S2023.08.01.3
Files > Conference Series > 2023 > Sumer 1
Bionatura Conference Series Vol 8. No 1. 2023
1st International Scientific Conference on Pure and Medical Sciences at Sumer University. Iraq,
Previous / Index / Next
Estimation of some immunological markers for patients with Hepatitis C and B viruses with B- thalassemia major in Babylon province

1Babylon College of Science for Women/University of Babylon, Babylon, Iraq . [email protected].
2Babylon College of Science for Women/University of Babylon, Babylon, Iraq . [email protected].
*Correspondence: [email protected] Tel.: (+964 7801919715)
Available from:http://dx.doi.org/10.21931/RB/CSS/S2023.08.01.2
ABSTRACT
Hepatitis C , B is a serious public health problem worldwide. Thalassemia patients depend on blood transfusions throughout and are at high risk of viral infections. The aim of this study was estimate the prevalence of hepatitis C infection and different clinical parameters of (HLA) in the multiplex thalassemia population. In this study, 66 patients with infectious complications beta thalassemia were enrolled and samples were collected from the Genetic Hematology Center at Babel Women and Children Hospital in Babylon Governorate / Iraq infected with HCV , serum ferritin, PCV and splenectomy were analyzed. The overall prevalence of hepatitis B virus and HCV was 3% and 55%, respectively, 100% of patients over 10-20 years of age had hepatitis C infection, and some episodes were presented by different HLA molecules, by amolecule HLA from HLA class II (HLA-DR) and HLA cass I (HLA-G), In this study, we review the role of the molecule and several parameters along the HLA-G and HLA-DR gene in viral hepatitis, studies of hepatitis suggest that even Human leukocyte antigen contributes to the pathogenesis of the disease. HLA classification combined with the study the regulatory elements of genes may help in understanding the influence of genetic background on susceptibility to disease..
Keywords. immunological markers (HLA), hepatitis , thalassemia
INTRODUCTION
Thalassemia is an inherited blood disorder. Spread in the (Mediterranean region). The Middle East, Africa, and Southeast Asia people have the potential to carry thalassemia genes (Bhandari et al, 2018). Anemia of varying degrees is caused by a genetic defect, and it may be a mutation or a deletion. Beta thalassemia includes three main types, thalassemia minor which is often called (BTT) or carrier beta thalassemia, (BTI) and thalassemia minor (BTM) which is often called Mediterranean anemia., 1 . The main cause of iron overload is blood transfusions., 2. Patients with beta-thalassemia major usually have severe anemia requiring frequent blood transfusions compared to beta-thalassemia intermediate ., 3 .Hepatitis C virus (HCV) is responsible for more than 85% of transfusion-related cases of hepatitis. The chance of an infection developing into a chronic condition increases by more than 50%, resulting in hepatocellular carcinoma or cirrhosis of the liver by 20%. Almost all countries in the world More than 172 million people are expected to be infected worldwide. In the United States, the prevalence of hepatitis C virus (HCV) has established its own database of hepatitis C virus ., 4. End stage liver disease in many areas. Regular blood transfusions in patients with genetics A, especially thalassemia, enhanced the overall survival rate, however, it does include some risks of infection with blood-borne viruses especially viral hepatitis (B & C) ., 5,6 In addition, in connection with the observed iron overload in the liver, which is usually unavoidable in patients with usual frequent blood transfusions, hepatitis C virus infection has been shown to have a strong effect on hepatic fibrosis in thalassemia patients ., 7. HCV was first characterized by ., 8. The hepatitis C virus genome includes more than 70 subtypes and 6 major genotypes ., 9. The six major genotypes of hepatitis C virus differ significantly based on age, geographic region, and pathological characteristics ., 10 as well as biological responses to treatment ., 11,12 For example, the genotype (1-b) is associated with rapid progression of liver damage and a lower response to antiviral alpha ., 13,14 multiple human leukocyte antigen (HLA) loci (Kamatani) treatment. ., 15,16. have been shown to be associated with hepatitis, and none of these associations have been shown to be conclusive. The mechanism of susceptibility to chronic persistent infection with hepatitis C virus has not been well elucidated. Since the outcome of HCV infection mainly depends on the immune response of the host, and HLA, an integral part of the immune response, plays an important role in the immune reaction to HCV infection ., 17, the species differed and to a high degree the HLA gene was considered to be multiple Polymorphisms as a suitable biosusceptibility gene associated with the development and progression of chronic HBV and HCV infection. Indeed, previous studies have demonstrated that HLA-DR polymorphisms influence individual immune responses, thus affecting disease outcome especially hepatitis, and that many different HLA alleles play a role in hepatitis C & B infection ., 18 In this meta-analysis, the identification of common HLA-DR and HLA-G alleles was examined by a systematic review of the literature followed by ELISA for all case-control studies. ELISA is a powerful method for quantitatively summarizing the results of various studies. One advantage is that the statistical power of results in ethnic and ancestral groups is enhanced and sample sizes are expanded, which may reduce the possibility of producing a false positive or false negative association by random error . 19.
