2020.05.01.4
Files > Volume 5 > Vol 5 No 1 2020
INVESTIGATION / RESEARCH
The Impact of Temperature-Dependent Sex Determination on the Population Dynamics of Green Sea Turtles (Chelonia mydas)
Candy Herrera1, Evelyn Guerra2, Victoria Penalver3, Andrea Rosas4, Yingying Wei5, Jack Pringle6, Baltazar Espinoza6, Baojun Song7
Available from: http://dx.doi.org/10.21931/RB/2020.05.01.4
ABSTRACT
The sex of the turtles is determined by the incubation temperature of the eggs during the mid-trimester of development. In green sea turtles (Chelonia mydas), recent studies show that sex ratios are changing, producing a female-biased sex ratio within the population. We developed a novel continuous model to analyze the dynamics of the green sea turtle population long-term. We determine the safe operating space for the proportion of eggs that become male at which the population of green sea turtle can exist without going to extinction. When the proportion of male eggs leaves this range the overall turtles’ population collapses. Additionally, we examined how temperature changes affect the sex ratios of the green sea turtle population.
Keywords: Green sea turtle, Sex ratio, Temperature-dependent sex determination, Population dynamics
1. INTRODUCTION
In this paper, we examine the sex ratio of sea turtles in relation to population collapse. We are particularly concerned with the population of green sea turtles (Chelonia mydas), in which temperature-dependent sex determination has been observed. Temperature-dependent sex determination (TSD) is a process where the temperature of an embryo’s environment results in the production of sex hormones that dictate the embryo’s sex development. This is an environmental sex determination system (ESD), meaning that the sex is determined depending on factors derived from the physical and biotic environments like temperature1,2. Every reptile species that exhibit TSD has a thermosensitive period during which the embryo sex is developed. For turtles, this period has been observed to take place during the mid-trimester of the embryo incubation period3.
Within the realm of TSD, there are three different possible patterns that a species uses for sex determination: FM-pattern, MF-pattern, and FMF-pattern. In the FM-pattern, female eggs are developed in low hatching temperatures while male eggs occur in high hatching temperatures; the MF-pattern is the inverse of the FM-pattern. The FMF-pattern dictates that female eggs develop in high and low temperatures while a medium range of temperature results in male eggs. Turtle embryos follow the MF-pattern, so at lower temperatures, the result is a mostly male hatchling population, and at higher temperatures, a primarily female hatchling population exists4. The temperature at which the sex differentiates is around 29.4◦C2,5. When the mean temperature of the nest during the thermosensitive period is at 29.4 ◦C, known as pivotal temperature, then we see an even distribution of male and female hatchlings occur. Whereas, when the mean temperature is above the vital temperature, then the hatchling sex population will be mostly female, and below it, will result in a primarily male population2,5,6. In recent years a disproportionate ratio of female to male turtle eggs has been observed in several different studies and has also been predicted to possibly lead to the extinction of sea turtles in the future5,7,8.
Since female sea turtles lay their eggs in chambers that they dig into the sand of their nesting beaches, it is reasonable to look into the factors that could be directly affecting these sand temperatures to understand why there is an imbalance in the ratio of female to male turtles. To determine these factors, researchers have proposed multiple hypotheses. One such hypothesis credits climate change with potentially leading to rising sand temperatures. This comes from the fact that global air temperatures are projected to increase, and there is a strong relationship between air temperatures and sand temperatures9–11. Another possible explanation for potential increases in temperature is an accumulation of microplastic on and within the beach sediment12.
