2021.06.01.19
Files > Volume 6 > Vol 6 No 1 2021
Pathogenicity of Moniliophthora roreri isolates from selected morphology groups in harvested cacao pods and in vitro sensitivity to compost tea.
María Gabriela Maridueña-Zavala1*, Maria Isabel Jimenez Feijoo2, Juan Manuel Cevallos-Cevallos1,2.
Available from: http://dx.doi.org/10.21931/RB/2021.06.01.19
ABSTRACT
Moniliopthora roreri is the frosty pod rot disease (FPD) and one of the most devastating cacao pathogens worldwide. However, M. roreri pathogenicity on harvested cacao pods and sensitivity to compost tea have not been fully described. Monosporic cultures of M. roreri from different morphology groups were obtained. The isolates’ pathogenicity was tested by inoculation onto harvested cacao pods, and symptoms were evaluated at 3-day intervals during 16 days before estimating the area under the disease progress curve (AUDPC). The sensitivity of M. roreri to compost tea was evaluated on potato dextrose agar (PDA) amended with 1 to 5 % compost tea. All morphology groups could infect harvested cacao pods during the 16 days with a disease severity index abode 75 %. Compost tea completely inhibited the growth of M. roreri when used at 4.5 % or higher. Results suggest a shortened biotrophic phase during the infection in harvested pods and a medium to high sensitivity of M. roreri to compost tea.
Keywords: Moniliasis, cacao national, biol, biotrophic, necrotrophic
INTRODUCTION
Cacao (Theobroma cacao) trees are usually categorized as “fine flavor” or “bulk/ordinary” depending on the aroma released by the fermented beans 1. Ecuador is the top delicate flavor cacao producer worldwide 2 with extensive production areas for the ‘Nacional’ and ‘CCN51’ types of cacaos. The Nacional cacao is preferred by consumers 3 but is more susceptible to the frosty pod rot disease (FPD) than CCN51 4. The FPD has caused significant losses in the cacao production of Latin American countries 1, and the production of Nacional cacao is partially being replaced by CCN51 in Ecuador 4 mostly because of FPD.
Moniliophthora roreri—the causal agent of FPD—has been classified into 14 morphology groups, and various characteristics, including growth rate and genetic patterns of each group, were recently described 5. To cause the disease, the pathogen infects plant-attached cacao pods and undergoes a biotrophic phase of up to 3 months before causing necrosis and spore masses over the pod’s surface 6. During the biotrophic phase, the pod is usually symptomless but, in some cases, may develop malformations before entering the necrotrophic phase 6,7. The pathogenicity of M. roreri strains from different origins has been assessed in plant-attached pods of various cultivars in which the necrosis was evidenced 8.
In addition to plant-attached pods, various diseases, including Phytophthora Pod Rot 9,10 and Botryodiplodia theobromae rot 11 can affect harvested cacao pods causing pod damage while becoming an inoculum source for spreading the disease. However, the pathogenicity of M. roreri from the different morphology groups has not been evaluated on harvested cacao pods.
In Ecuador, a significant proportion (about 7600 ha in 2007) of the cacao production is organic 12,13, and fungicides or other chemicals cannot be applied in the fields. As an alternative, the use of biological control agents 14 and the application of compost teas 15 have been proposed to control plant pathogens in organic plantations. Compost teas are usually obtained through fermentation of agricultural waste and effectively control several plant pathogens, including M. roreri 16. However, the sensitivity to compost teas of M. roreri isolates belonging to the different morphology groups has not been reported.
This research aimed to assess harvested cacao pods’ pathogenicity and the sensitivity to compost tea of M. roreri isolates from selected morphology groups.
MATERIALS AND METHODS
Isolation and classification of M. Roreri.
Isolates of M. Roreri were obtained as indicated in previous reports 5. Briefly, mature cacao pods showing FPD symptoms, including pod lesions with white to creamy mycelium, and internal pod tissues showing necrosis 17, were collected from plantations located on Ecuador’s coast. Healthy pods were also randomly collected and analyzed as controls. Sampled pods that showed symptoms of other diseases were not considered for this study.
