2021.06.02.8
Files > Volume 6 > Vol 6 No 4 2021 > Vol 6 No 2 2021
Biocompatible thermo-responsive N-vinylcaprolactam based hydrogels for controlled drug delivery systems
J. Fernanda Romero1; Antonio Díaz-Barrios1,*; Gema González2,3
Available from: http://dx.doi.org/10.21931/RB/2021.06.02.8
ABSTRACT
The treatment of several diseases requires drugs commonly administered orally or intravenously. Said administration has several drawbacks, such as low control of the necessary drug levels in plasma, making the treatment ineffective and, furthermore, side effects and low compatibility with the patient. Recently, the use of stimuli-responsive hydrogels in controlled Drug Delivery Systems (DDSs) has been considered an excellent alternative because of its inherent biocompatibility, responsiveness to physiological changes in the body, and diversity of both natural and synthetic material options.
The present work focuses mainly on the synthesis, characterization, and drug release capacity of poly (N-vinyl caprolactam) (PVCL) and poly (N-vinyl caprolactam) microgels crosslinked with various concentrations of poly (ethylene glycol) diacrylate (PEGDA), which show temperature stimuli-responsiveness near the physiological temperature of the human body. For that reason, changes in the average hydrodynamic particle diameter at different temperatures are estimated and correlated with the drug release rate. The model drug chosen for releasing studies is colchicine, a potential drug for gout disease treatment, currently in disuse because of its low therapeutic index. It is expected that the use of the control release procedure by drug encapsulation in this polymer overcomes this drawback. The synthesis of PVCL homopolymer and three VCL-co-PEGDA hydrogels varying the PEGDA crosslinker concentration was successfully carried out by emulsion polymerization. Their characterization was performed by DLS and FTIR spectroscopy. Polymerization yields were estimated by total solids analysis, and UV-VIS determined the cloud points. Finally, the drug loading and release over time were monitored by HPLC and UV-VIS spectroscopy showing that drug release profiles obtained corresponded to a sustained drug delivery system.
Keywords: thermo-responsive polymers, lower critical solution temperature (LCST), nanogels, drug delivery system, colchicine
INTRODUCTION
The hydrogel's swelling behavior allows the hydrogel to absorb a water volume, which penetrates the gel matrix, causing gel-solvent interactions to be stronger than gel-gel interactions. Nevertheless, it does not mean that the water explicitly dissolves the polymer.1–4 Dušek and Patterson 5 argued that it is possible to control the swelling property reversibly with external condition changes, such as physical or chemical stimuli. Recently, thermo-responsive gels are one of the most studied hydrogels due to their physiological importance, since they can take advantage of the temperature difference between the environment, the human body (37°C), the presence of fever (>37.5°C), or the presence of an intratumoral environment (40–44°C).6,7 For these reasons, they have an excellent performance for cancer therapy 7, transdermal drug therapy 8, and oral drug delivery 9. Thermo-sensitive hydrogels exhibit a phase transition when there is a change in temperature, so gel volume increases or decreases depending on having an Upper Critical Solution Temperature (UCST) or Lower Critical Solution Temperature (LCST).10,11
In particular, LCST hydrogels present an interesting phase separation when heating the system above a specific temperature named LCST (Figure 1). Below LCST, the polymer chains interact with water by H-bonding, so the hydrogen bond energy predominates in the system, and the polymer is miscible in the water showing a swollen state (single-phase). Upon heating, the hydrogen bond interactions become weaker, while polymer-polymer hydrophobic interactions become more robust due to the molecular agitation, and polymer phases out of the solution. That phase transition is visually perceptible because the solution passes from transparent to turbid. In this way, LCST polymers are excellent candidates to design polymeric particles for controlled, targeted, and sustained drug delivery on the nanometric scale, also called nanocarriers.12 The release mechanism for drug delivery consists of (1) the encapsulation at temperatures below LCST and (2) the delivery when the polymer shrinks at a temperature above LCST.
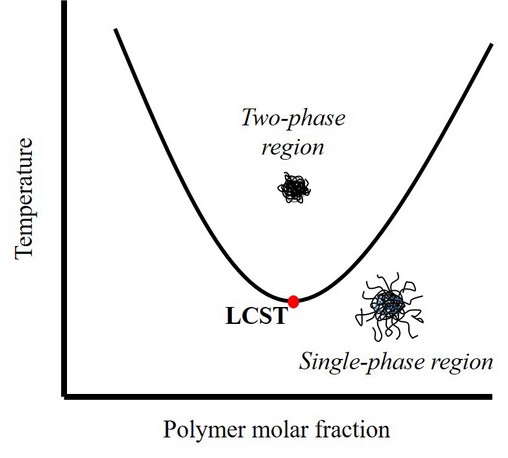
Figure 1. Phase diagram of LCST thermo-responsive hydrogels.
One of the synthetic LCST hydrogels most studied is the poly (N-isopropyl acrylamide) (PNIPAM), a vinyl polymer with secondary amide pendant groups that shows an LCST around 32°C. However, this polymer induces cellular cytotoxicity at 37°C, and the secondary amide group can produce toxic amines if hydrolysis occurs. Consequently, it is not biocompatible.13 Thus, it has been a challenge to develop a biocompatible thermo-responsive hydrogel, and poly(N-vinyl caprolactam) (PVCL) based polymers are considered an excellent alternative to PNIPAM for controlled and biocompatible drug delivery systems.