This problem naturally lends itself to investigation via a sex-structured population model. An overview of the history of sex-structured models was described by Iannelli et al. .13. The discussion of sex-structured models first arose after it was realized that the one-sex stable population theory was insufficient in answering questions about the existence and interrelations of the sexes. It was found that trying to apply a one-sex model for both genders at the same time resulted in contradictory results. Kuczynski14 first noted this inconsistency. These inconsistencies between male and female reproduction rates were further noted and explored by Karmel15, making him the first to observe many of the ideas of two-sex population modeling. Whilst Karmel15 introduced many of the two-sex population ideas, Kendall16 brought the first significant dynamic model. The work done by Kendall16 on two-sex populations more accurately accounted for the dynamics of observed systems leading to further research-based on his work13. Although Kendall’s work has inspired several further studies into sex-structured models, the majority of published population models are unstructured concerning sex17. Some of the papers that use sex-structured models are Lee et al. 18 and Mignatti et al. 19. In both articles, the sex-structure modeling framework was utilized because it was noticed that the sexual dimorphism was critical in understanding the dynamics of the population as a whole.
In this paper, we developed a system of ordinary differential equations (ODE’s) model for general green sea turtles focusing on the impacts of the TSD on the overall population dynamics to determine a male to female sex ratio that leads to extinction.
2. METHODS
2.1. Model Development
The life cycle of green sea turtles starts on land when they emerge from their nests as hatchlings and immediately travel to the sea. Upon entering the ocean, the hatchlings are relatively unseen until they reappear as juveniles in the open sea20. This period between the hatchling and juvenile stage is composed of several years and is the life-stage that remains relatively unknown20. Thus, scientists have coined the term “lost years” to describe this stage of life20,21. Since these two first stages of the life cycle of the turtles do not carry relevant information for the development of the model, a simplified life cycle can be seen in Figure (1), which shows the “egg” stage as each individual that has not yet reached sexual maturity and the adult stage as only the sexually mature adults.

Figure 1. Compartmental diagram of the simplified life cycle of green marine turtle (Chelonia mydas). The eggs with undefined sex will develop either in male or female, depending on the incubation temperature. Once hatchlings hatch, they will mature and will reach sexual maturity. After mating, new offspring are produced.
For green sea turtles, the mean age of sexual maturity is estimated to be within the range of 40-60 years22–24. When sea turtles reach sexual maturity, females and males return to their natal beaches to mate and nest. Male sea turtles usually breed every year or every two years, whereas female sea turtles generally breed every 2 to 5 years25,26. During mating season, sea turtles have multiple mates and lay several clutches of eggs at approximately 2-week intervals27. This scheme of the life cycle focuses mainly on the relationship between adults during the mating period. This interaction eventually will result in the production of eggs that can later develop into either female or male mature sea turtles. Also, it shows how the death of the adults and the movement out of the reproductive stage decreases the population of adult sea turtles, while the end of eggs and juveniles reduces the people of the “eggs” class.
On the other hand, the successful mating of adults will increase the number of eggs deposited every season. Each clutch can be divided into female and male eggs depending on the incubation temperatures7. If eggs reach maturity, this will increase the number of adults in the population.
To describe the long-term behavior of the green sea turtle population, we study the dynamics of the community with a continuous model. The main objective is to figure out the sex ratio that would ultimately lead the overall population of green sea turtles to extinction. We chose the continuous model to be able to witness the change within the turtle’s entire life cycle (60-70 years)24 and for the whole population over time. Modeling the distinctive aspects of the reproductive biology and nesting behavior of green sea turtles poses a significant challenge. The mating process is a complex interaction between male and female sea turtles that involves a variety of factors that could strongly affect the birth rate of the population. To clearly understand the principles involved in this interaction, we consider the mating process as a functional response, similar to continuous-time predator-prey models28.
Such as in predator-prey models, we can conveniently classify most of our variables involved in the mating process: (1) density of female population, (2) density of male population, (3) behavioral response of females during mating process, (4) characteristics of the males, e.g., searching efficiency29. To represent the successful number of mating interactions between males and females per unit time, which will eventually result in the production of eggs, we used the Holling type II functional response29,30. In predator-prey models, researchers suggest that ratio-dependence is suitable when the predator must seek its prey and consequently compete for food 31. In modeling the process of mating interactions between green sea turtles, we have to consider that male sea turtles have to search for females, and that male turtles will compete with one another to mate with a female32. Moreover, we have to consider some aspects, such as coupling time, associated with each female being found by a male and the fact that not every male will interact with every female. Thus, it made more sense to use a ratio-dependent system to produce more accurate results31.