.
MATERIALS AND METHODS
Prepare all reagents, standard solutions and samples as instructed. Bring all reagents to room temperature before use. The assay is performed at room temperature. 2. Determine the number of strips required for the assay. Insert the strips in the frames for use. The unused strips should be stored at 2-8°C. 3. Add 50μl standard to standard well. Note: Don’t add biotinylated antibody to standard well because the standard solution contains biotinylated antibody. 4. Add 40μl sample to sample wells and then add 10μl anti-HLA-DR &HLA-G antibody to sample wells, then add 50μl streptavidin-HRP to sample wells and standard wells ( Not blank control well ). Mix well. Cover the plate with a sealer. Incubate 60 minutes at 37°C. 5. Remove the sealer and wash the plate 5 times with wash buffer. Soak wells with 300ul wash buffer for 30 seconds to 1 minute for each wash. For automated washing, aspirate or decant each well and wash 5 times with wash buffer. Blot the plate onto paper towels or other absorbent material. 6. Add 50μl substrate solution A to each well and then add 50μl substrate solution B to each well. Incubate plate covered with a new sealer for 10 minutes at 37°C in the dark. 7. Add 50μl Stop Solution to each well, the blue color will change into yellow immediately. 8. Determine the optical density (OD value) of each well immediately using a Microplate reader set to 450 nm within 10 minutes after adding the stop solutio
RESULTS
Serum patients results
HCV Ab positive thalassemia patients were divided into two groups:β- thalassemia patients with hepatitis by gender in male (26:5.9 ± 3.2) ,in female (24:12.15 ± 15.4) and Positive Control( Thalassimic ) also higher (5.9 ± 3.2) than the Control (healthy)(4.0 ± 1.7) be low in P value 0.007. and also by age and vaccinated in control healthy (4.0 ± 1.7) is lower than the patients in result of age 1 - 9 Years (4. ± 0.6) , 10 - 19 Years (12.4. ± 19.6) , 20 - 29 Years (7. 8± 5.0) in P value 0.87and in vaccinated ranging (7. 5± 4.7) when in the non-vaccinated (9. 8± 14.5) This shows the importance of the vaccine in the immunity represented by HLA . however statistically in this result show that no significant difference at P. Value >0.05 as in the table (2.1(. showed a significant decrease in thalassemia patients and thalassemia patients with hepatitis ,the results Hb in patients (6.94 ± 1. 13) , Positive Control (6.63 ± 1.28) lower than the Control (healthy) (11.9 ± 2.23). the same is true for PCV in patients (21.63 ± 5.38) and in Control (healthy) (35.53 ± 5.5).. The text continues here.. to the serum patients results to(HLA-G), HCV Ab positive thalassemia patients were divided into two groups:β- thalassemia patients with hepatitis by gender in male (723.2 ± 571.2) ,in female (24:907.15 ± 706.4) and Positive Control( Thalassimic ) also higher (22081.9 ± 95291.2) than the Control (healthy)(561.0 ± 394.7) be low in P value 0.007. and also by age and vaccinated in control healthy (561.0 ± 394.7) is lower than the patients in result of age 1 - 9 Years (483. ± 81.6) , 10 - 19 Years (888.4. ± 697.6) , 20 - 29 Years (840. 8± 670.0) in P value 0.87and in vaccinated ranging (647. 5± 591.7) when in the non-vaccinated (956. 8± 679.5) This shows the importance of the vaccine in the immunity represented by HLA . however statistically in this result.