PVCL shows a similar LCST near 32°C.14,15 Additionally, Vihola et al. 13 demonstrated that cell cultures successfully tolerated PVCL polymer after three hours of incubation, at concentrations in the range of 0.1–10.0 mg/ml, at room temperature and physiological temperature (37°C). Therefore, PVCL is biocompatible.13–16
Many researchers have been developing hydrogels based on PVCL because of the reversible volume transition from micro- to nano-scale.12,17–20
This work aims to synthesize PVCL-based polymers to obtain suitable nanocarriers for drug delivery applications. PEGDA is the chosen crosslinker due to its biocompatibility, hydrophilicity, and ability to prevent protein adsorption and cell adhesion.21–23 In the present work, the model drug for drug release studies is colchicine due to the critical application for gout treatment and its low therapeutic index24, which motivates to overcome this limitation.
METHODS
Materials
Monomers: N-vinyl caprolactam (VCL; Sigma Aldrich, 98%), and Poly(ethylene glycol) diacrylate (PEGDA; Sigma Aldrich, Mn 250 g/mol). Initiator: ammonium persulfate (APS; FMC Corporation,>99%). Emulsifier: sodium dodecyl sulfate (SDS; STEOL®CS-230 Stepan). Buffer: sodium hydrogen carbonate (Sigma-Aldrich, ≥99.7%) used as provided. Colchicine (Sigma Aldrich ≥ 95%), potassium dihydrogen phosphate (Fisher Scientific, 99.6%), and methanol (LiChrosolv®, HPLC grade) were also used as provided. All aqueous solutions were prepared with double deionized water (DDI) produced by aDirect-Q®3 UV Water Purification System.
Synthesis of hydrogels
Four thermo-responsive microgels, one PVCL homopolymer, and three poly(N- Vinylcaprolactam -co-PEGDA) copolymers were synthesized by emulsion polymerization of VCL and PEGDA in a flat bottom flask equipped with a reflux condenser, using PEGDA as a crosslinker, SDS as an emulsifier, APS as initiator, and sodium hydrogen carbonate as a buffer (Figure 2).
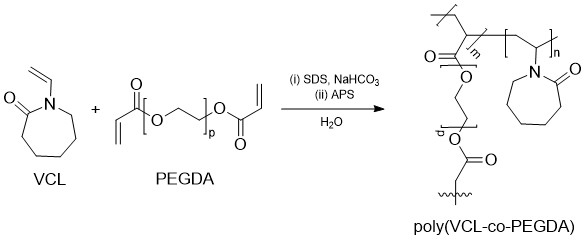
Figure 2. Scheme of VCL-PEGDAx synthesis reaction.
Once the monomers, emulsifier, and buffer were loaded, the system was heated to 70°C and stirred at 350 rpm. Then, the initiator was added, and after a short period, the reaction system became turbid, showing that polymerization began. The reaction was allowed to continue for 7h with stirring at 70°C . Once polymerization finished, the reaction was allowed to cool down to room temperature and stirring continued for 12h to avoid agglomeration. The final products were dialyzed against DDI water at least three times a day to remove unreacted reagents and impurities until the solvent showed conductivity of DDI (1.7μS). Recipes and reaction conditions are resumed in Table 1.
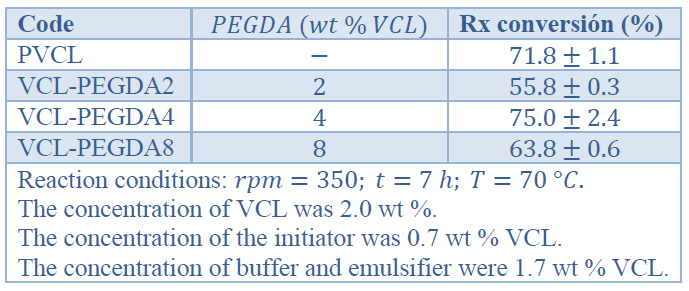
Table 1. Polymerization conditions and reaction conversion
Total Solids (TS) Analysis
The concentration of hydrogels was estimated by Total Solids (TS) analysis by weighing the solid present in a known volume of a purified hydrogel. The analysis was carried out by weighing a Petri dish, filling it with a known hydrogels volume, and evaporating the water in a drying oven at 105°C for 2h to constant weight. Finally, the Petri dish was weighted, and concentration (C) of purified hydrogels was calculated by Equation (1), Where mi is the weight of the empty Petri dish, mf is the weight of Petri dish with residual gel, and V is the volume of hydrogel added.
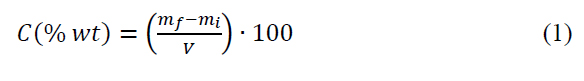
The yield of polymerization was calculated gravimetrically by Equation (2), where CTS is the concentration of hydrogels obtained from total solids (TS) analysis, and CTS, theoretical is the theoretical total solids of hydrogels
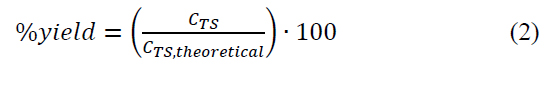
Characterization and Methods
Dialysis
Purification of hydrogels and drug uptake/delivery systems were carried out by dialysis against DDI water using Carolina ™ Dialysis Tubing (MWCO: 12.000 - 14.000 Daltons) as a dialysis membrane.
