2023.08.03.108
Files > Volume 8 > Vol 8 No 3 2023
Physiological responses of Helianthus annulus L. plants under allelopathic effect of Cucurbita moschata
1Department of Biology, College of Science, Mosul University, Mosul, Iraq
2Department of Biology, College of Education for Girls, Mosul University, Mosul, Iraq
E-mail: [email protected]
2,* Department of Biology, College of Science, Mosul University, Mosul, Iraq; E-mail: [email protected]
* Correspondence: [email protected]
Available from: http://dx.doi.org/10.21931/RB/2023.08.03.108
ABSTRACT
Cucurbita moschata L.(pumpkin) is a creeping herbaceous plant in all Arab countries. This plant Grows through the year and multiplies by seeds. It can be a small area for cultivation. The research was conducted in the Department of Biology, College of Science greenhouse to determine the allelopathic effect of C. moschata L. added to the soil at 5,10 and 15% w:w. they were incubated for two weeks on seed germination and growth of Helianthus anuus compared to the ground without residues. Results showed that the highest percentage of inhibition was 20.5% at a 15% ratio. An increase in the shoot and root length and their dry weights was treated with C. moschata residues at the three ratios, and the highest stimulation percentage reached (110,105.6,140,27.4) at the ratio of 15%. With an increase in the ratio, the rate of inhibition. Results showed the effect of C. moschata incubated on the chlorophyll a and b content and total chlorophyll in H. anuus. The highest stimulation percentage was 8.6% in chlorophyll b at 5% ratio. This is reflected by an increase in leaf Area of some treatments and a reduction in others.
Keywords: Allelopathy, Cucurbita moschata, Crops, secondary chemicals
INTRODUCTION
Allelochemicals play a vital part in the adaptation of many species. They participate in several chemical groups and act in various ways: 1 . Through biochemical pathways, allelopathy describes the inhibitory and stimulatory interactions at the soil-plant interface.
By altering soil conditions, It is acknowledged as a stressor for processes like diversification, succession, and invasion2. Allelochemicals can obstruct receiver plants' ability to perform fundamental functions such as photosynthesis, cell division, respiration, and protein synthesis3. Through leaching, volatilization, root exudation, and residue degradation, secondary chemicals are released into the environment, speeding up allelopathic interactions that impede agriculture, growth, and development; it contains excellent nutritional and medical benefits4,5. The effects of diverse plant allelochemicals, extract concentration, plant tissue type, and environmental conditions on the target plants might be positive or negative. Numerous studies have been done on releasing allelopathic substances through the breakdown of plant residues in soil. Economic importance of C. moschata: it is considered the world's third most important oil crop. This crop, which is a member of the Cucurbitaceae family and is grown for ornamental purposes and its edible seeds, is distinguished by its large radial flowers, adaptability to various summer and winter climates, and capacity to grow in multiple soil types. It also contains selenium, a mount of oil, and vitamins. The most important active compounds found in C. moschata are Aspartic acid, cysteine, lysine, valine, arginine, alanine praline and glutamic acid6.
The study included conducting a greenhouse experiment to discover the allelopathic effects of C. moschata residues added to the soil on seed germination and some growth characteristics of H. anuus.
preparing for the experiment
The vegetative parts of the plant were collected from one of the fields planted with this crop in the village of Al-Rahmaniya in Nineveh, sliced into little pieces, put in an oven at a temperature of 70°C for 72hours and then ground with a Blender; then powder was kept until use7.
Preparing the seeds for planting
Seeds of American sunflowers were obtained from the Department of Seed Testing and Certification. The viability of the seeds was tested at 25°C, and it was 95%. Residues of C. moschata were mixed with three ratios 5, 10 and 15% w:w, mixed with soil incubated for 2 weeks, used and placed in 1kg plastic pots with a diameter of 20cm and a height of 20 cm, then planted with 10seeds of H. annus in each pot, with three replicates for each treatment. Watered as needed, after 10 days of planting, the number of seedlings was reduced to 5, which was measured7.
The germination % = number of germinated seeds \ number of cultivated seeds x 100
After the end of the experiment period, plants were uprooted, and the vegetative part was separated from the root. The measured length of each part was then dried in an electric oven at 70 °C for 72 hours, and their weights were recorded. Number of leaves: Three plants from each treatment are selected at random. The area of the leaf was calculated according to the following formula :
leaf area cm2/sheet = leaf weight x small section area \ Small section weight of8.
