2023.08.04.29
Files > Volume 8 > Vol 8 no 4 2023
Genetic map of the isolated Cryptosporidium parasite from children with diarrheal in the city of Mosul
Hibba Alobide1,*, Firas Alkhashab2,Hiyam Altaee3
1 Department of Biology/ University of Mosul/ College of Education For Girls / Mosul / Iraq;
2 Department of Biology/ University of Mosul/ College of Education For Girls / Mosul / Iraq; [email protected].
Department of Biology/ University of Mosul/ College of Science/ Mosul / Iraq;
* Correspondence: [email protected]; Tel.: +9647709998261
Available from. http://dx.doi.org/10.21931/RB/2023.08.04.29
ABSTRACT
The current study was conducted between the beginning of October 2022 and the end of March 2023 to identify the prevalence of Cryptosporidium parivum infection among children under five years using microscopic and molecular methods. The study recorded 43(37.71%) infected children diagnosed using the Modified Zeihl Neelsen Stain and Gimsa stain. On the other hand, a molecular examination of stool samples using PCR showed that 25(21.92%) children out of 115 collected stool samples were infected with this parasite. DNA Sequencing was performed for specimens diagnosed with Cryptosporidium parasite, and the new isolates were registered for the first time in Nineveh governorate in NCBI under the accession number 757376.
Keywords: Cryptosporidium disease, Genetic map.
INTRODUCTION
Cryptosporidium is a single-cell protozoa parasite and is a Eukaryotic belonging to the phylum Apicomplexa1 and includes the full development of Cryptosporidium on asexual reproduction and Gametogenesis and forms an oocyst containing four Sporozoites, that occurs within the same host 2 and is the leading cause of Cryptosporidiosis which affects the digestive and respiratory tract of types of hosts , including humans 3,4 as well as the cause of watery or lipophilic diarrhoea with colic 5,6 which affects the digestive and respiratory tract of the kinds of hosts , including humans 7 as well as the cause of watery or lipophilic diarrhoea with colic, Intestinal parasite infections such as Cryptosporidium parasites are among the most critical problems experienced by humanity, causing diarrhea in children and the elderly, and sometimes the infection is particularly severe in people with weak immunity 8,9,10, Since individuals who are immune competent , the parasite will live for a short time because Cell-mediated immunity and Humoral immunity is able to resist infection11 and most individuals who have immune efficiency do not show symptoms of disease Asymptomatic carrier, 12. The risk is in people who have weak immunity, such as infants as well as malnourished people infected with AIDS as well as those taking Immunosuppressive Therapy. These cases are prone to chronic infections with Cryptosporidium And prone to dehydration and malabsorption 13. more than half of all diseases are water-borne 14, starting with exposure to oocysts, which have the potential to survive prolonged periods in the environment because they are resistant to known household disinfectants and can travel through physical water treatment processes 1,10.
Collection of Stool Samples
Stool samples were collected from patients (children), male and female, who reviewed the Ibn Al-Atheer Children's Hospital in Nineveh governorate and laboratory tests for parasite detection were carried out, 114 samples were collected with a size of 15-20 g and placed in sterile plastic packages with a tight cover to maintain moisture and prevent the sample from drying out as well as the patient's name, age, gender and address were recorded and transferred to the research laboratory (Parasite Laboratory) at the Faculty of Education for Girls (University of Mosul) after which the samples were examined in kind by observing (color, texture, smell of feces, presence and absence of blood and mucus) Then go for microscopic examination in the modified Ziehl-Neelson and Giemsa stain methods to confirm the presence of the parasite. The remainder of the sample is performed using the flotation method to obtain the egg cysts and is placed in sterile tubes. It was stored in a potassium bichromate solution with a 2.5% concentration added to each ml of the feces sample and 1 ml of the solution. The samples were kept in the refrigerator at 4 ° C until the DNA parasites were isolated and the PCR chain polymerization reaction examined 15.
MATERIALS AND METHODS
Microscopic examination
The staining method by modified Ziehl-Neelson according to Henrisken and Pohlenz, Khudhair 16,17.