In our model, we focus on the proportion of eggs allocated to males and females in the population, which is influenced by the temperature they are exposed to during the incubation period. Since the juvenile stage does not provide relevant information to the main objective of this investigation, the population is divided into two groups: eggs and adults, where “eggs” refers to the pre-reproductive life stages of the turtles. We denote the egg population and adult population for males and females as AM(t), AF(t), and EM(t) and EF(t) at time t.
2.2. Model Description

 .
.

Table 1. Parameters description and values.





4. RESULTS
The following simulations were developed by using parameter estimations, as seen in Table (1). All parameter values in Table (1) are kept constant except for values of p .

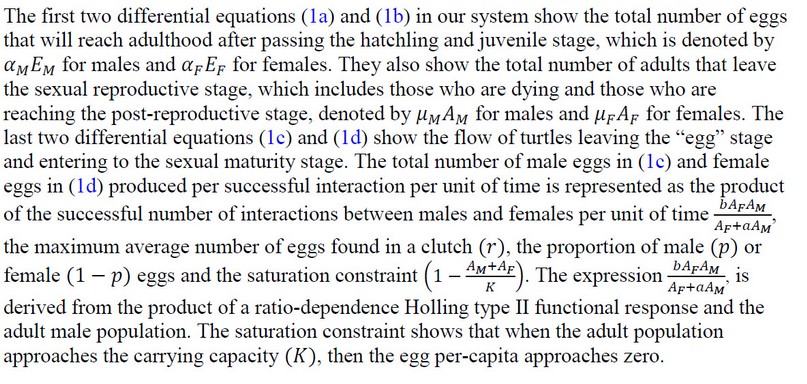
Figure 2. The population of individuals in the adult and egg stages over time. Variations in the population are due to changes in the proportion of male eggs (p) . When the equilibrium point (I*) is locally asymptotically stable; for any deviation of p , the population numbers change until they reach a steady-state. Because we are examining values of that are within our existence condition for the proportion of males, our population remains viable.

Figure 3. Sex ratios of individuals in adult (3(a)) and egg (3(b)) stages overtime when the interior equilibrium exists. Variation in sex ratios is due to changes in the proportion of male eggs (p) .
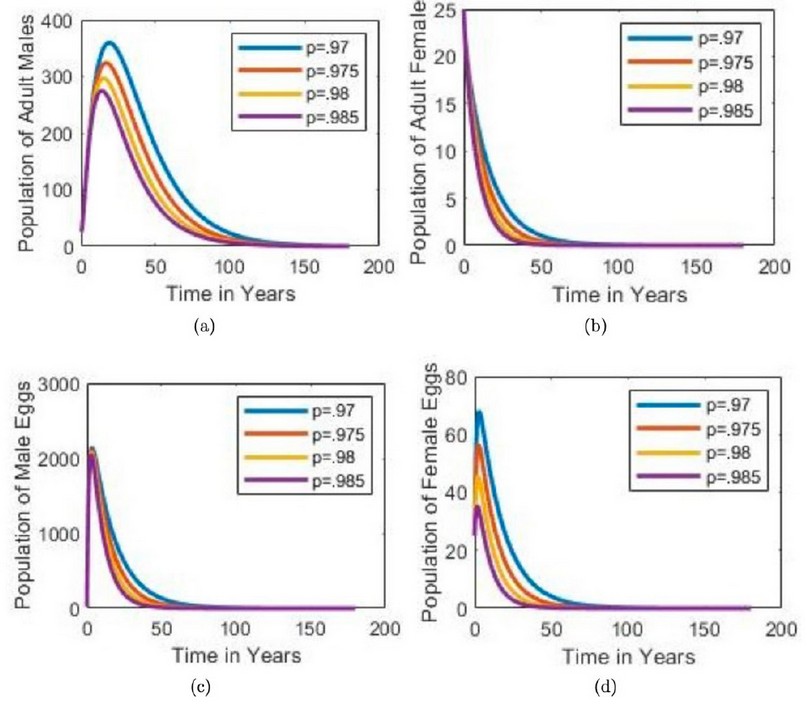
Figure 4. The population of individuals over time for variations of outside of the existence condition for p. When the existence condition is not satisfied, the interior equilibrium (I*) does not exist and system (1) only has an equilibrium point at (0.0.0.0.). For values outside of the existence condition, the adult and egg populations approach zero towards extinction.