Sampled pods were cut into small sections and placed onto MEA (malt extract agar, Oxoid) and incubated at 27°C for 48 h to allow the growth of M. roreri. Mycelia were then transferred to fresh MEA plates and incubated at 27°C for 7 days. Meiospores formed on the MEA media were then mixed in water (106 meiospores/mL), and 10 µL of the mix were transferred to WA (water agar, Oxoid) and incubated at 27°C for 24 to 48 h. Using a stereomicroscope, germinated meiospores were selected and transferred to MEA plates followed by incubation at 27°C for 20 days. Mycelia samples were then transferred to MEA and incubated at 27°C for 15 days. The identity of each isolate was confirmed by PCR amplification and sequencing of the ITS region. All of the obtained isolates were classified into one of the 14 morphology groups defined in previous reports 5 using the classification scale shown in Table 1.
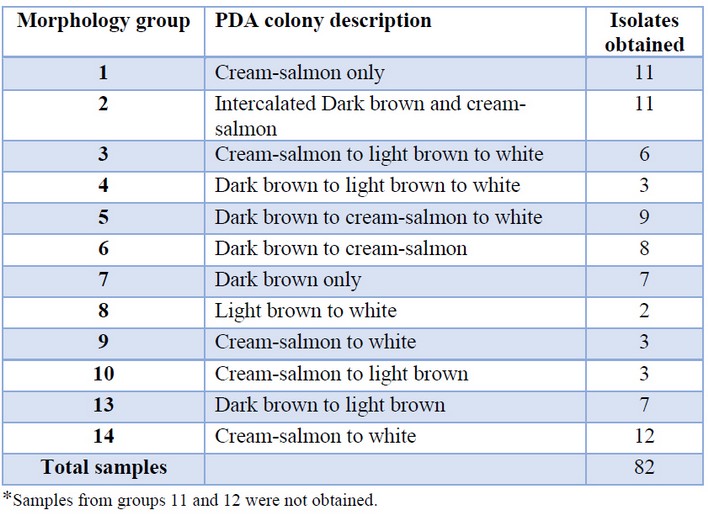
Table 1. Isolates sampled from each of the morphology groups defined by Maridueña-Zavala (2016)*.
Pathogenicity assessment.
Pathogenicity tests were conducted on harvested pods using the dry conidia method 18. Briefly, M. roreri meiospores were attached to a pin’s head and deposited onto a two square centimeters area of a cocoa pod previously marked and moistened with sterile water. Inoculated pods were placed in a moist chamber to promote the germination of conidia. External severity of symptoms was assessed using the disease severity scale (SS) from 0 to 5 based on previous studies 8 and shown in Table 2.
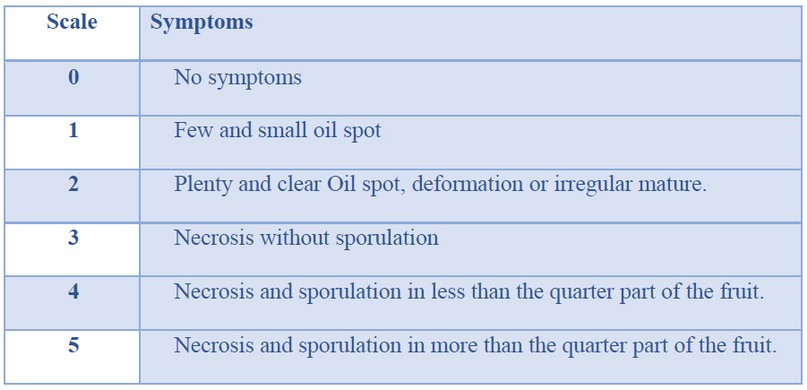
Table 2. Severity scale of cocoa pods infected with M. roreri
The disease severity index (DSI) was calculated using Equation 1 19.
Where ni is the number of pods showing symptoms at the severity scale i, and N is the total number of pods inoculated.
Values of DSI were recorded at 0, 3, 7, 12, and 16 days after inoculation, and the area under the disease progress curve (AUDPC) was estimated using Equation 2 20:

Where the DSI values were recorded at the time intervals ti, all the experiments were run in triplicate.