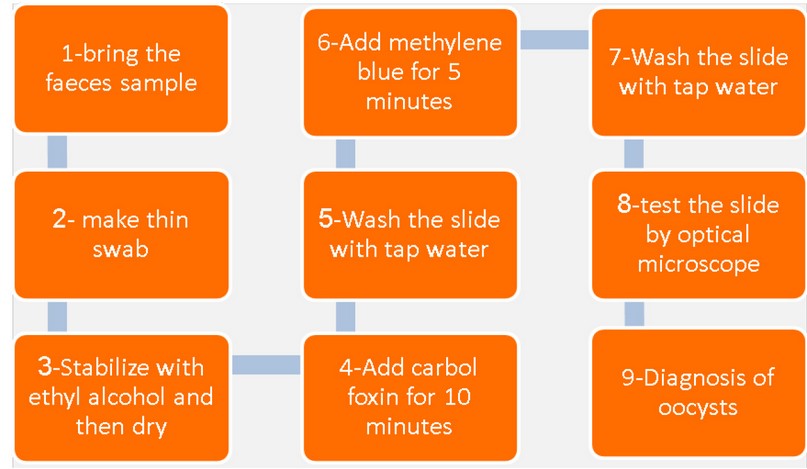
Figure 1. The staining method by modified Ziehl-Neelson stain
After the procedure of staining operation by modified Ziehl-Neelson MZN and Diagnosis of oocysts, the remainder of the stool sample Enters a Phase Flotation by Sheather's Sugar Solution according to method 18.
Molecular examination
Extracting DNA from Cryptosporidium spp parasite
Instructions from Geneaid company directed the Assay Procedure to extract DNA installed on the Kit.
Preparation of Agarose Gel and Electrophoresis of DNA
The Agarose gel is prepared at a concentration of 1% to deploy and detect DNA. This concentration is dissolved by 0.5 g of Acarose powder in (50) ml of X1 TBE, and the addition of 3 microliters of red safe dye is done using a hot plate magnetic stirrer until boiling and left to cool to a temperature of (60-50)C. The gel solution is poured into the tray with the electrophoresis device after the special comb is installed to form Wells drilling at the gel's limbs, considering that the casting is quiet to avoid forming bubbles. If included, it is removed using the absorbent, and then the gel is left until hardened.
Then, the tray is placed in the electrophoresis device basin containing an appropriate amount of X1 TBE solution, after which the comb is quietly lifted. Relay samples are prepared by mixing (5) microliters of DNA sample with (3) microliters of loading solution. The electrophoresis device is powered by an Electric Current supply with a voltage difference (5) V/cm, which takes (2-1.5) hours. Then, the gel is filmed under ultraviolet light using the Gel Imaging Device Gel Documentation to see the DNA packages and the PCR reaction product.
The PCR reaction
The DNA concentration in all study samples was adjusted by mitigation by a TE buffer solution to obtain the required concentration for PCR interactions, which was (100) ng/microliters per sample. The Master Reaction mixture is prepared for each PCR reaction by blending the DNA sample and the unique prefix for each gene with the Pre-mix components within the 0.2 ml Eppendorf tube equipped by Biolaps English company. The reaction volume has been stabilized to 20 microliters with distilled water. The combination is discarded in the Microfuge for between (5-3) seconds to ensure that the interaction components are blended. Then, the reaction tubes are introduced into the Thermocycler to conduct the multiplier reaction using the particular program of each response. The sample was then loaded into Etch the agarose gel at 2% concentration with the addition of the Ladder DNA volume guide equipped by Biolaps in one etch. The samples are then relayed by running the electrophoresis device for between 70 and 60 minutes, after which the gel is filmed using the gel documentation device.
Molecular diagnosis of Cryptosporidium spp parasite based on 18S rDNA region
The presence of the enlarged area has been revealed with the addition of 4 microliters (100 nanograms) of DNA template and 1 microliter (10 picomol) from each unique prefix in the gene to the contents of the Master mix.

Table 1. The prefixes used to amplify the COWP gene to Cryptosporidium parasite.
Then, the reaction tubes were inserted into the Thermocycler device to perform the multiplier reaction using the particular response program.
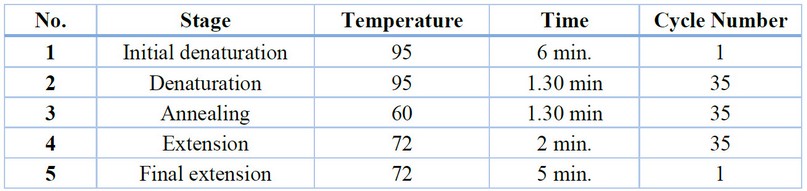
Table 2. Shows the special program of polymerase chain reaction used in the current study.
The sequence of nitrogen bases for the samples under study was determined as the products of the pre-mentioned PCR reaction of the samples were sent with the special prefixes in the resulting beam. The sequence was read for the genes based on the 3130 Genetic Analyzer device equipped by the Japanese company Hitachi.
