang Y, Ye X, He J. Hypertension and breast cancer risk: a systematic review and meta-analysis. Scientific reports. 2017 Mar 20;7(1):1-9.
Received: May 15, 2023/ Accepted: June 10, 2023 / Published: June 15, 2023
Citation: Hussein, H.A.; Algburi, F.S. The role of Renin angiotensin aldosterone system in women with breast cancer before and after treatment. Revis Bionatura 2023;8 (2) 68. http://dx.doi.org/10.21931/RB/2023.08.02.68