Determination of chlorophyll content
9 Chl-a (mg/gm leaf) = (12.7) (Abs663) -(2.69) (Abs645)/mg leaf tissue
Chl b(mg/gm leaf)=(22.9)(Abs645)-(4.68)(Abs663)/mg leaf tissue.
Total Chl(A+B) =20.2 (Abs 645) +9.02) (Abs663) *V/1000*W.Abs
Reading of the extracted chlorophyll optical density at 663 and 645 nm, respectively
(V= final volume of acetone at a concentration of 80%, W= wet weight in grams of plant tissue used)10.
Statistical analysis
The experiment was carried out according to a completely randomized design C.R.D., according to the SAS program using Duncan's Duncan's Duncan's Duncan'sDuncan's test was used to distinguish between averages11. Average inhibition or stimulation in H. anuus growth was calculated to evaluate the allelopathic potential of C. moschata
Reduction or stimulation from comparesion% = 100 -A / B × 100 (12)
A= measurement for plants in treatment
B = measurement for plants in control
Table 1 shows the effect of incubated C. moschata residues at (5,10 and 15) % w: w ratios. on H. anuus seeds germination and growth observed significant differences between treatments, inhibited seed germination, highest inhibition reached 70% at the ratio % w;w. On the other hand, results showed the effect of residues on the number of H.anuus leaves. There was a reduction in the number of leaves at the three ratios; the highest reduction was 9 % at the 5% ratio. The results were received with a decreased germination rate. And some growth characteristics. The findings revealed a considerable decrease in the seed germination and growth of wheat plants grown in soil. Incubated with residues of cucumber, melon and C. moschata crops Cu. melo.L C.sativus and C. pepo.L on Triticum aestivum.L compared to control soil ,13
The results also showed a reduction in the number of leaves at the three ratios. On the other hand, adding C. moschata residues to the growth characteristics of H. anuus caused growth stimulation, indicating the highest shoot length reached 39.5 cm, and the highest weight was 0.96 gm. At the ratio 15% w;w. The present results also showed an increase in root length and dry weight of the root system with increased ratios of residues. The highest root length reached 22 cm and in the root dry weight was 0.21gm at a ratio 15%.
Fourteen noticed decreased Zea mays seed germination by leaf extracts of C. pepo L. and E. citriodora. All fractions of the extract were moderate to lightly toxic to Z. mays L. seedling 15; water extract of rape at low treatment ratios increased the length of the corn seedling'sseedling's roots and stems, which were far higher than the control. According to our findings, rape water extract had the most substantial allelopathic inhibitory impact on H. anuus, followed by corn and oat.
A significant increase with the increase in the addition ratios of residues is attributed to C. moschata; residues contain chemical compounds during their decomposition in soil, which affected the growth of H. anuus that released especially its effect on cell division and elongation, and some phenolic compounds are effective in secretion auxins, which leads to their accumulation and caused an increase in lengths. The effect differs due to a difference in the quality and quantity of the chemical compounds in the residues. According to their ratios and distribution within plants, IAA , which in turn works on increasing cell division and elongation and the formation of roots, then increasing their dry weight. Corn root exudates significantly increased potato bud fresh weight and length, with high ratios having the best growth-promoting effects. A similar pattern was seen with soybean root exudates.it was found effect of aqueous extracts of Cucurbita and Cucumis at ratios (0,3,5) %w: v on germination and seedling growth of Cucumis; the highest percent of reduction was (51.8,61.1 and 53.5%), respectively at (3%) of Cucurbita extracts. As ratios of the aqueous extract of C.falcata increased, the germination reduced. At ratios of 10% and above, According to the findings, C.falcata had a highly potent allelopathic effect on the germination of the plants under investigation16. The level of inhibition of germination rate and growth increased with the increase in the ratios of the incubated Cucurbita residues.