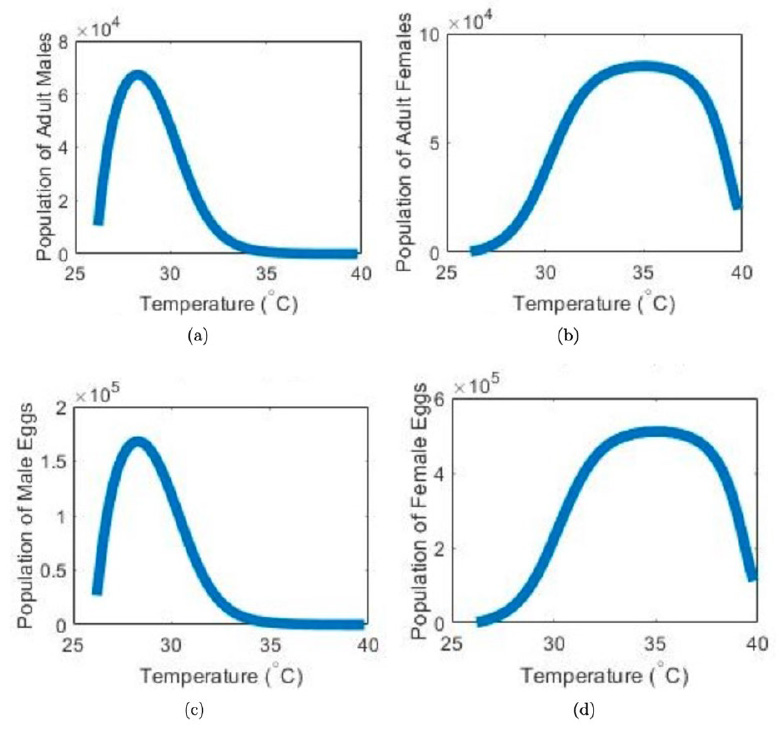
Figure 5. Population of adults and eggs as daily mean temperature increases. The changes in the population are due to variations in the proportion on male eggs caused by temperature p(T).
We only analyzed our system for values of p that satisfy the equilibrium existence condition. In other words, since is a function of T, we analyzed our system for temperatures that allowed p(T)to still be within the equilibrium condition (8). Since our system is now analyzed with the new p(T)function, the graphs in Figure (5) reflect how temperature changes lead to different proportions of males and how those proportions lead to different population numbers for the different stages.

Figure 6. Taking the ratio of (5(c)) and (5(d)), we obtain the changes in the male to the female sex ratio of eggs as daily mean temperature increases. The graph reflects that as temperatures increase, the sex ratio decreases because the proportion of male eggs becomes smaller than the proportion of female eggs.