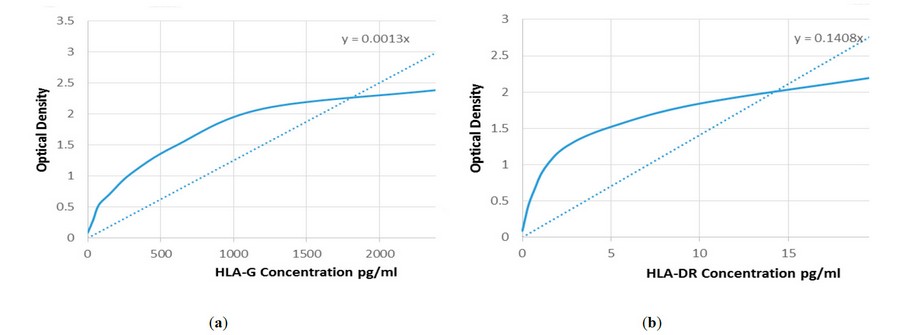
Figure 1. Relationship of HLA-G and HLA-DR with ferritin for β- thalassemia Patients with hepatitis, shows a positiveRelationship, Relationship of HLA-G and HLA-DR with Hb for β- thalassemia Patients with hepatitis. The Hb results for patients hepatitiswere low compared to the control, and this is an opposite result when compared with the increase in the expression of the HLA-DR,G level,. Relationship of HLA-G and HLA-DR with pcv for β- thalassemia Patients with hepatitis The PCV results of hepatitis patients were low compared to the control, and this is counterproductive if compared with the increased expression of the HLA DR, G level
Figure 2. Curve the average of optical density of standard (450 nm) with theHLA-G concentrion U/ml: (a) Curve the average of optical density of standard (450 nm) with the HLA-DR concentrion U/ml
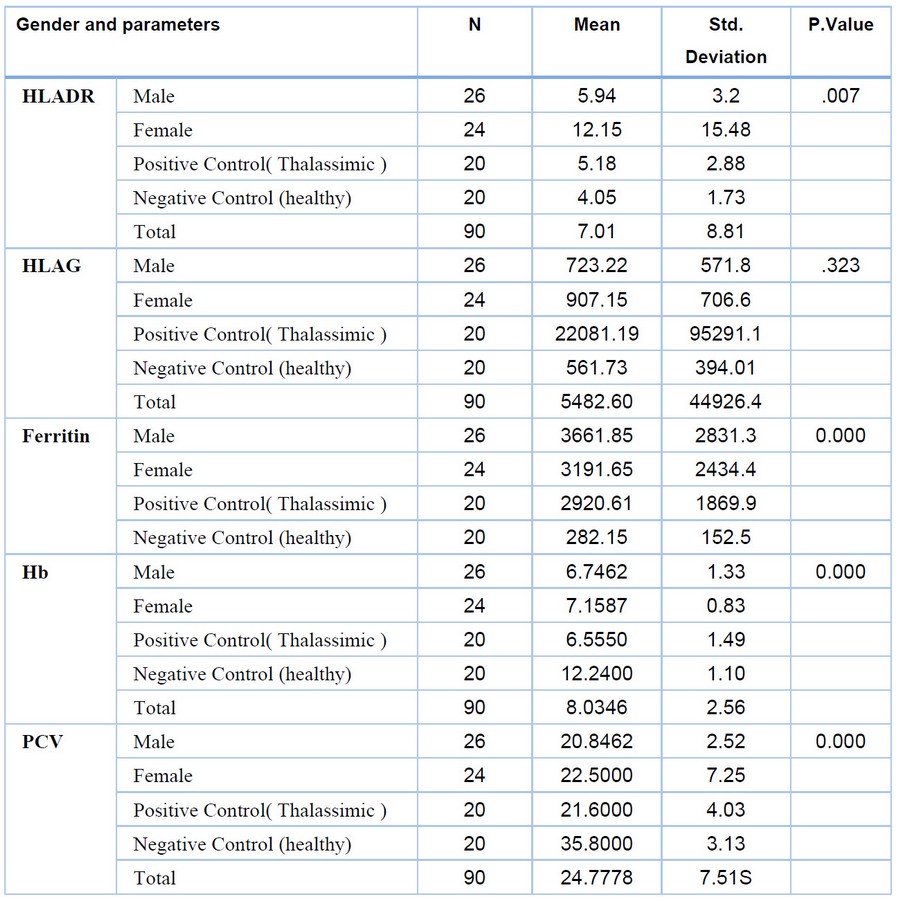
Table 1. Result of Relationship gender and many parameters of B-thalassemia patients with hepatitis and control.