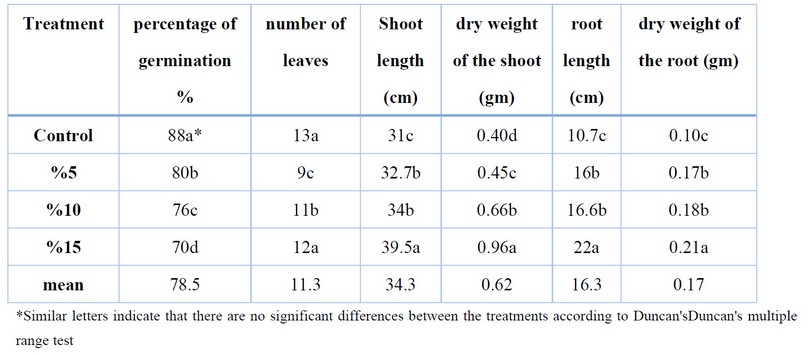
Table 1. Effect of Cucurbita moschata on germination and growth of H. anuus
Table 2 shows the percentages of increase or reduction of seed germination % and growth characteristics of H.anuus. The highest percentage % of stimulation, in general, was in the dry weight of the shoot-at ratio.15 %, while the highest increase reached 30.8% in leaves at 5% ratio. The highest value is 27.4%, 140% in dry weight at 15%. The results also showed an increase in root length and dry weight of the root system with the increased ratios of residues. The highest percentage of increase in root length reached 105.6%, and in the dry weight of the root system 110 % at 15%. ratios.
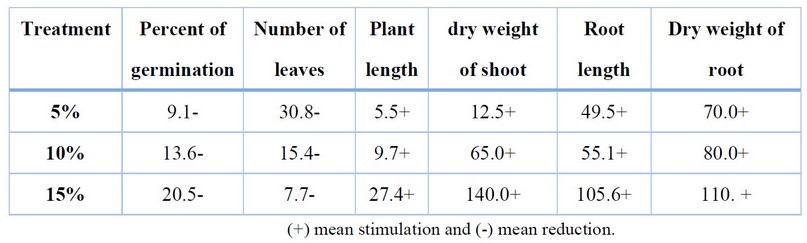
Table 2. Percentage of Stimulation or Reduction of Seed Germination and G characteristics of H.anuus when adding C. moschata residues
When comparing the results of Table 3 regarding the effect of adding c.moschata residues on the content of chlorophyll a, b and total chlorophyll. There is a significant difference between the ratios of residues and the chlorophyll content. The increase and decrease in chlorophyll a or b content and total at the ratio.10% and15%. The previous effects of C. moschata residues were reflected in the effect on the content of chlorophyll a,b and total chlorophyll in H.anuus, gave an increase in the content of chlorophyll a, b and total in H.anuus, eggplant and pepper leaves.
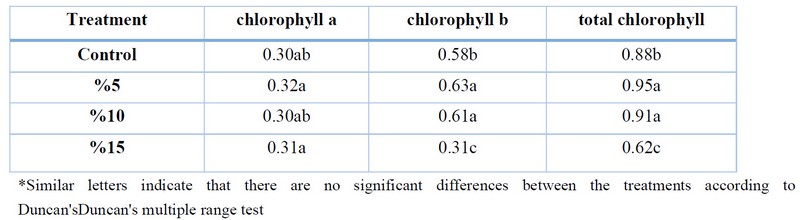
Table 3. Effect of Cucurbita moschata on chlorophyll in H. anuus
Figure (1) shows the effect of C.moschata residues incubated at (5,10,15) % ratios on the leaf area of the Helianthus anuus. An increase in the leaf area of plants was observed at a ratio of 10%, while a reduction occurred at a ratio (15, 5)%.
Stress from phenolic acid has an impact on crop seedlings ' physiological indicators. The amount of chlorophyll in C. coronarium was reduced as the ratios of benzoic acid and cinnamic acid increased. Cinnamic acid's allelopathic effect was much more significant than benzoic acid's. Additionally, during the seedling growth stage, the chlorophyll content of the leaves exhibited a trend of first increasing and then decreasing, reaching the peak value on the seventh day. This trend was attributed to high ratios of allelochemicals and prolonged exposure to allelochemicals. This resulted in impaired chlorophyll synthesis, decreased stomatal opening, and decreased stomatal opening ratio in leaves 17.