5. DISCUSSION
According to the International Union for Conservation of Nature (UICN) Red List Threatened Species41, Green sea turtle is considered as an endangered species and its population is continuously decreasing. This population decline is due to many factors like illegal trade of eggs, turtle-shell trade, plastic, and other marine debris, ocean pollution, and global warming42–45. Most studies focus on how these factors harm the adult population and have determined that we should maintain current efforts to reduce mortality of long juvenile and adult stages to keep a sustainable population46. Our concern, in contrast, focuses on some aspects at the earliest stages of the life cycle that can harm the adult population long-term. Due to this, we studied how the temperature at the mid-trimester of the incubation period impacts the sex ratios of the population. Fluctuations in the incubation temperature can cause a female or male-biased sex ratios in the population. With this model, we analyzed how the sex ratio is decisive for the population growth and how this is affected by the temperature. We determined a safe operating space at which temperature can alter the sex ratios without taking the population to collapse. It has been shown that different populations of sea turtles have female-biased offspring production due to temperature increases during the incubation period47,48. Regardless of that, our results show that the proportion of female eggs can go to extreme values without having a severe impact on the turtle population. However, our numerical results depend completely on the values that our parameters take since many of these are not found in the literature; most of them had to be estimated affecting the accuracy of the results. To avoid this, more fieldwork is necessary to strengthen the parameter estimations.
A system of ordinary differential equations was used to represent the life cycle of the turtle population. The emphasis of our study is the mechanics of sex structure on population stability. A closed-form analytic condition for the temperature to ensure species persistence was found. The previous literature relating to this subject has been relatively sparse, but our results seem to be in general in accordance with prior publications49. At the moment, the lack of studies on the subject has left us without any current contrary findings.
Since we simplified our model to denote all pre-reproductive stages as the egg stage and the reproductive stage as the adult stage, we think that this might propose limitations on the details of each sub-stage in the life cycle, as well as including the post-reproductive stage. A dearth of information has been reported with regard to the searching efficiency and coupling time of green sea turtles. We believe that having inaccurate parameter estimations and a simplified model probably caused our condition on and our condition on to be so large, thus giving us a broad range where highly skewed proportions are viable. We also based our model in a very general sense, rather than focusing on a specific area such as a beach or ocean. Male sea turtles often never come back to land after they are born and females only come to lay their eggs, so much is unknown about green sea turtles, thus proposing further limitations for us to study population dynamics, this suggests uncertainties in our mathematical model. The well-mixed, homogeneous assumption has far more severe limitations based on our understanding of sea turtle biology than in other cases. Green sea turtles both male and female return, mate, and lay their eggs at their natal beaches. A discrete-time model of a single beach or a discrete-time model that takes into spatial dynamics would be far more appropriate. The limitations of our continuous-time approach cannot be stated until these other methods are explored.
Our model can be used as a basis for a more complex model involving details about each stage in the life cycle of males and females and determine which stage is the most critical for the survival of the population. It can also be used in future research to include the thermodynamics of microplastic in the sediment and see how it is directly affecting the sea turtle population sex-ratio. We believe that one can incorporate climate change into the model to show that many species and ecosystems are being affected by it. We also think it would be very useful to create a proportion of male eggs as a time-dependent function of temperature p(T(t)) to replace p(T). First, the random variation from mean daily temperatures in June and July would be the most appropriate complication added to the model. Secondly, it would be essential to include a general trend of climate change into the stochastic yearly variation as this is an observable trend. If we could find better information about the impact of microplastic proliferation on beaches on soil thermodynamics, then we could begin to include some heuristic to capture this effect. Lastly, a much more complicated model might be interested in studying how cyclical climate variations like El Niño on the long-term stability of turtle populations. It will be essential in the future to have broad trans-disciplinary collaborations to properly account for the edaphoclimatic factors that impact the turtles TSD. Lastly, it will be necessary for further models to include egg death induced by extreme temperatures49.
We found that there is a limited safe operating space of temperature for green sea turtle sustainability, as seen from Equation (8). If this condition is not met, then the total population approaches extinction. However, it is essential to note that this threshold is substantial, so the probability that the temperature would be outside of this range is unlikely. Based on our simulations in Figure (5), we found that the proportion of eggs that become male can decrease dramatically without a severe impact on turtle populations. When the balance of males p approaches zero, the population of females can still survive in high numbers. Highly skewed sex ratios do not necessarily mean that the population is no longer viable because males will breed with many females, suggesting that just a few males are sufficient to father multiple clutches50.