DISCUSSION
In this study, the regulation of HLA-DR antigen in serum HCV was investigated in thalassemia patients infected with hepatitis C virus and has been widely used for viral infection studies . The results showed that HCV Ab expression from a construct increased or higher HLA-DR in patients. The presence of HCV leads to the down-regulation of MHC class II antigen presentation at the cell surface. The results provided further evidence that HLA-DR exposed to HCV had increased expression, and that acute hepatitis infection significantly impaired its ability to present antigen at the surface. The observations are consistent with previous reports that MHC class II patients with chronic HCV do not respond to maturation stimuli antigens and maintain an immature phenotype . Together, these results suggest that the negative effects of hepatitis C virus on APC function can lead to decreased immunity in vivo. It has been previously reported that MHC maturational defects result from chronic hepatitis C infection with thalassemia ., 20,21,22, , On the other hand, hepatitis C (HCV) has been shown to infect not only hepatocytes but also liver tissue, and this would explain how patients with chronic hepatitis C show selective deficits in anti-HCV immunity while maintaining a normal immune response. For unrelated infections. Antigens: Cellular immune responses are essential for HCV clearance . Failure to download a robust and extensive T-cell repertoire response leads to persistent HCV infection. It has been suggested that hepatitis C compromises cellular immunity by raise or increases MHC II in stimulated patients, which in turn leads to HLA-DR activation ., 23. Serum ferritin in this study was significantly increased in thalassemia and in thalassemia patients with hepatitis than in those with control. Iron excess or iron overload, there will be an excess of iron ferritin complex ., 24, Frequent blood transfusions and this may be the most important cause of elevated ferritin in the blood. These results indicate iron overload in the patient group because serum ferritin is used to indicate iron overload diseases as plasma ferritin is considered the best single indicator of total body iron ., 25The hematological characteristics of the study groups appeared, as all CBC and PCV variables as well as the indicators evaluated in this study indicated a significant difference between the control group and thalassemia patients with hepatitis. Presentations of severe anemia have been identified in patients with beta thalassemia. Low levels of (erythrocytes, hemoglobin, leukocytes, PCV) were significantly decreased in thalassemia patients because beta thalassemia is an hereditary hemoglobin synthesis disorder in severe anemia ., 26. This result was similar to other research ., 27. There was a decrease in the patients' hemoglobin levels compared to the level recorded in the controls since the patients and controls examined in this study share the same genetic background as well as healthy contris. In this study it was suggested that a significant increase in HLA-G expression in plasma may play a role in non-response to combined therapy, chronic infection and cirrhosis. Increased expression of hepatocytes HLA-G in HCV-infected liver samples has been associated with milder stages of fibrosis and hemosiderin deposits ., 28Besides hepatocytes, HLA-G expression has been observed on mast cells located in areas of cirrhosis ., 28. Increased plasma HLA-G levels in chronic HCV infection have been associated with genetic alterations in thalassemia patients and increased ferritin levels ., 29. , infiltrating cells may play an important role in maintaining chronic infection and induction of chronic complications. One study associated increased HLA-G expression in hepatocytes with HBV and HCV viral load ., 30. Various studies have associated increased levels of sHLA-G in blood plasma with hepatitis virus infection ., 30, 31, ., which was associated with an increased percentage of CD4 regulatory and HLA-G monocytes in patients showing They include acute or chronic hepatitis ., 31, active hepatitis B and C virus infection 31and HBeAg-negative hepatitis, and hepatocellular carcinoma. The results suggest that HLA-DR may play a protective role in HCV infection. Among normal hepatitis C patients. The HLA-protective susceptibility found in this study may be under the influence of a specific genetic disorder and immune abnormality of thalassemia patients. The possible mechanism by which this immunogenicity may enhance the effect of some HLA molecules in the context of thalassemia, may be higher or lower than certain HLA alleles. In some diseases, the role of the heritable characteristics or the immune profile of patients is in the particular regulation of genes linked or independently diverse to the HLA-class II region either to increase the expression of certain HLA class II and class l alleles. mentioned. Further investigations are needed to study the pathways through which these alleles contribute to hepatitis C clearance or persistence and the role of other immune-regulating genes that act synergistically with them. Identification of these factors may be important for the development of vaccines and treatment strategies, as well as elucidating the observed immune.