ATR-FTIR Spectroscopy
An Attenuated Total Reflectance Fourier Transformed Infrared (ATR-FTIR) Agilent Cary 630 spectrometer was used to characterize the obtained polymers. Measurements were carried out for PVCL, VCL-PEGDAx hydrogels, VCL and PEGDA dried samples.
Cloud Point Determination
The cloud point (or LCST) of hydrogels was determined by a ZUZI® 4211/50 spectrophotometer monitoring the hydrogel's light transmittance upon cooling the hydrogels from 40°C until room temperature. The measurements were performed at 650 nm to avoid the absorbance of light by the polymeric gels. The temperature was recorded with a digital thermometer.
Particle Size Determination
The mean particle diameters of hydrogels' aqueous dispersion were measured by Dynamic Light Scattering (DLS) method using a BI-90 Plus Brookhaven Instrument Corporation particle size analyzer. The device was equipped with a standard solid state laser light source (35 mW and 659 nm)
All measurements were carried out five-times at two temperatures (RT and T >LCST) to give an average hydrodynamic diameter and size distribution. The plastic cuvette was filled with approximately 1 mL of the hydrogels previously purified by dialysis. The mean particle diameter and polydispersity index (PDI) parameters were calculated using ZetaPlus Particle Sizing Software. Two Nanosphere™ size standards were used: (1) 90 nm (Duke Scientific Corporation), and (2) 20 nm (Thermo Scientific).
High-Pressure Liquid Chromatography (HPLC)
An UltiMate 3000 HPLC apparatus equipped with an autosampler, a quaternary pump, a column compartment, and a UV-VIS detector was used as a drug uptake/release analytical method. The analysis was carried out using a reverse-phase Hypersil GOLD™ (150 mm 4.6 mm, 5 μ particle size) C-18 column. The mobile phase was prepared by mixing potassium dihydrogen phosphate (450 mL, 6.8 g/L) and methanol (530 mL).25 The mixture was cooled to room temperature and made up to 1000 ml with methanol. Then, the pH was adjusted to 5.5 with diluted phosphoric acid. The final mixture was filtered through Titan 47 mm Membrane Disc filters. The mobile phase flow rate was 1.0 mL/min, and the injection volume was 20 μL. The column temperature was kept at 30 °C, and detection was carried out at 254 nm.
Standard solutions and calibration curves
A standard stock solution of colchicine (1000 μg/mL) was prepared. From this stock solution, standards with concentrations of 2, 5, 15, 16, 20, 40, 65, 100 μg/mL were also prepared. Two calibration curves were constructed over the concentration ranges of 2-20 and 5-100 μg/mL, both with five concentration levels. Chromeleon™ Chromatography Data System Software was used to obtain the corresponding calibration curves.
Drug Uptake Studies
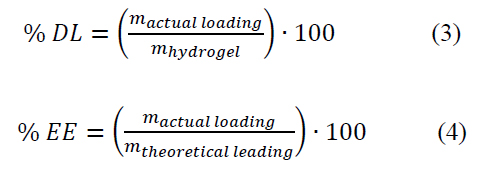
The method was to take 9 mL of the hydrogel, placed it in a 50 mL glass recipient, and dry at 50 °C. The dried hydrogel was allowed to swell in 5 mL of drug solution (1000 μg/mL). The mixture was sonicated for 20 min at RT and allowed to stand for 48 h to reach equilibrium. Then, the mixture was subjected to dialysis overnight to remove the non-loaded drug from the system. Posteriorly, the dialysate solution was recollected, filtrated with 0.45 μm syringe filters, and quantified by the analytical method previously described. Thus, the % of drug loading (DL) and encapsulation efficiency (EE) was determined using Equation (3) and Equation (4), respectively, where is the mass of hydrogel in the encapsulation process, is the encapsulated drug, and is the total amount of drug initially added.
Drug Release Studies
The previously dialyzed solutions were placed in a dialysis membrane and again dialyzed to release the drug against DDI water (35 mL) at approximately 38°C; this is above the LCST. At regular intervals of time, dialysates containing the released drug were collected in test tubes. After every sample collection, the solvent was refreshed. Finally, all recollected samples were filtered with 0.45 μm syringe filters and quantified by the previously described HPLC analytical method.
RESULTS AND DISCUSSION
One PVCL homopolymer and three poly(VCL-co-PEGDA) water-soluble hydrogels were synthesized by emulsion polymerization with different crosslinker amounts (2, 4, 8 wt. %) relative to the VCL monomer. During the reactions, the appearance of turbidity was observed with the APS initiator's addition, showing that polymerization started. After 7h of reaction, the polymers were cooled down, and turbidity slowly disappeared. The transition to transparency from turbidity confirmed that volume phase transition had occurred, and the resulting polymer materials were thermo-responsible. 26
Once polymerization had been finished, the hydrogels were purified by dialysis to remove unreacted reagents until dialysate showed conductivity of DDI water (1.7 μS), approximately. The polymerization reactions show relatively good yields as can be appreciated in Table 1
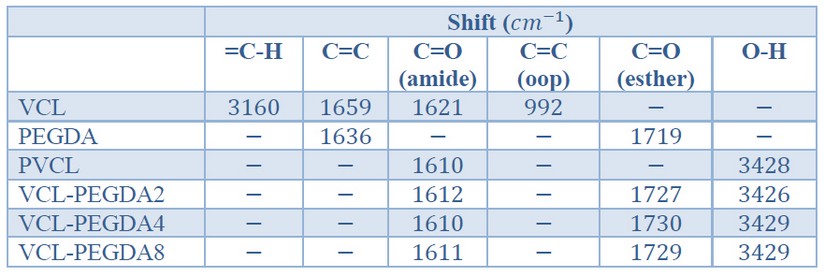
Table 2. ATR-FTIR Peak assignments for VCL, PEGDA, PVCL and for VCL-PEGDAx copolymers
ATR-FTIR Studies
The chemical structure of the poly (VCL-co-PEGDA) hydrogels was confirmed by ATR-FTIR spectroscopy. The characteristic peaks of VCL monomer, PEGDA crosslinker, and VCL-PEGDAx (x = % crosslinker amount) hydrogels are listed in Table 2.