Moreover, male sea turtles breed twice as frequently as female sea turtles, meaning females can continue to find mates even when males are low in numbers49. However, when the male proportion reaches zero, then the whole population of Green sea turtles will go extinct. Our research indicates that it is necessary to urge policymakers and environmental managers about the importance of monitoring beach temperatures to ensure the continued existence of Green sea turtles. If temperatures get too high and exceed our threshold, then the population will go extinct, thus hurting the two ecosystems that they are a part of.
CONCLUSION
Expression (8) give us a safe operator space of temperature where green sea turtle population is sustainable. When temperature increases and exceeds the boundaries of this condition, then the overall population will decline until extinction. According to our simulations the probability that temperature reaches values outside the range is unlikely. A highly skewed population towards females do not necessarily mean that the population will be no longer viable because males are able to breed with many females, suggesting that just few males are sufficient to father multiple clutches50. However, these results could be due to an inexact parameter estimation, since the majority of these are combination of other parameters that are not easily found or are not present in the literature. Our research indicates that it is necessary more field work to obtain highly reliable parameters. Additionally, it is essential to impulse policy makers and environmental managers about the value of supervising beach temperatures to safeguard the existence of the green sea turtle population.
REFERENCES
1. Moeller, K. T. Temperature-Dependent Sex Determination in Reptiles. Embryo Proj. Encycl. (2013).
2. Matsumoto, Y. & Crews, D. Molecular mechanisms of temperature-dependent sex determination in the context of ecological developmental biology. Mol. Cell. Endocrinol. 354, 103–110 (2012).
3. Beckwith, V. K. & Fuentes, M. M. P. B. Microplastic at nesting grounds used by the northern Gulf of Mexico loggerhead recovery unit. Mar. Pollut. Bull. 131, 32–37 (2018).
4. Yamaguchi, S. & Iwasa, Y. Temperature-dependent sex determination, realized by hormonal dynamics with enzymatic reactions sensitive to ambient temperature. J. Theor. Biol. 453, 146–155 (2018).
5. ÖZD\.ILEK, Ş. Y., SÖNMEZ, B. & Kaska, Y. Sex ratio estimations of Chelonia mydas hatchlings at Samandağ Beach, Turkey. Turkish J. Zool. 40, 552–560 (2016).
6. Wright, L. I. et al. Turtle mating patterns buffer against disruptive effects of climate change. Proc. R. Soc. B Biol. Sci. 279, 2122–2127 (2012).
7. King, R., Cheng, W.-H., Tseng, C.-T., Chen, H. & Cheng, I.-J. Estimating the sex ratio of green sea turtles (Chelonia mydas) in Taiwan by the nest temperature and histological methods. J. Exp. Mar. Bio. Ecol. 445, 140–147 (2013).
8. Spotila, J. R., Standora, E. A., Morreale, S. J. & Ruiz, G. J. Temperature dependent sex determination in the green turtle (Chelonia mydas): effects on the sex ratio on a natural nesting beach. Herpetologica 74–81 (1987).
9. Fuentes, M., Limpus, C. J. & Hamann, M. Vulnerability of sea turtle nesting grounds to climate change. Glob. Chang. Biol. 17, 140–153 (2011).
10. Tomillo, P. S. et al. Global analysis of the effect of local climate on the hatchling output of leatherback turtles. Sci. Rep. 5, 16789 (2015).
11. Hays, G. C., Broderick, A. C., Glen, F. & Godley, B. J. Climate change and sea turtles: a 150-year reconstruction of incubation temperatures at a major marine turtle rookery. Glob. Chang. Biol. 9, 642–646 (2003).
12. Lusher, A. Microplastics in the marine environment: distribution, interactions and effects. in Marine anthropogenic litter 245–307 (Springer, Cham, 2015).
13. Iannelli, M., Martcheva, M. & Milner, F. A. Gender-structured population modeling: mathematical methods, numerics, and simulations. vol. 31 (Siam, 2005).