Sensitivity to compost tea
A compost tea with antifungal properties was prepared exactly as described in previous reports 15. Compost tea doses of 0, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5 and 5 % were prepared in PDA. Three-millimeter disks of M. roreri mycelia from morphology groups 3, 8, and 14 were transferred onto the PDA containing the different compost tea concentrations. The plates were then incubated at 27 °C, and the mycelial diameter was measured at 3, 6, 9, and 12 days after inoculation. Other morphology groups were not tested as the preliminary data suggested no significant differences in the sensitivity to compost tea among the different groups.
Statistical analyses.
Analysis of variance ANOVA compared values of AUDPC obtained by each morphology group tested, and significance was reported for P < 0.05. Inhibitory concentrations 50 (IC 50) were estimated from the mycelial growth data obtained at the different compost teas concentrations using Prism 7 software (GraphPad, La Jolla, CA 92037 USA). T-test was used to compare the means of IC50, and significance was reported at p < 0.05.
RESULTS
A total of 82 isolates were obtained and the identity was confirmed as M. roreri by the DNA sequence of the ITS region.
Pathogenicity test.
All isolates could infect the harvested cacao pods and cause identical symptoms to FPD reported in plant-attached pods (Figure 1). Isolates from morphology groups 1, 2, 4, 6, 8, 10, 13, and 14 caused the first FPD symptoms 3 days after pod inoculation, whereas pods inoculated with isolates from groups 3, 5, 7 and 9 showed FPD symptoms from day 7 onwards (Figure 2). Similarly, all isolates produced DSI of 75 % or higher within 16 days after the pathogen’s inoculation on the harvested pods (Figure 2) and yielded AUDPC values of 25 or above (Figure 3). The lowest AUDPCs were caused by groups 3 and 5 (P < 0.05) while the rest of the groups produced similar AUDPC values (Figure 3), but no correlation was found between the AUDPC values and the morphology groups.

Figure 1. Typical FPD symptoms produced on harvested cacao pods inoculated with M. roreri.
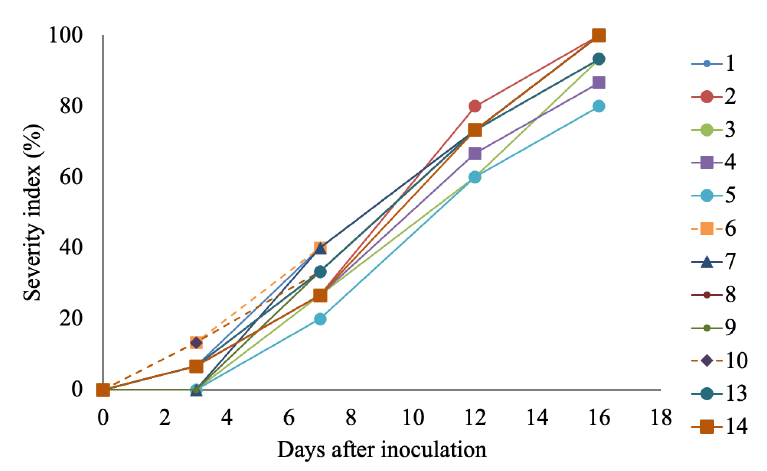
Figure 2. FPD disease progression on harvested cacao pods inoculated with M. roreri isolates belonging to the morphology groups described in Table 1.
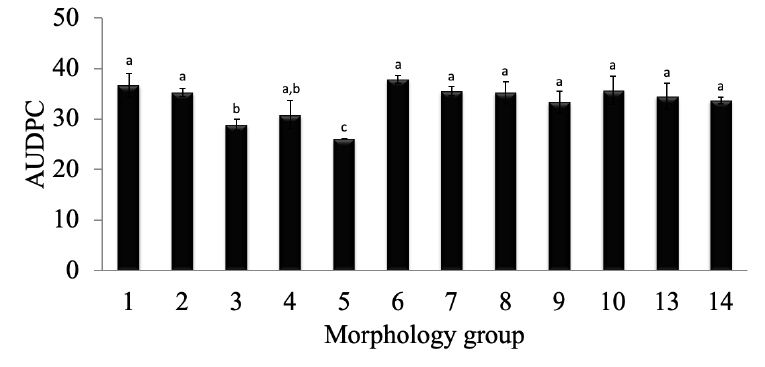
Figure 3. Values of the area under the disease progress curve (AUDPC) for M. roreri groups are described in Table 1. Different letters represent significant differences at p < 0.05
Compost tea significantly inhibited the growth of M. roreri at concentrations of 1 % or above and was able to fully inhibit the growth of all isolates tested at 4.5 % or higher (Figure 4). The IC50 values were above 4 % for all isolates tested (Figure 5), but no significant differences were found when comparing the IC50 values of the different morphology groups.