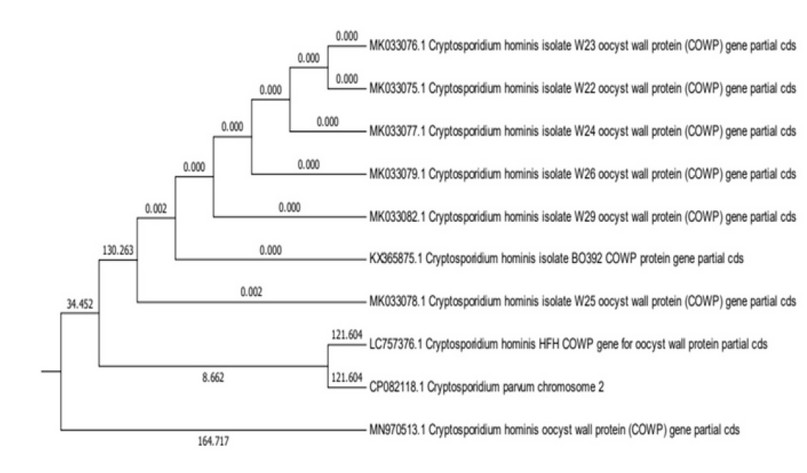
Figure 2. The evolutionary tree and the relationship between local isolates and global isolates
Gene sequences were matched with gene sequences documented at the National Center for Biotechnology Information NCBI, and the results were analyzed based on the BLAST program.
RESULTS AND DISCUSSION
Microscopy
The result of microscopic examination of the 114 stool samples in the city of Mosul. There were 43 children with Cryptosporidium disease (37.71%), according to the microscopic examination of modified Diehl-Neelson stain MZN, as well as Flotation operation with Sheather's Sugar Solution was the infection (31.57%).
The results of the current study Also show a difference in the rates of infection between the ages of 5 months and 5 years, when the highest incidence is recorded(3-4 years). The lowest proportion was in the age group (5 months/year), which agreed with a study by Hussein 19. The age factor plays a role in the spread of the parasite because children's immune system is incomplete. They are constantly exposed to contamination, both food contaminants and even other environmental contaminants, due to frequent movement and mixing with other children and possibly contact with stored animals and carriers of parasites, as well as the residential area in which they are located, Especially if animal husbandry abounds is a source of infection in addition to lack of hygiene and health awareness 20.
The discrepancy also appeared in the infection rates according to sex in children, as the percentage of males (72.09%) infected with females (30.23%). Many studies have confirmed, such as a study, that there is no relationship between sex and the rate of infection with the Cryptosporidium parasite. Still, the reason may be due to the type of food Or males eating exposed foods contaminated with oocysts in public places because they are in contact with external environment conditions that play a role in parasite transmission and infection, as well as smoking with break and lack of attention to personal hygiene all increase the chance of infection with Cryptosporidium more than females 21.
The highest incidence of infections among children was found in November (37.2%), and the lowest rate of infections (2.32%) in February. This matched the study of Abbas & Resan 22, which recorded the highest infection in winter. The reason was the rain that drifts the soil contaminated with parasite oocysts into the stool of animals and mixes with water sources such as rivers and streams, causing the Cryptosporidium disease.
The results indicated that the Cryptosporidium parasite was more prevalent in rural areas (67.44%) and less in urban areas (32.55%). This study came in agreement with several studies of which Mohanad 23 and Al-Kubaaisy 24, For many reasons, including the low level of health and cultural awareness due to the lack of schools and hospitals, and the use of river water for drinking, cooking and swimming, and raising and touching animals and fertilizing crops increased.
Molecular examination
Results emerged from one package (single band) in the Agarose gel with a molecular size of 650bp in DNA extracted from samples of individuals with Cryptosporidium. It is considered a reference to the patient's infection. The current study showed that the infection with the Cryptosporidium parasite was (21.92%) close to the study of Abdul-Sada 25, Tahvilder & Salehi 26 and Gawad et al. 27 as (24.3%) (20.8%) (21.0%), respectively.