14. Kuczynski, R. R. Bankers’ profits from German loans. (The Brookings institution, 1932).
15. Karmel, P. H. The relations between male and female reproduction rates. Popul. Stud. (NY). 1, 249–274 (1947).
16. Kendall, D. G. Stochastic processes and population growth. J. R. Stat. Soc. Ser. B 11, 230–282 (1949).
17. Berec, L. Mate search and mate-finding Allee effect: on modeling mating in sex-structured population models. Theor. Ecol. 11, 225–244 (2018).
18. Lee, H.-H. et al. Sex-structured population dynamics of blue marlin Makaira nigricans in the Pacific Ocean. Fish. Sci. 80, 869–878 (2014).
19. Mignatti, A., Casagrandi, R., Provenzale, A., von Hardenberg, A. & Gatto, M. Sex-and age-structured models for Alpine ibex Capra ibex ibex population dynamics. Wildlife Biol. 18, 318–333 (2012).
20. Bolten, A. B. & Balazs, G. H. Biology of the early pelagic stage–the “lost year.” Biol. Conserv. Sea Turtles, Revis. Ed. Smithson. Inst. Press. Washington, DC 579, (1995).
21. Carr, A. Notes on the behavioral ecology of sea turtles. Biol. Conserv. sea turtles 19–23 (1982).
22. Gerosa, G., Aureggi, M. & others. Sea Turtle Handling Guidebook for Fishermen–Teaching Book. UNEP/MAP RAC/SPA, Tunis, Tunis. (2001).
23. Bjorndal, K. A. & Zug, G. R. Growth and age of sea turtles. Biol. Conserv. Sea Turtles 599–600 (1995).
24. NOAA, Green turtle, ://www.fisheries.noaa.gov/species/green-turtle
25. Limpus, C. J. The green turtle, Chelonia mydas, in Queensland: breeding males in the southern Great Barrier Reef. Wildl. Res. 20, 513–523 (1993).
26. Wibbels, T., Owens, D. W., Limpus, C. J., Reed, P. C. & Amoss Jr, M. S. Seasonal changes in serum gonadal steroids associated with migration, mating, and nesting in the loggerhead sea turtle (Caretta caretta). Gen. Comp. Endocrinol. 79, 154–164 (1990).
27. Hirth, H. F. & Samson, D. A. Nesting behavior of green turtles (Chelonia mydas) at Tortuguero, Costa Rica. Comportamiento de anidamiento de las tortugas verde (Chelonia mydas) en Tortuguero, Costa Rica. Caribb. J. Sci. 23, 374–379 (1987).
28. Arditi, R. & Ginzburg, L. R. Coupling in predator-prey dynamics: ratio-dependence. J. Theor. Biol. 139, 311–326 (1989).
29. Holling, C. S. The components of predation as revealed by a study of small-mammal predation of the European pine sawfly. Can. Entomol. 91, 293–320 (1959).
30. Holling, C. S. Some characteristics of simple types of predation and parasitism. Can. Entomol. 91, 385–398 (1959).
31. Hsu, S.-B., Hwang, T.-W. & Kuang, Y. Global analysis of the Michaelis–Menten-type ratio-dependent predator-prey system. J. Math. Biol. 42, 489–506 (2001).
32. Okuyama, J., Kagawa, S. & Arai, N. Random mate searching: male sea turtle targets juvenile for mating behavior. Chelonian Conserv. Biol. 13, 278–281 (2014).
33. Zug, G. R. & Glor, R. E. Estimates of age and growth in a population of green sea turtles (Chelonia mydas) from the Indian River lagoon system, Florida: a skeletochronological analysis. Can. J. Zool. 76, 1497–1506 (1998).
34. Limpus, C. J. Notes on growth rates of wild turtles. Mar. Turt. Newsl. 10, 8 (1979).
35. Fowler, L. E. Hatching success and nest predation in the green sea turtle, Chelonia mydas, at Tortuguero, Costa Rica. Ecology 60, 946–955 (1979).