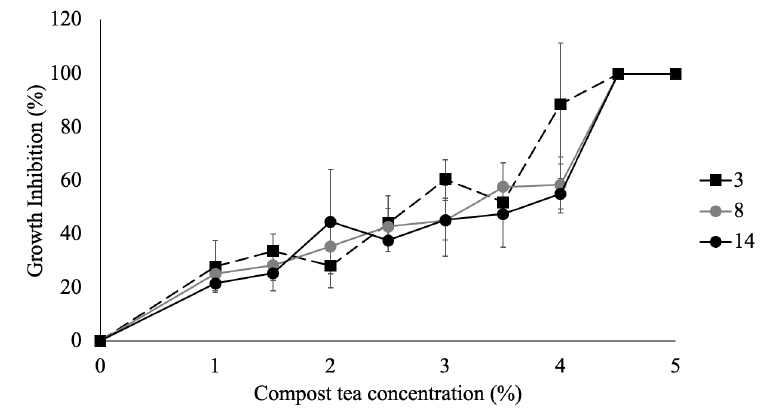
Figure 4. Growth inhibition of M. roreri from morphology groups 3, 8, and 14 by compost tea.
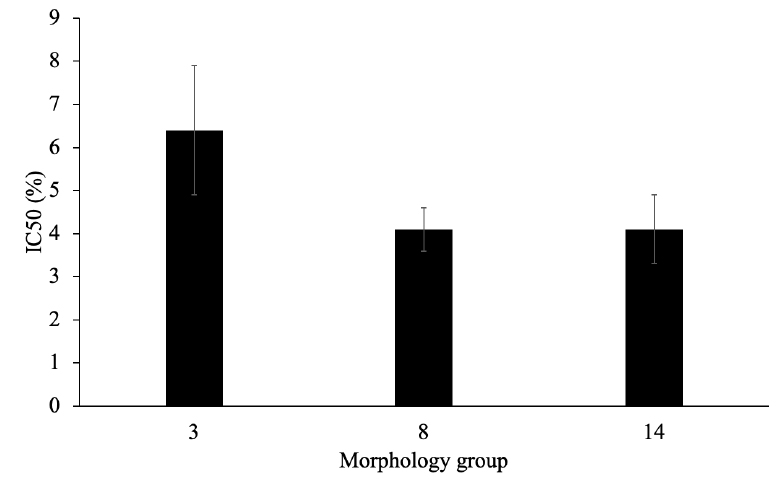
Figure 5. Inhibitory concentration 50 (IC50) values of M. roreri from morphology groups 3, 8, and 14 by compost tea.
DISCUSSION
Most isolates caused the first FPD symptoms on day 3 after inoculation and all isolates were able to fully produce the FP symptoms on the harvested cacao pods within 16 days after inoculation. This disease progression is significantly faster than that observed for plant-attached pods, in which progression times of 9 weeks 7,8 to 3 months 6 have been reported. The data suggest that the asymptomatic-biotrophic phase of FPD was absent or significantly shortened in harvested ripe cacao pods inoculated with M. roreri. Results are similar to those observed in other fungal pathogens such as Colletotrichum spp. When infecting ripe harvested fruits, Colletotrichum spp. can produce fruit rot with a shortened biotrophic phase. However, this phase can be extended if Colletotrichum spp. infects unripe harvested fruits 21. Further research is needed to assess the pathogenicity of M. roreri in harvested cacao pods of different ripening levels.
No correlation between pathogenicity and fungal morphology was observed. Results agree with previous reports in which isolates of M. roreri with different levels of aggressiveness showed no correlation with genetic variation 8. Additionally, various fungal species’ pathogenicity can be dissociated from morphological switching and in vitro growth rate 22.