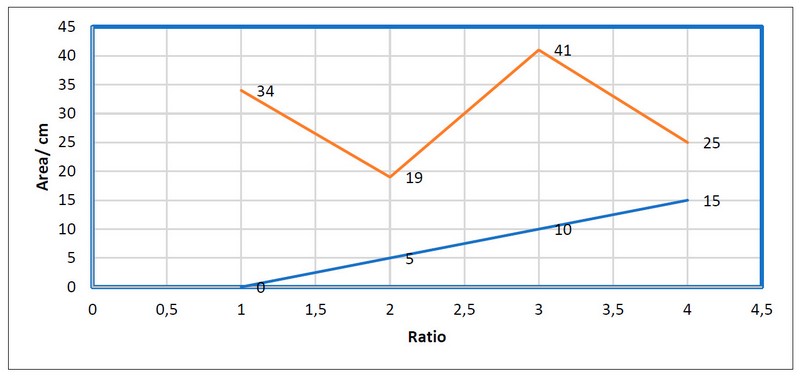
Figure 1. Effect of Cucurbita moschata residues incubated on Helianthus anuus leaf area.
Results showed that the reduction in chlorophyll content for some treatments may be attributed to a defect in the ion exchange process of mineral elements such as iron and magnesium, which are essential in forming chlorophyll, as for the effect on leaf area. Substances are unstable in the soil due to their microbial decomposition. The toxic plant activity of antagonistic substances is affected by many factors that affect the behavior of compounds18,19 revealed the effects of the behavior of antagonistic compounds in the soil and in both the donor and the receptor plant, such as the composition of the soil, and that the allelopathic activity is significantly influenced by the antagonistic chemicals contained in water because the receptor plants can absorb directly20.
Through biochemical pathways, allelopathy describes the inhibitory and stimulatory interactions at the soil-plant interface. Results showed significant differences between treatments, inhibited seed germination, and the effect of residues on the number of H.anuus leaves. On the other hand, the effect of adding C. moschata residues on the growth characteristics of H. anuus caused stimulate in growth. The present results also showed an increase in root length and dry weight of the root system with increased ratios of residues.
Funding: "This research received no external funding,""
Acknowledgments: The author would like to thank the Dean and Head of the Department of Biology at the College of the Science / University of Mosul for providing all the facilities to complete this research.
Conflicts of Interest: "The authors declare no conflict of interest."
1. Cheng, F. and Cheng, Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front Plant Sci:2015, 6:102003. DOI org/10.3389/fpls.2015.01020.
2. Moradi, P.; Vitalini, S. and Iriti, M.Allelopathic Interactions between Seeds of Portulaca oleracea L. and Crop Species. Appl. Sci.,2021, 11(8), 3539; Doi.org/10.3390/app11083539.
3. Abdallah, A. and Amine, H. m. Allelopathic effects of Cucurbita pepo L. and Eucalyptus citriodora H. plant extract fractions on seed germination of corn and some associated weeds. Tanta University Journal of environment:2016, 9:220-227. DOI:10.13140/RG.2.1.1735.0641.
4. Novakoski, A. D. S.; Coelho, É. M. P. ; Ravagnani, G.T.; Costa, A.; Rocha, S.A.; Zucareli, V. and Lopes, A.D. Allelopathic potential of plant aqueous mixtures on Euphorbia heterophylla. Agriculture.2020,10(10):449. Doi.org/10.3390/agriculture10100449.
5. Bajwa, A. A.; Nawaz, A. and Farooq, M.Allelopathic crop water extracts application improves the wheat productivity under low and high fertilizer inputs in a semi-arid environment. Int J Plant Prod.2020, 14:23–35. DOI.org/10.1007/s42106-019.
6. Linfeng, C.; Yi, W.; Lulu, S.; Jingchan, Z. and Wenhuai, W. Identification of allelochemicals from pomegranate peel and their effects on Microcystis aeruginosa growth, Environmental Science and Pollution Research:2019, volume 26, pages22389–22399.
7. Al-Doree, E. K.; Al-Sffar, R. S. and Jasim, I. R. Phenotypic and genotypic investigation of three weeds residues allelopathic effect on the growth of three hybrid wheat cultivars.j. Bionatura:2022, Volume 7 Issue 4 (64). DOI i.org/10.21931/RB/2022.07.04.64
8. Blanco, F. F. and Folegatti, M.V. ""A new method for estimating the leaf area index of cucumber and tomato plants"". Horticultura Brasileira.2023, 21 (4): 666–669. Doi:10.1590/S0102-05362003000400019.