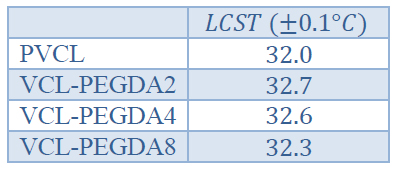
Table 3. Lower Critical Solution Temperature (LCST) values for PVCL and VCL-PEGDAx polymers
In the FTIR spectrum of the monomer VCL (Figure 3a), it can be observed the characteristic vinyl peaks of =C-H stretching at 3160 and C=C stretching at 1659 . The characteristic peak of carbonyl (C=O) stretching for an amide group is generally found at 1700-1640 . Cyclic amides (lactams) decrease the C=O frequency for increasing ring size. Indeed, in the monomer spectrum, C=O is shown at a lower frequency (1621 ) due to the 7-membered lactam
Figure 3b shows the characteristic peaks of the crosslinker PEGDA. In general, the C=O stretching band of an ester group appears at 1750-1735 . Conjugation of the carbonyl group with , unsaturations shifts the stretching C=O vibration by about 15 to 25 to lower frequencies, and C=C vibration to lower frequency, too. Thus, the C=O band of the ester groups is found at 1719 and C=C stretching band at 1636
The FTIR spectra of VCL based hydrogels are shown in Figure 3c. PVCL corresponds to N-vinyl caprolactam (VCL) homopolymer, while VCL-PEGDAx (x=2,4,8) corresponds to the copolymer of VCL and PEGDA with 2, 4, and 8 % of PEGDA.
The FTIR spectra of PVCL homopolymer and VCL-PEGDAx (x=2,4,8) polymers are shown in Figure 3c. The Four spectra showed the absence of C=C stretching (1659 ), =CH stretching (3100 ), and C=C out-of-plane bending (992 ) in comparison to the monomer VCL spectrum, indicating that polymerization occurred. The intense C=O stretching band of the VCL amide group is shown at ~1610 , and the C-N stretching at ~1477 in all spectra. Both peaks showed a displacement to lower frequencies compared to monomer VCL that might be due to the changes in the molecules' conformation and interaction of molecules upon polymerization. , The C=O (ester) stretching band at ~1730 , and its displacement to higher frequency indicates the absence of conjugation, and that it could be associated with the presence of crosslinking on VCL-PEGDA hydrogels.
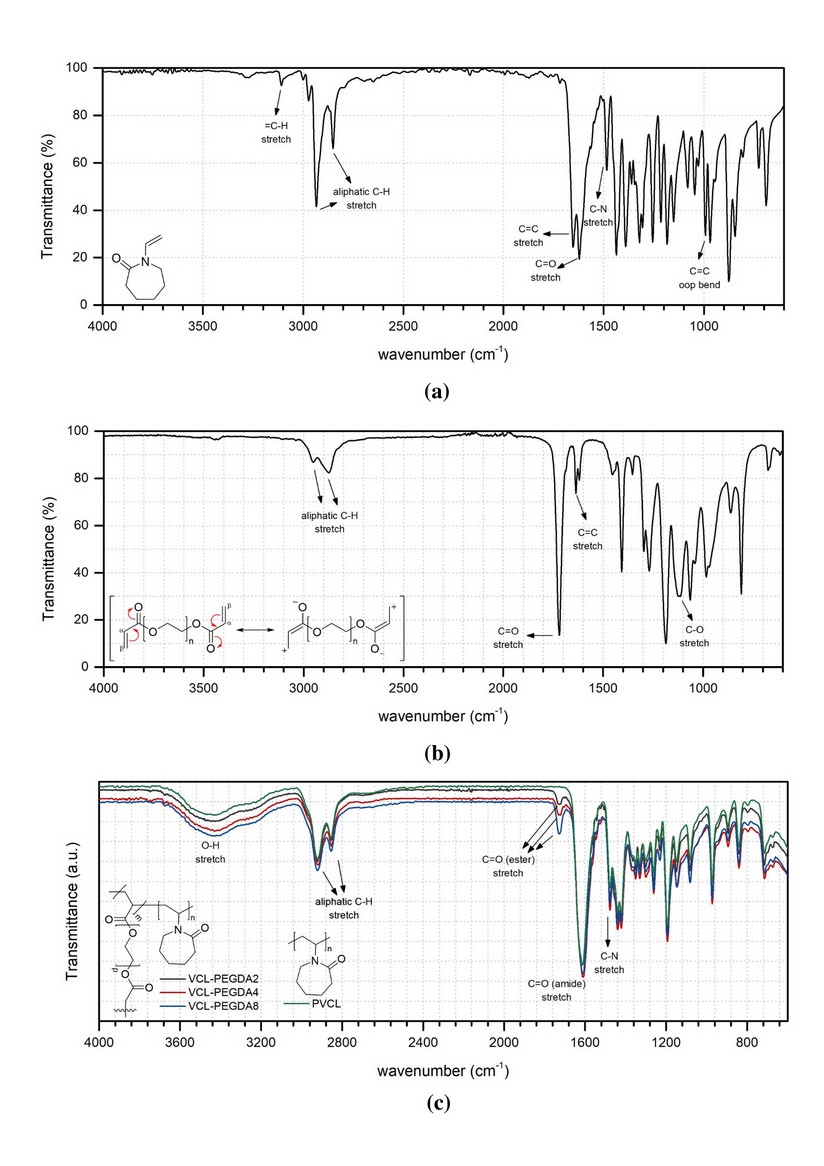
Figure 3. ATR-FTIR spectra of (a) VCL, (b) PEGDA, and (c) VCL-PEGDAx.