The compost tea significantly inhibited the growth of all M. roreri isolates at concentrations of 1 % or above, reaching full inhibition at concentrations of 4.5 % or greater. The compost tea concentrations effective against M. roreri were lower than the 5 to 20 % needed for the control of other fungal pathogens such as Alternaria solani 23, Alternaria alternate, Botrytis cinereal, and Pyrenochata lycopersici 24 but higher than the 2.5 % required to inhibit the growth of M. perniciosa 25, suggesting an intermediate to high sensitivity of M. roreri to compost tea. Further research is needed to assess the sensitivity of M. roreri to compost tea in the field.
CONCLUSIONS
All the morphology groups of M. roreri were able to produce FPD necrotrophic symptoms in harvested pods during the 16-day evaluation period. Therefore, the biotrophic phase of the disease was likely absent or significantly shortened. Compost tea effectively controlled the pathogen's growth in vitro at concentrations from 1 to 4.5 %, showing the potential for applications in organic plantations. This is the first report presenting M. roreri isolates’ pathogenicity from different morphology groups on harvested cacao pods.
Funding information
VLIR-UOS financed this research under grant VLIR Network Ecuador
Acknowledgment
Not applicable
Competing interest
There are no competing or financial interest associated to this research
REFERECES
1. Argout X, Fouet O, Wincker P, et al. Towards the understanding of the cocoa transcriptome: Production and analysis of an exhaustive dataset of ESTs of Theobroma cacao L. generated from various tissues and under various conditions. BMC Genomics. 2008;9(1):512. doi:10.1186/1471-2164-9-512
2. Freire J. Proyecto de Reactivacion del Cacao Nacional Fino o de Aroma. Cocoa connect. http://www.cocoaconnect.org/presentation/proyecto-de-reactivacion-del-cacao-nacional-fino-o-de-aroma. Published 2012.
3. CacaoWeb. About the Cacao Tree. About the Cacao Tree. http://www.cacaoweb.net/cacao-tree.html. Published 2003.
4. Loor RG, Risterucci a. M, Courtois B, et al. Tracing the native ancestors of the modern Theobroma cacao L. population in Ecuador. Tree Genet Genomes. 2009;5(3):421-433. doi:10.1007/s11295-008-0196-3
5. Maridueña-Zavala MG, Villavicencio-Vásquez ME, Cevallos-Cevallos JM, Peralta EL. Molecular and morphological characterization of Moniliophthora roreri isolates from cacao in Ecuador. Can J Plant Pathol. 2016;38(4):460-469. doi:10.1080/07060661.2016.1261372
6. Bailey BA, Evans HC, Phillips-Mora W, Ali SS, Meinhardt LW. Moniliophthora roreri , causal agent of cacao frosty pod rot. Mol Plant Pathol. 2018;19(7):1580-1594. doi:10.1111/mpp.12648
7. Bailey BA, Crozier J, Sicher RC, et al. Dynamic changes in pod and fungal physiology associated with the shift from biotrophy to necrotrophy during the infection of Theobroma cacao by Moniliophthora roreri. Physiol Mol Plant Pathol. 2013;81:84-96. doi:10.1016/j.pmpp.2012.11.005
8. Phillips-Mora W, Castillo J, Krauss U, Rodriguez E, Wilkinson MJ. Evaluation of cacao (Theobroma cacao) clones against seven Colombian isolates of Moniliophthora roreri from four pathogen genetic groups. Plant Pathol. 2005;54(4):483-490. doi:10.1111/j.1365-3059.2005.01210.x
9. Nyadanu D, Akromah R, Adomako B, et al. Host Plant Resitance to Phytophthora Pod Rot in Cacao. Int J Bot. 2012;8(1):13-21. http://worldcocoafoundation.org/wp-content/files_mf/nyadanu2012.pdf. Accessed 27 July, 2015.
10. Iwaro AD, Sreenivasan TN, Umaharan P. Cacao resistance to Phytophthora: Effect of pathogen species, inoculation depths and pod maturity. Eur J Plant Pathol. 1998;104(1):11-15. doi:10.1023/A:1008622115731
11. Peter T, Godfried O-M, Emmanuel M. The rot fungus Botryodiplodia theobromae strains cross infect cocoa, mango, banana and yam with significant tissue damage and economic losses. African J Agric Res. 2014;9(6):613-619. doi:10.5897/AJAR2013.7528
12. Quingaísa E. Estudio de caso: denominación de origen “cacao arriba.” 2007:70.
13. Naturland – Association for Organic Agriculture. How to Grow Organic Cocoa.
14. Milton B, Alfonso V, Andrea M, et al. Comportamiento Agroproductivo De 31 Clones De Cacao Nacional (Theobroma Cacao L.) Con La Aplicación De Un Biocontrolador Para Moniliasis (Moniliophthora Roreri). Investig y Saberes. 2016;V(1):39-54.