Genetic isolates were compared with other Cryptosporidium parvum by finding the genetic tree. A new type of Genotype of Cryptosporidium parvum was recorded in Nineveh governorate and registered in the NCBI genetic Bank with the researchers' names. DNA sequencing of 10 samples chosen from Polymerase chain reaction products was analyzed from an original sample 25 which showed a positive result for the target area of the DNA of the products with the primary sequence of the DNA of the parasite Cryptosporidium reference gene published in the Nitrogen base Database of the GenBank website using BLAST Program of The National Center For Biotechnology Information (NCBI) The rate of matching was high with the isolation recorded in the America, while the rate of matching was lower with other isolations registered and the ratio of match ranged from 83% _ 88% Results show that the local isolation that recorded at the Gene Bank in sequence (757376) has a high convergence rate with the American isolation that sequence (082118) of about 88% This shows the origin of an evolutionary development associated with these isolations despite the geographical location of the parasite isolation zones of the two similar strains, but studies have confirmed that travel and movement between countries have played a role in the development and transmission of isolations by infected people to many different countries, especially in recent times, He stated 28 that Cryptosporidium has become an epidemiological disease and called Traveller Diarrhea (TD), because it is transmitted among travelers through public health facilities at the world's airports, where the parasite was found this way by 20 _ 60% in North America and Europe. The strain's emergence could be due to its transmission during the United States military's presence in Mosul after the events of 2003.
CONCLUSIONS
In conclusion, the microscopic examination of stool samples in Mosul revealed a high prevalence of Cryptosporidium infection among children, with the highest incidence occurring in the age group of 3-4 years. The infection rates were higher in males than females, and the highest incidence of infections was observed in November. Additionally, the study found that the Cryptosporidium parasite was more prevalent in rural areas, and a new genotype of Cryptosporidium parvum was recorded in Nineveh governorate. The genetic analysis showed a high convergence rate with American isolates, suggesting the possibility of transmission through travel and movement between countries.
Funding: This research received no external funding.
Acknowledgments: I'm incredibly grateful to the council of the University Of Mosul/ College Of Education For Girls. It would not have been possible without their support and nurturing.
Conflicts of Interest: The authors declare no conflict of interest.
REFERENCES
1. Ryan, U.; Paparini, A.; Monis, P.; Hijjawi, N. It's official–Cryptosporidium is a gregarine: What are the implications for the water industry?. Water Research. 2016 Nov 15;105:305-13.
2. Cruz-Bustos, T.; Feix, A.S.; Ruttkowski, B.; Joachim, A. Sexual development in non-human parasitic apicomplexa: just biology or targets for control?. Animals. 2021 Oct 4;11(10):2891.
3. Fayer, R. Taxonomy and species delimitation in Cryptosporidium. Experimental parasitology. 2010 Jan 1;124(1):90-7.
4. Ryan, U.N.; Fayer, R.; Xiao, L. Cryptosporidium species in humans and animals: current understanding and research needs. Parasitology. 2014 Nov;141(13):1667-85.
5. Chen, X.M.; Keithly, J.S.; Paya, CV; LaRusso, N.F. Cryptosporidiosis. New England Journal of Medicine. 2002 May 30;346(22):1723-31.
6. Kosek, M.; Alcantara, C.; Lima, A.A.; Guerrant, R.L. Cryptosporidiosis: an update. The lancet infectious diseases. 2001 Nov 1;1(4):262-9.
7. Wiser, MF; Nutrition and protozoan pathogens of humans: A primer. Nutrition and infectious diseases: shifting the clinical paradigm. 2021:165-87.
8. Platts-Mills, J.A.; Babji, S.; Bodhidatta, L.; Gratz, J.; Haque, R.; Havt, A.; McCormick, B.J.; McGrath, M.; Olortegui, M.P.; Samie, A.; Shakoor, S. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). The Lancet Global Health. 2015 Sep 1;3(9):e564-75.
9. Khalil, I.A.; Troeger, C.; Rao, P.C.; Blacker, B.F.; Brown, A.; Brewer, T.G.; Colombara, D.V.; De Hostos, EL; Engmann, C.; Guerrant, R.L.; Haque, R. Morbidity, mortality, and long-term consequences associated with diarrhoea from Cryptosporidium infection in children younger than 5 years: a meta-analyses study. The Lancet Global Health. 2018 Jul 1;6(7):e758-68.
10. Wang, Z.D.; Liu, Q.; Liu, H.H.; Li, S.; Zhang, L.; Zhao, Y.K.; Zhu, X.Q. Prevalence of Cryptosporidium, microsporidia and Isospora infection in HIV-infected people: a global systematic review and meta-analysis. Parasites & vectors. 2018 Dec;11:1-9.
11. Riggs, M.W. Recent advances in cryptosporidiosis: the immune response. Microbes and Infection. 2002 Aug 1;4(10):1067-80.