Thermo-responsiveness Studies
During the synthesis process, copolymers showed a phase transition from turbid to transparent solutions when cooling down. For this reason, turbidity measurements were carried out to estimate the cloud point temperatures (also called LCST) of hydrogels at 650 nm. Figure 4 shows the transmittance curves of PVCL and VCL-PEGDAx hydrogels, and Table 3 resumes the LCST values of hydrogels
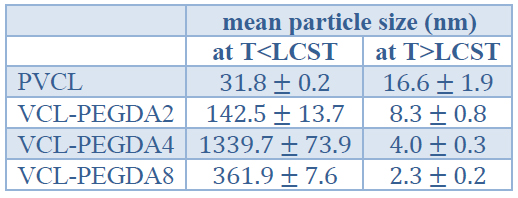
Table 4. Mean particle size of PVCL and VCL-PEGDAx polymers at T < LCST and at T > LCST.
According to the literature, the LCST can be defined as the temperature at which the polymer solution becomes turbid, so it has a transmittance closer to 0%. For poly(N-vinyl caprolactam) (PVCL), the LCST value is reported between and is slightly affected by the concentration. On the other hand, polyethylene glycol (PEG) derivatives present LCST values around in water. However, it can be said that the use of PEGDA, a derivative of PEG, as crosslinker influences LCST values, 27,28 as observed in the present work.
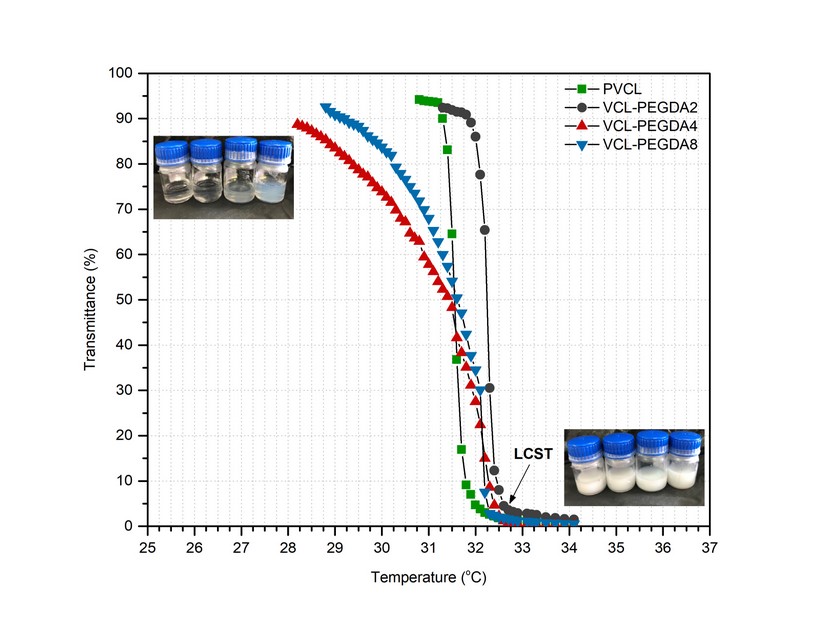
Figure 4. Transmittance curves as a function of temperature for VCL-PEGDAx.
Thus, PVCL showed a sharp phase transition at LCST value, which is in good agreement with the literature.12 VCL-PEGDA2 showed a similar phase transition behavior but slightly higher LCST value at, while VCL-PEGDA4 and VCL-PEGDA8 showed a continuous phase transition over a broad temperature range from 29 to 32 °C and the LCST values, according to the 0% transmission criteria are and , respectively
Particle Size Studies
The thermal-responsiveness studies demonstrated that PVCL and VCL-PEGDAx hydrogels show a phase transition LCST when the aqueous solution's temperature is around 32 °C. Thus, above LCST the polymer collapses going out of phase and this has been attributed to polymer-polymer hydrophobic interactions overcoming hydrogen bonding in the polymer-solvent interactions. On the other side, below LCST, VCL-based hydrogels swell, and this can be attributed to hydrogen bonding governing the polymer-solvent interactions 6,29. Sample VCL-PEGA4 presents a considerable size, which might be associated with aggregation phenomena.