CONCLUSIONS
This study was conducted on the serum of thalassemia patients with hepatitis, and it was measured(HLA-DR and HLA-G) by ELISA technique, and it showed no significant differences and effects HLA type on patients, especially in females.
REFERENCES
1. Bhandari, R., Chand, S., & Lal, V. (2018). BETA THALESSEMIA MAJOR; RARE HAEMATOLOGICAL DISORDER.
2. Demosthenous, C., Vlachaki, E., Apostolou, C., Eleftheriou, P., Kotsiafti, A., Vetsiou, E., ... & Sarafidis, P. (2019) Beta-thalassemia: renal complications and mechanisms: a narrative review. Hematology, 24(1), 426-438....
3. Papanikolaou, G., Tzilianos, M., Christakis, J. I., Bogdanos, D., Tsimirika, K., MacFarlane, J., ... & Nemeth, E. (2005). Hepcidin in iron overload disorders. Blood, 105(10), 4103-4105
4. Bajwa, H., & Basit, H. (2019). Thalassemia.
5. Gupta, E., Bajpai, M., & Choudhary, A. (2014). Hepatitis C virus: Screening, diagnosis, and interpretation of laboratory assays. Asian journal of transfusion science, 8(1), 19,
6. Alberti, A., & Benvegnu, L. (2003). Management of hepatitis C. Journal of hepatology, 38, 104-118
7. Alavian, S. M., Adibi, P., & Zali, M. R. (2005). Hepatitis C virus in Iran: Epidemiology of an emerging infection
8. Choo, Q. L., Kuo, G., Weiner, A. J., Overby, L. R., Bradley, D. W., & Houghton, M. (1989). Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science, 244(4902), 359-362
9. Lindenbach, B. D., & Rice, C. M. (2013). The ins and outs of hepatitis C virus entry and assembly. Nature Reviews Microbiology, 11(10), 688-700
10. Chitturi, S., Weltman, M., Farrell, G. C., McDonald, D., Liddle, C., Samarasinghe, D., ... & George, J. (2002). HFE mutations, hepatic iron, and fibrosis: ethnic‐specific association of NASH with C282Y but not with fibrotic severity. Hepatology, 36(1), 142-149.
11. Chayama, K., Tsubota, A., Kobayashi, M., Okamoto, K., Hashimoto, M., Miyano, Y., ... & Kumada, H. (1997). Pretreatment virus load and multiple amino acid substitutions in the interferon sensitivity–determining region predict the outcome of interferon treatment in patients with chronic genotype 1b hepatitis C virus infection. Hepatology, 25(3), 745-749.
12. Keyvani, H., Fazlalipour, M., Monavari, S. H. R., & Mollaie, H. R. (2012). Hepatitis C virus-proteins, diagnosis, treatment and new approaches for vaccine development. Asian Pacific Journal of Cancer Prevention, 13(12), 5917-5935
13. Kew, M. C., Yu, M. C., Kedda, M. A., Coppin, A. L. I. S. O. N., Sarkin, A. N. D. R. E. W., & Hodkinson, J. O. H. N(1997). The relative roles of hepatitis B and C viruses in the etiology of hepatocellular carcinoma in southern African blacks. Gastroenterology, 112(1), 184-187.
14. Guo, X., Zhang, Y., Li, J., Ma, J., Wei, Z., Tan, W., & O'Brien, S. J. (2011). Strong influence of human leukocyte antigen (HLA)‐DP gene variants on development of persistent chronic hepatitis B virus carriers in the Han Chinese population. Hepatology, 53(2), 422-428.
15. Godkin, A., Davenport, M., & Hill, A. V. (2005). Molecular analysis of HLA class II associations with hepatitis B virus clearance and vaccine nonresponsiveness Hepatology, 41(6), 1383-1390.
16. Singh, R., Kaul, R., Kaul, A., & Khan, K. (2007). A comparative review of HLA associations with hepatitis B and C viral infections across global populations World journal of gastroenterology: WJG, 13(12), 1770
17. Blettner, M., Sauerbrei, W., Schlehofer, B., Scheuchenpflug, T., & Friedenreich, C. (1999). Traditional reviews, meta-analyses and pooled analyses in epidemiology. International journal of epidemiology, 28(1), 1-9.