12. Wang, Y.; Cao, J.; Chang, Y.; Yu, F.; Zhang, S.; Wang, R.; Zhang, L. Prevalence and molecular characterization of Cryptosporidium spp. and Giardia duodenalis in dairy cattle in Gansu, northwest China. Parasite. 2020;27.
13. Hunter, P.R.; Nichols, G. Epidemiology and clinical features of Cryptosporidium infection in immunocompromised patients. Clinical microbiology reviews. 2002 Jan;15(1):145-54.
14. Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, RF; Faruque, A.S. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. The Lancet. 2013 Jul 20;382(9888):209-22.
15. Khudhair, M.S.; Al-Niaeemi, N.E. Experimental study of heat-killed oocysts of Cryptosporidium Parvum in Balb/c Mice. J. Educ. Sci. 2020 Jun 1;29(2):158-73.
16. Henriksen, S.A.; Pohlenz, J.F. Staining of cryptosporidia by a modified Ziehl-Neelsen technique. Acta veterinaria scandinavica. 1981;22(3-4):594.
17. Khudhair, M.S. Experimental study of heat -killed oocysts of Cryptosporidium parvum in Balb/c Mice. Journal of Education and Science (ISSN 1812 – 125X),Vol:29,No:2,2020 ( 158 – 173).
18. Freire-Santos, F.; Oteiza-Lopez, A.M.; Vergara-Castiblanco, C.A.; Ares-Mazas, M.E. Effect of salinity, temperature and storage time on mouse experimental infection by Cryptosporidium parvum. Veterinary Parasitology. 1999 Nov 1;87(1):1-7.
19. Hussein, Z.A. Percentage of infection with Cryptosporidium parvum in children At Al-Zahra Maternity and children Hospital. 2011;MSc. Nursing. Collage., Al-KufaUniv.:8pp.
20. Al-Sultan, Sh.H. Diagnostic epidemiological and pathological study of Cryptosporidium parvum in children under five years of age in some areas of Nineveh Governorate (MSc,Education for Girls.Collage., MosulUniv.:2021; 67pp.
21. Al-Mamouri, A.K. Epidemiology of intestinal parasites and head lice in pupils of some primary school at Al-Mahaweel district, Babylon province (Doctoral dissertation, MSc. Thesis. Collge of Science. Babylon University. 2000: 122).
22. Sabri, SM Determining the spread of Cryptosporidium by traditional methods in Al-Diwaniyah Governorate-Iraq. Iraqi Journal of Humanitarian, Social and Scientific Research. 2022.
23. Mohanad, M.M. The study of Cryptosporidium parvum dispersal in children less than five years old in Ramadi province. J. Al-Anbar Uni. Sci. 2008;2(2):84-8.
24. Waqar, AL; Hassanain, A.L.; Alyaa, A.K. Intestinal parasitic diarrhea among children in Baghdad–Iraq. Tropical biomedicine. 2014 Sep;31(3):499-506.
25. Abdul-Sada, K.M. Molecular and Epidemiological Study of Cryptosporidium spp. in Mid-Euphrates Area. Kufa Journal for Nursing Sciences. 2015 Apr 25;5(1):167-78.
26. Tahvildar-Biderouni, F.; Salehi, N. Detection of Cryptosporidium infection by modified ziehl-neelsen and PCR methods in children with diarrheal samples in pediatric hospitals in Tehran. Gastroenterology and hepatology from bed to bench. 2014;7(2):125.
27. Gawad, SS; Ismail, M.A.; Imam, N.F.; Eassa, A.H. Detection of Cryptosporidium spp. in diarrheic immunocompetent patients in Beni-Suef, Egypt: insight into epidemiology and diagnosis. The Korean journal of parasitology. 2018 Apr;56(2):113.
28. Shinta, T.., Oda, T., & Arizono, N. Imported cryptosporidiosis report of a case in Japan and the literature. Kansen, Shogaku. Zasshi., 2004;68(7):941945.
Received: 15 June 2023 / Accepted: 23 August 2023 / Published:15 December 2023
Citation: Al-Obide, H.; Alkhashab, F. Genetic map of the isolated Cryptosporidium parasite from children with diarrheal in Mosul. Revis Bionatura 2023;8 (4)29. http://dx.doi.org/10.21931/RB/2023.08.04.29
Publisher's Note: Bionatura stays neutral concerning jurisdictional claims in published maps and institutional affiliations.
Copyright: © 2023 by the authors. Submitted for possible open-access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