Table 4 shows the mean particle size of PVCL and VCL-PEGDAx polymers at temperatures below and over LCST. As shown below, LCST the gels showed random values of average particle size concerning crosslinker concentration; however, these values are much higher than particle size values above LCST. In general, a decrease in particle size for PVCL and VCL-PEGDAx hydrogels is observed when the temperature rises above the previously determined LCST, which supports the phase transition from swollen to a collapsed state. The mean particle size above LCST does show some differences within the same order of magnitude, and the observed trend is a decrease in mean particle size when the PEGDA crosslinker amount is increased. It has been reported that PEGDA acts as a polymer surfactant stabilizer 22 and causes that polymer-polymer interactions overcome polymer-solvent interactions. Therefore, the presence of PEGDA, due to this stabilization effect, might contribute to the decrease in the mean particle size of VCL-PEGDA copolymers.
Uptake and Release Studies
Colchicine loading and encapsulation efficiency of PVCL and VCL-PEGDAx hydrogels are shown in Table 5. As can be observed, the drug loading did not change meaningfully by the crosslinker addition, ranging from (1.1 ± 0.2) % to (1.7 ± 0.2) %. On the other hand, the encapsulation efficiency tends to decrease when the crosslinked amount increases.
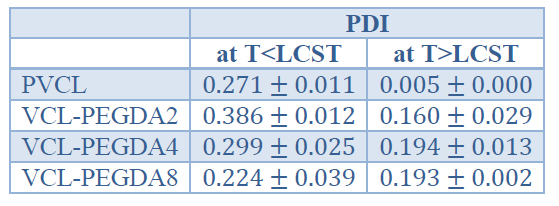
Table 5. Drug Loading (% DL) and Encapsulation Efficiency (% EE) of VCL-PEGDAx hydrogels.
The drug release profiles for the colchicine-loaded PVCL and VCL-PEGDAx hydrogels are shown in Figure 5. In general, a sustained release was obtained for all VCL-PEGDA hydrogels used in this study. They showed a gentle slope at the beginning, followed by a plateau whose appearance and maximum value depends on the hydrogel composition
Thus, the maximum % of cumulative release appears to be a function or crosslinker content in the hydrogel and follows this order: VCL-PEGDA8 > VCL-PEGDA4 > VCL-PEGDA2, corresponding to PEGDA crosslinker content of 8, 4, and 2 wt. %, respectively. As previously commented, this trend can be associated with more collapse polymer and smaller gel average particle size with higher polymer PEGDA crosslinker content. This is in agreement with the polymeric surfactant stabilization effect previously mentioned 22. PVCL as a homopolymer with a different chemical structure does not show a large particle size contraction, however it has a good drug release comparable to gel VCL-PEGDA4 and above gel VCL-PEGDA2.
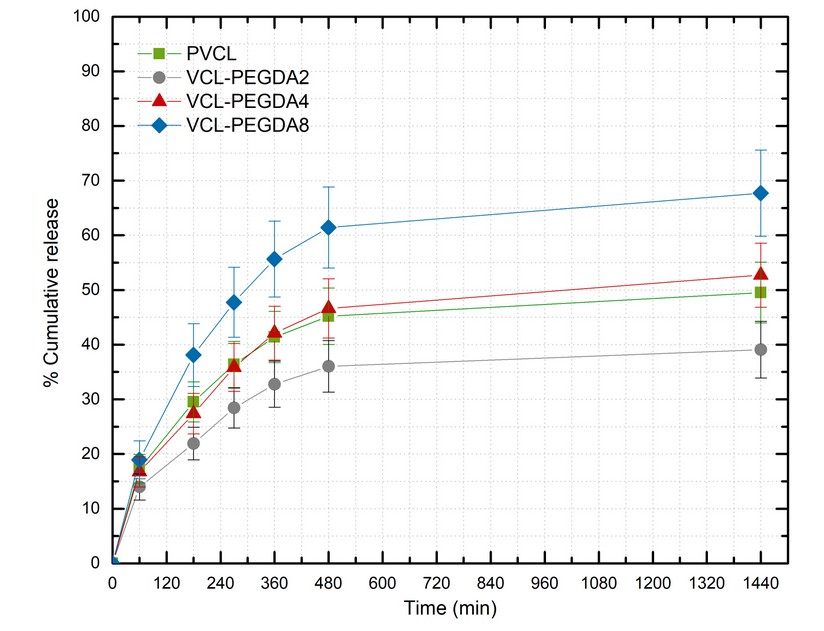
Figure 5. Cumulative release (%) of colchicine at T = 38 °C.
It has been stated that smart hydrogels are recommended in the following situations: (a) a sustained constant concentration of the drug in the body is desired, (b) the bio-active compound has a very short half-time, (c) the drug has strong side-effects or stability problems, (d) it is necessary to achieve better patient compliance, and (e) the drug has to be taken in frequent dosage.30 The design of the hydrogels prepared in this study fulfills the requirement of a sustained release of the colchicine as a drug with a low therapeutic index.24 Similar drug release profiles have been observed for other PVCL-based hydrogels.9,17,29,31
CONCLUSIONS
Four thermo-responsive polymeric hydrogels were synthesized through emulsion homopolymerization of N-vinyl caprolactam and copolymerization of VCL and PEGDA, the latter acting as the crosslinker. FTIR results confirmed the chemical structures of the above-mentioned polymeric hydrogels. The cloud point determination of all four VCL based hydrogels showed values around 32°C, related to their LCST, and confirmed their thermo-responsive behavior. As shown by the cloud point values, the phase transition is slightly affected by the crosslinker amount in the polymeric hydrogel. For PVCL and low crosslinked VCL-PEGDA2 a sharp phase transition is observed, while for the higher content of PEGDA, VCL-PEGDA4, and VCL-PEGDA8, continuous phase transition over a wide temperature range is observed.