18. Mao, C., D. Davies, I. M. Kerr, and G. R. Stark. 1993. Mutant human cells defective in induction of major histocompatibility complex class II genes by interferon gamma. Proc. Natl. Acad. Sci. USA 90:2880–2884
19. Reith, W., LeibundGut-Landmann, S., & Waldburger, J. M. (2005). Regulation of MHC class II gene expression by the class II transactivator. Nature Reviews Immunology, 5(10), 793-806
20. Brady, M. T., A. J. MacDonald, A. G. Rowan, and K. H. Mills. 2003.Hepatitis C virus non-structural protein 4 suppresses Th1 responses by stimulating IL-10 production from monocytes. Eur. J. Immunol. 33:3448–3457
21. Ayed K, Ayed-Jendoubi S, Sfar I, Labonne MP, and Gebuhrer L: HLA class I and class II phenotypic gene andhaplotypic frequencies in Tunisians by using molecular typing data. Tissue Antigens 2004;64:520–532
22. Al-Hakeim, H. K. A. H. and Al-Hakany, M. F. M. (2013) ‗The Effect of Iron Overload on the Function of Some Endocrine Glands in β-Thalassemia Major Patients.‘, Al-Kufa University Journal for Biology. University of Kufa, 5(2), pp.104–123
23. Kalender, B. et al. (2002) ‗The effects of acute phase proteins on serum albumin, transferrin and haemoglobin in haemodialysis patients.‘, International journal of clinical practice, 56(7), pp. 505–508
24. Nienhuis, A. W. and Nathan, D. G. (2012) ‗Pathophysiology and clinical manifestations of the β-thalassemias‘, Cold Spring Harbor perspectives in medicine. Cold Spring Harbor Laboratory Press, 2(12), p. a011726
25. Auffermann-Gretzinger, S., E. B. Keeffe, and S. Levy. 2001. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C.virus infection. Blood 97:3171–3176
26. Weng PJ, Fu YM, Ding SX, Xu DP, Lin A, Yan WH. Elevation of plasma soluble human leukocyte antigen-G in patients with chronic hepatitis C virus infection. Hum Immunol (2011) 72(5):406–11. doi:10.1016/j.humimm.2011.02.008
27. Amiot L, Vu N, Rauch M, L’Helgoualc’h A, Chalmel F, Gascan H, et al. Expression of HLA-G by mast cells is associated with hepatitis C virus-induced liver fibrosis. J Hepatol (2014) 60(2):245–52. doi:10.1016/j.jhep.2013.09.006
28. Souto FJ, Crispim JC, Ferreira SC, da Silva AS, Bassi CL, Soares CP, et al. LiverHLA-G expression is associated with multiple clinical and histopathologicalforms of chronic hepatitis B virus infection. J Viral Hepat (2011) 18(2):102–5.doi:10.1111/j.1365-2893.2010.01286.x
29. Shi WW, Lin A, Xu DP, Bao WG, Zhang JG, Chen SY, et al. Plasma soluble human leukocyte antigen-G expression is a potential clinical biomarker inpatients with hepatitis B virus infection.HumImmunol (2011) 72(11):1068–73.doi:10.1016/j.humimm.2011.06.012
30. Han Q, Li N, Zhu Q, Li Z, Zhang G, Chen J, et al. Association of serum soluble human leukocyte antigen-G levels with chronic hepatitis B virus infection. Clin Exp Med (2014) 14(1):35–43. doi:10.1007/s10238-012-0214-5
31. Park, Y., Park, Y., Lim, H. S., Kim, Y. S., Hong, D. J., & Kim, H. S. (2012). Soluble human leukocyte antigen‐G expression in hepatitis B virus infection and hepatocellular carcinoma. Tissue antigens, 79(2), 97-103
Received: 26 September 2022 / Accepted: 15 October 2022 / Published:15 February 2023
Citation: Alwan E; Almamory A; Naeem, A. Estimation of some immunological markers for patients with hepatitis C and B viruses with B- thalassemia major in Babylon province. Revis Bionatura 2023;8 (1) 2. http://dx.doi.org/10.21931/RB/CSS/S2023.08.01.2