9. Karthic, T.; Sarkar, g.; Babu, S.; Amalraj, L. D. and Jayasri, M. A.Preparation and evaluation of liquid fertilizer from Turbinaria ornate and Ulva reticulate. Biocatalysis and Agricultural Biotechnology,2020, 28,101712. DOI.org/10.1016/j.bcab.2020.101712.
10. Dikio, E. D. and Isabirye, D. Isolation of chlorophyll a form spinach leaves. Bulletin of the Chemical Society of Ethiopia:2008, 22(2). DOI:10.4314/bcse. v22i2.61302.
11. Steel, R.G.D.and Torrie, J.H. Principle and procedures of ststistics.2nd MC-GrawHillcompany, Inc., London.1980.
12. Chung, M. K.; Worsley, K. J.; Paus, T.; Cherif, C. ; Collins, D. C. ; Gieddd, J. N. ; Rapoportd, J. L. and Evansb, A. C.Unified Statistical Approach to Deformation-Based Morphometry, NeuroImage:2001, V 14, 3, 595-606.doi.org/10.1006/nimg.08620064-6.
13. Abid-Aljabar, F. A. and Saeed, J. A. The Residues Effect of some Crop Plants on Germination and Growth of Five Wheat Cultivars Triticum aestivum L. Department of Biology/ College of Science/ University of Mosul. Journal of Sciences of Rafidain,2001, 28, Number 4, 8-22. DOI 10.33899/rjs.2019.163298.
14. Sabry, A. and Amine, H. M. Allelopathic effects of Cucurbita pepo L. and Eucalyptus citriodora H. plant extract fractions on seed germination of corn and some associated weeds Tanta University, Egypt. Ordinal: sativus On germination and some growth characteristic. Basic education journal,2016,12(4). DOI:10.13140/RG.2.1.1735.0641.
15. Ying, Yong S. and tai, xue b. Allelopathy of rape on seed germination and seedling growth of three crops, The Journal of Applied Ecology,2020, 31(12):4153-4160. DOI: 10.13287/j.1001-9332.202012.002.
16. Tarbali, R. ; Aliloo, A. and Farjami, N. M.Allelopathy of Ceratocephalus falcata on enzymatic activity of some crops seeds in germination stage. Journal of Crop Production,2020, 13(1), 67-84. DOI:10.52547/yujs.7.2.1.
17. Y, H.; FU, W, X. QU, AN*, CC, LI C Y, QIAN F H, TANG and X J CHEN. Allelopathic effects of phenolic acids on seedling growth and photosynthesis in Rhododendron delavayi Franch. Photosynthetica:2019,57 (2): 377-387, 2019. DOI: 10.32615/ps.2019.045.
18. Kocira, S.; Szparaga, A.; Kocira, A.; Czerwi, ''nska E.; Depo, K. ;Erlichowska, B. and Deszcz, E. Effect of applying a bio stimulant containing seaweed and amino acids on the content of fiber fractions in three soybean cultivars. Legume Res.,2019, 42, 341–347.
19. Kocira, A.; Świeca, M.; Kocira, S.; Złotek, U. and Jakubczyk, A. Enhancement of yield, nutritional and nutraceutical properties of two common bean cultivars following the application of seaweed extract (Ecklonia maxima). Saudi J. Biol. Sci.2018, 25, 563–571. Doi.org/10.1016/j.sjbs..01.039.
20. Ibrahim ,R.A.; Yaqub ,H.M.; Jasim,I.R. Effect of orange Peel (Citrus sinensis) L. powder on germination and growth of some oremental plant. Annals of forest Reseaech.,2022 65(1): 6328-6332. DOI: 10.5281/zenodo.7410182.
Received: 25 June 2023/ Accepted: 26 August 2023 / Published:15 September 2023
Citation: Iman Radha J , Rawnaq Ahmed I, Hala Muzher Y. Physiological responses of Helianthus anuus L. plants under allelopathic effect of Cucurbita moschata. Revis Bionatura 2023;8 (3) 108 http://dx.doi.org/10.21931/RB/2023.08.03.108