A sustained release was observed for PVCL and VCL-PEGDAx hydrogels. The hydrogel containing the lower amount of PEGDA showed a better drug loading capacity, while the increment of PEGDA crosslinker contributes to a better drug release profile. The maximum % of cumulative colchicine release appears to be a function of crosslinker content in the hydrogel and follows the following order: VCL-PEGDA8 > VCL-PEGDA4 > VCL-PEGDA2. This trend can be associated with a more collapsed gel state and smaller mean particle size with higher PEGDA crosslinker content in the polymer. Similar drug release profiles have been observed for other PVCL based hydrogels reported in the literature.9,29,31,32
Acknowledgments
We thank the School of Chemical Sciences and Engineering at Yachay Tech University for providing laboratory facilities. Also, we acknowledge the helpful assistance of Paulina Romero and Salomé Galeas during DSL measurements at Escuela Politécnica Nacional de Quito. We are grateful to Floralba López, Hortensia Rodríguez, and Kamil Makowski for material provision and useful discussions, to Ruth Oropeza and Terencio Thibault for HPLC and UV-VIS measurements, respectively.
REFERENCES
1. Soni KS, Desale SS, Bronich TK. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J Control Release [Internet]. 2016;240:109–26. Available from:
2. Ahmed EM. Hydrogel: Preparation, characterization, and applications: A review. J Adv Res [Internet]. 2015;6(2):105–21. Available from: http://www.sciencedirect.com/science/article/pii/S2090123213000969
3. Jagur-Grodzinski J. Polymeric gels and hydrogels for biomedical and pharmaceutical applications. Polym Adv Technol. 2009;21:27–47.
4. Wack H. Method and Model for the Analysis of Gel-Blocking Effects during the Swelling of Polymeric Hydrogels. Ind Eng Chem Res - IND ENG CHEM RES. 2006;46.
5. Dušek K, Patterson D. Transition in swollen polymer networks induced by intramolecular condensation. J Polym Sci B Polym Phys. 1968 Jul;6(7):1209–16.
6. Teotia AK, Sami H, Kumar A. 1 - Thermo-responsive polymers: structure and design of smart materials. In: Zhang Z, editor. Switchable and Responsive Surfaces and Materials for Biomedical Applications [Internet]. Oxford: Woodhead Publishing; 2015. p. 3–43. Available from: http://www.sciencedirect.com/science/article/pii/B9780857097132000018
7. Alsuraifi A, Curtis A, Lamprou DA, Hoskins C. Stimuli Responsive Polymeric Systems for Cancer Therapy. Pharmaceutics [Internet]. 2018 Aug 22;10(3):136. Available from: https://www.ncbi.nlm.nih.gov/pubmed/30131473
8. Chatterjee S, Hui PC, Kan C, Wang W. Dual-responsive (pH/temperature) Pluronic F-127 hydrogel drug delivery system for textile-based transdermal therapy. Sci Rep [Internet]. 2019;9(1):11658.doi.org/10.1038/s41598-019-48254-6
9. Vihola H, Laukkanen A, Tenhu H, Hirvonen J. Drug release characteristics of physically crosslinked thermosensitive poly(N‐vinylcaprolactam) hydrogel particles. J Pharm Sci [Internet]. 2008;97(11):4783–93. Available from: http://www.sciencedirect.com/science/article/pii/S0022354916327782
10. Shin J, Braun P V, Lee W. Fast response photonic crystal pH sensor based on templated photo-polymerized hydrogel inverse opal. Sensors Actuators B Chem [Internet]. 2010;150(1):183–90. Available from: http://www.sciencedirect.com/science/article/pii/S0925400510005939
11. Mohammed M, Yusoh K, Shariffuddin J. Poly(N-vinyl caprolactam) thermoresponsive polymer in novel drug delivery systems: A review. Mater Express. 2018;8:21–34.
12. Cortez-Lemus NA, Licea-Claverie A. Poly(N-vinylcaprolactam), a comprehensive review on a thermoresponsive polymer becoming popular. Prog Polym Sci [Internet]. 2016;53:1–51. Available from: http://www.sciencedirect.com/science/article/pii/S007967001500091X
13. Vihola H, Laukkanen A, Valtola L, Tenhu H, Hirvonen J. Cytotoxicity of thermosensitive polymers poly(N-isopropylacrylamide), poly(N-vinylcaprolactam) and amphiphilically modified poly(N-vinylcaprolactam). Biomaterials [Internet]. 2005;26(16):3055–64. Available from: http://www.sciencedirect.com/science/article/pii/S0142961204008051
14. Dalton M, Halligan S, Killion J, Murray K, Geever L. Smart Thermosensitive Poly (N-vinylcaprolactam) Based Hydrogels for Biomedical Applications. Adv Environ Biol. 2014;8:1–6.