36. Troëng, S. & Chaloupka, M. Variation in adult annual survival probability and remigration intervals of sea turtles. Mar. Biol. 151, 1721–1730 (2007).
37. Wood, J. R. & Wood, F. E. Reproductive biology of captive green sea turtles Chelonia mydas. Am. Zool. 20, 499–505 (1980).
38. Sánchez, Y. F., D\’\iaz-Fernández, R. & Fernández, R. D. Caracter\’\isticas de la anidación de la tortuga verde Chelonia mydas (Testudinata, Cheloniidae) en la playa Caleta de los Piojos, Cuba, a partir de marcaciones externas. Anim. Biodivers. Conserv. 30, 211–218 (2007).
39. Booth, J. & Peters, J. A. Behavioural studies on the green turtle (Chelonia mydas) in the sea. Anim. Behav. 20, 808–812 (1972).
40. Girondot, M. Statistical description of temperature-dependent sex determination using maximum likelihood. Evol. Ecol. Res. 1, 479–486 (1999).
41. Seminoff, J. A. Chelonia mydas. The IUCN Red List of Threatened Species. Southwest Fisheries Science Center, U.S. (2004).
42. Balazs, G. H. & Chaloupka, M. Thirty-year recovery trend in the once depleted Hawaiian green sea turtle stock. Biol. Conserv. 117, 491–498 (2004).
43. Fuentes, M. et al. Proxy indicators of sand temperature help project impacts of global warming on sea turtles in northern Australia. Endanger. Species Res. 9, 33–40 (2009).
44. Bugoni, L., Krause, L. & Petry, M. V. Marine debris and human impacts on sea turtles in southern Brazil. Mar. Pollut. Bull. 42, 1330–1334 (2001).
45. Schuyler, Q. A. et al. Risk analysis reveals global hotspots for marine debris ingestion by sea turtles. Glob. Chang. Biol. 22, 567–576 (2016).
46. Crouse, D. T., Crowder, L. B. & Caswell, H. A stage-based population model for loggerhead sea turtles and implications for conservation. Ecology 68, 1412–1423 (1987).
47. Mrosovsky, N. & Provancha, J. Sex ratio of loggerhead sea turtles hatching on a Florida beach. Can. J. Zool. 67, 2533–2539 (1989).
48. Broderick, A. C., Godley, B. J., Reece, S. & Downie, J. R. Incubation periods and sex ratios of green turtles: highly female biased hatchling production in the eastern Mediterranean. Mar. Ecol. Prog. Ser. 202, 273–281 (2000).
49. Hays, G. C., Mazaris, A. D., Schofield, G. & Laloë, J.-O. Population viability at extreme sex-ratio skews produced by temperature-dependent sex determination. Proc. R. Soc. B Biol. Sci. 284, 20162576 (2017).
50. Lee, P. L. M. & Hays, G. C. Polyandry in a marine turtle: females make the best of a bad job. Proc. Natl. Acad. Sci. 101, 6530–6535 (2004).
Received:20 December 2019
Accepted:20 January 2020
Candy Herrera1, Evelyn Guerra2, Victoria Penalver3, Andrea Rosas4, Yingying Wei5, Jack Pringle6, Baltazar Espinoza6, Baojun Song7
1Yachay Tech University, School of Biological Sciences and Engineering
2California State Polytechnic University, Pomona, College of Science
3University of Hawaii West Oahu, Department of Mathematics
4California State University, Fullerton, College of Natural Sciences and Mathematics
3University of Hawaii West Oahu, Department of Mathematics
4California State University, Fullerton, College of Natural Sciences and Mathematics
5University of Shanghai for Science and Technology, College of Science
6Arizona State University, School of Human Evolution and Social Change
7Montclair State University, College of Science and Mathematics
6Arizona State University, School of Human Evolution and Social Change
7Montclair State University, College of Science and Mathematics