15. Jimenez M, Van der Veken L, Neirynck H, Rodríguez H, Ruiz O, Swennen R. Organic banana production in Ecuador: Its implications on black Sigatoka development and plant–soil nutritional status. Renew Agric Food Syst. 2007;22(04):297-306. doi:10.1017/S1742170507001895
16. Magdama F. Estudio del efecto de Bioles y cepas de Trichoderma sp . aisladas de zona cacaotera, como alternativa de control de Moniliophthora roreri, en condiciones in vitro. 2010.
17. Phillips-Mora W, Cawich J, Garnett W, Aime MC. First report of frosty pod rot (moniliasis disease) caused by Moniliophthora roreri on cacao in Belize. Plant Pathol. 2006;55(4):584-584. doi:10.1111/j.1365-3059.2006.01378.x
18. Phillips-Mora W. Evaluación de Resistencia de cultivares de cacao (Theobroma cacao L.) a Moniliopthora roreri (cif. y Par) Evans et al..Tesis de Magister scientiae. 1986.
19. Dissanayake MLMC, Kashima R, Tanaka S, Ito S. Pathogenic variation and molecular characterization of Fusarium species isolated from wilted Welsh onion in Japan. J Gen Plant Pathol. 2008;75(1):37-45. doi:10.1007/s10327-008-0135-z
20. Madden L. The Study of Plant Disease Epidemics -. 2nd ed. St. Paul, Minnesota: American Phytopathological Society; 2007. https://www.google.com.ec/webhp?sourceid=toolbar-instant&hl=en&ion=1&qscrl=1&rlz=1T4GGHP_enUS470US470&gws_rd=cr&ei=ZwpiUqraCYrI9QSuvoAg#hl=en&q=The Study of Plant Disease Epidemics&qscrl=1.
21. Alkan N, Fortes AM. Insights into molecular and metabolic events associated with fruit response to post-harvest fungal pathogens. Front Plant Sci. 2015;6(OCTOBER):889. doi:10.3389/fpls.2015.00889
22. Magee PT. Fungal pathogenicity and morphological switches. Nat Genet. 2010;42:560-561.
23. Mengesha WK, Gill WM, Powell SM, Evans KJ, Barry KM. A study of selected factors affecting efficacy of compost tea against several fungal pathogens of potato. J Appl Microbiol. 2017. doi:10.1111/jam.13530
24. Pane C, Celano G, Villecco D, Zaccardelli M. Control of Botrytis cinerea, Alternaria alternata and Pyrenochaeta lycopersici on tomato with whey compost-tea applications. Crop Prot. 2012;38:80-86. doi:10.1016/j.cropro.2012.03.012
25. Maridueña-Zavala MG, Freire-Peñaherrera A, Espinoza-Lozano RF, Villavicencio-Vasquez M, Jimenez-Feijoo M, Cevallos-Cevallos JM. Genetic characterization of Moniliophthora perniciosa from Ecuador and in vitro sensitivity to compost tea. Eur J Plant Pathol. 2019;154(4):943-959. doi:10.1007/s10658-019-01714-1
Received: 20 November 2020
Accepted: 2 January 2021
María Gabriela Maridueña-Zavala1*, Maria Isabel Jimenez Feijoo2, Juan Manuel Cevallos-Cevallos1,2.
1Escuela Superior Politecnica del Litoral, ESPOL. Centro de Investigaciones Biotecnológicas del Ecuador (CIBE). Campus Gustavo Galindo Km 30.5 vía perimetral. Apartado 09015863, Guayaquil-Ecuador
2Escuela Superior Politecnica del Litoral, ESPOL. Facultad de Ciencias de la Vida (CIBE). Campus Gustavo Galindo Km 30.5 vía perimetral. Apartado 09015863, Guayaquil-Ecuador
*Correspondence to [email protected]