15. Sun S, Wu P. Infrared Spectroscopic Insight into Hydration Behavior of Poly(N-vinylcaprolactam) in Water. J Phys Chem B [Internet]. 2011 Oct 13;115(40):11609–18. Available from: https://doi.org/10.1021/jp2071056
16. Lau ACW, Wu C. Thermally Sensitive and Biocompatible Poly(N-vinylcaprolactam): Synthesis and Characterization of High Molar Mass Linear Chains. Macromolecules [Internet]. 1999 Feb 1;32(3):581–4. Available from: https://doi.org/10.1021/ma980850n
17. Rao KM, Mallikarjuna B, Rao KSVK, Siraj S, Rao KC, Subha MCS. Novel thermo/pH sensitive nanogels composed from poly(N-vinylcaprolactam) for controlled release of an anticancer drug. Colloids Surfaces B Biointerfaces [Internet]. 2013;102:891–7. Available from: http://www.sciencedirect.com/science/article/pii/S0927776512005218
18. Maudens P, Meyer S, Seemayer CA, Jordan O, Allémann E. Self-assembled thermoresponsive nanostructures of hyaluronic acid conjugates for osteoarthritis therapy. Nanoscale [Internet]. 2018;10(4):1845–54. Available from: http://dx.doi.org/10.1039/C7NR07614B
19. Panja S, Dey G, Bharti R, Kumari K, Maiti TK, Mandal M, et al. Tailor-Made Temperature-Sensitive Micelle for Targeted and On-Demand Release of Anticancer Drugs. ACS Appl Mater Interfaces [Internet]. 2016 May 18;8(19):12063–74. Available from: https://doi.org/10.1021/acsami.6b03820
20. Sudhakar K, Rao KM, Subha MCS, Rao KC, Sadiku ER. Temperature-responsive poly(N-vinylcaprolactam-co-hydroxyethyl methacrylate) nanogels for controlled release studies of curcumin. Des Monomers Polym [Internet]. 2015;18(8):705–13. Available from: https://doi.org/10.1080/15685551.2015.1070497
21. Son KH, Lee JW. Synthesis and Characterization of Poly(Ethylene Glycol) Based Thermo-Responsive Hydrogels for Cell Sheet Engineering. Materials (Basel) [Internet]. 2016;9(10). Available from: https://www.mdpi.com/1996-1944/9/10/854
22. Imaz A, Forcada J. N-vinylcaprolactam-based microgels: Effect of the concentration and type of crosslinker. J Polym Sci Part A Polym Chem [Internet]. 2008;46(8):2766–75. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/pola.22609
23. Imaz A, Forcada J. N-vinylcaprolactam-based microgels: Synthesis and characterization. J Polym Sci Part A Polym Chem [Internet]. 2008;46(7):2510–24. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/pola.22583
24. Niel E, Scherrmann J-M. Colchicine today. Jt Bone Spine [Internet]. 2006;73(6):672–8. Available from: http://www.sciencedirect.com/science/article/pii/S1297319X06001837
25. Gowda BG. High-performance liquid chromatographic determination of colchicine in pharmaceutical formulations and biological fluids. Int J Pharm Pharm Sci. 2014;6:335–7.
26. Gola A, Niżniowska A, Musiał W. The Influence of Initiator Concentration on Selected Properties on Poly-N-Vinylcaprolactam Nanoparticles. Nanomater (Basel, Switzerland) [Internet]. 2019 Nov 7;9(11):1577. Available from: https://www.ncbi.nlm.nih.gov/pubmed/31703338
27. Bordat A, Boissenot T, Nicolas J, Tsapis N. Thermoresponsive polymer nanocarriers for biomedical applications. Adv Drug Deliv Rev [Internet]. 2019;138:167–92. Available from: http://www.sciencedirect.com/science/article/pii/S0169409X18302539
28. Liu F, Kozlovskaya V, Kharlampieva E. Poly( N -vinylcaprolactam): From Polymer Synthesis to Smart Self-assemblies: Chemistry, Properties and Applications. In 2018. p. 93–120.
29. Wang Y, Nie J, Chang B, Sun Y, Yang W. Poly(vinylcaprolactam)-Based Biodegradable Multiresponsive Microgels for Drug Delivery. Biomacromolecules [Internet]. 2013 Sep 9;14(9):3034–46. Available from: https://doi.org/10.1021/bm401131w
30. Bajpai AK, Shukla SK, Bhanu S, Kankane S. Responsive polymers in controlled drug delivery. Prog Polym Sci [Internet]. 2008;33(11):1088–118. Available from: http://www.sciencedirect.com/science/article/pii/S0079670008000609
31. Madhusudana Rao K, Krishna Rao KSV, Sudhakar P, Chowdoji Rao K, Subha MCS. Synthesis and characterization of biodegradable poly (vinyl caprolactam) grafted on to sodium alginate and its microgels for controlled release studies of an anticancer drug. J Appl Pharm Sci. 2013;3(6):61–9.
32. Madhusudana Rao K, Mallikarjuna B, Krishna Rao KSV, Siraj S, Chowdoji Rao K, Subha MCS. Novel thermo/pH sensitive nanogels composed from poly(N-vinylcaprolactam) for controlled release of an anticancer drug. Colloids Surfaces B Biointerfaces [Internet]. 2013;102:891–7. Available from: http://dx.doi.org/10.1016/j.colsurfb.2012.09.009
Received: 8 November 2020
Accepted: 18 January 2021
J. Fernanda Romero1; Antonio Díaz-Barrios1,*; Gema González2,3
1School of Chemical Sciences and Engineering, Yachay Tech University, Urcuquí, Ecuador, 2School of Physical Sciences and Nanotechnology, Yachay Tech University, Urcuquí, Ecuador 3Centro de Ing. Materiales y Nanotecnología, Instituto Venezolano de Investigaciones Científica, Caracas, Venezuela
*Corresponding author: [email protected]
