2022.07.02.9
Files > Volume 7 > Vol 7 No 2 2022
Soluble (PD-1) and Autophagy protein (ATG-5) in patients with tuberculosis
Suhaib Waad Oklah Musa1 , Ayad M. Gaidan2*, Reyam F. Saleh3
1 Department of Biology- College of Science - Tikrit University, Iraq e-mail: [email protected]
2 Department of Biology- College of Science - Tikrit University, Iraq
3 Department of Biology- College of Science - Tikrit University, Iraq; e-mail: [email protected]
*Correspondence: e-mail: [email protected]
Available from: http://dx.doi.org/10.21931/RB/2022.07.02.9
ABSTRACT
Immune checkpoints such as programmed cell death protein (PD-1) and autophagy-related protein 5 (ATG5) can directly affect the immune response and thus, interfere with infection with Mycobacterium tuberculosis (Mtb). This study aimed to determine the role of soluble ATG5 and soluble PD-1 proteins in tuberculosis (TB) patients. The study included a collection of 90 blood samples (47 samples from patients with confirmed tuberculosis and 43 samples from age and gender-matched healthy subjects to estimate the concentration of soluble autophagy (ATG-5) and programmed cell death protein (PD-1). Enzyme-linked immunosorbent assay was used to estimate the serum levels of these markers. The median value of ATG5 level in patients was 2.08 µg/ml, which is higher than the level of the control group (median = 0.93 µg/ml) with a highly significant difference. Similarly, the median value of PD-1 level in patients was 259.4 ng/ml (range 107.76-924.25 ng/ml), which was higher than that of the control (median = 146.4 ng/ml, range = 50.32-1117.1 ng/ml) and with a highly significant difference. It can be concluded that both soluble ATG5 and PD-1 increased significantly in patients with TB compared with controls.
Keywords: tuberculosis, M. tuberculosis, Autophagy, Programmed cell death protein-1
INTRODUCTION
Tuberculosis (TB) is considered one of the essential epidemic diseases, and it is caused by Mycobacterium tuberculosis (Mtb) which commonly attacks the lungs and other organs such as bones, lymph and joints 1. Patients often present with pulmonary symptoms such as a bloody cough (hemoptysis), chest pain and shortness of breath (SOB), and general clinical symptoms such as fever, poor appetite, weight loss and night sweats 2.
According to World Health Organization (WHO) statistics in 2018, one-third of the world's population is infected with MTB, 90% of whom have latent infection. With most severe cases are in developing countries. The prevalence is associated with poor treatment, drug resistance and limited vaccines 3.
Understanding the host response to Mtb infection is a critical factor in efforts to eradicate TB by developing effective vaccines and immune therapeutics. The ability of Mtb to survive and replicate in host macrophages is an essential step for its pathogenesis, with intracellular growth being often associated with virulence 4,5.
Autophagy is an intracellular self-digestion process whereby cytoplasmic constituents are delivered to and degraded by lysosomes 6. Besides being crucial for quality control and energy supply, autophagy plays critical roles in immune defenses against invading bacterial and viral pathogens, such as regulation of inflammation, antigen presentation and microorganism capture and degradation 7-9.
Programmed cell death protein 1 (PD-1) is a 288 amino acid cell surface molecule that has been designated as a membrane protein of the immunoglobulin superfamily in humans. This protein is expressed on T cells, pro-B cells, and myeloid-derived dendritic cells, leading to negative regulation of the proliferation and activity of these cells 10.
Increasing evidence suggests PD-1 expression 5and some autophagy proteins such as ATG5 are upregulated in patients with active TB 11-13. This study aimed to measure the serum levels of soluble PD-1 and ATG5 in patients with TB.
MATERIALS AND METHODS
The Study Population
The study involved 47 patients with TB attending the TB control center/ Baghdad during the period from 1st January 2019 to 31st October 2020. The TB diagnosis was made first with acid-fast stain (figure 1), then confirmed with culturing on Löwenstein–Jensen (LJ) culture and biochemical test specific for Mtb.
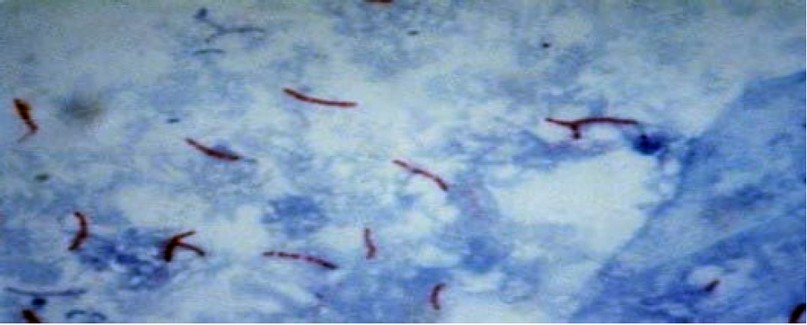
Figure 1: Acid fast bacilli of M. tuberculosis 100X.
Other age- and gender-matched 43 apparently healthy subjects where selected to represent controls. Blood samples were obtained from patients and controls. The sera were separated and stored at -20⁰C for immunological studies. Demographic data (age, gender, socioeconomic status, smoking habit) were collected from each participant through direct interview. The ethical committee approved the study of the College of Science, Tikrit University.
Serum Level of Soluble PD-1 and ATG5
Ready commercial kits (SunLong Biotech Co., LTD/ China) were used to measure serum levels of soluble ATG5 and PD-1 through enzyme-linked immunosorbent assay (ELISA). This assay is based on the presence of antibodies (for PD-1 orATG-5) attached to microliter wells. Soluble PD-1 or ATG-5 reacts with these antibodies. An enzyme-conjugated anti- PD-1 orATG-5 (horseradish peroxidase (HRP) is then added to the reaction. The enzyme acts as an amplifier of the detection signal by converting a substrate that results in color changes in the wells. The density of color is proportional to the serum concentration of PD-1 or ATG-5. The manufacturer's instructions were followed precisely. A fully automated apparatus read the results. These assays were performed at the Medical Research Unit, College of Medicine, Al-Nahrain University/ Baghdad.
Statistical Analysis
Statistical analyses were performed using SPSS software version 25.0 (SPSS, Chicago). Continuous data were subjected to a normality test (Shapiro Wilk test); Data with normal distribution were presented as mean and standard deviation and analyzed with a Student t-test. Data with non-normal distribution were presented as median and range and analyzed with the Mann Whitney U test. Categorical variables were expressed as numbers and percentages and analyzed with the Chi-square test. The receiver operating characteristic curve (ROC) was used to evaluate the diagnostic value of PD-1 and ATG5 in the discrimination TB patients and controls. A p-value less than 0.05 was considered to indicate a statistically significant difference.
RESULTS
Demographic characteristics of the study population
The mean age of the patients was 40.94 ± 16.79 years, which did not differ significantly from the control group (39.0 ± 16.21 years). Similarly, the distribution of females and males, the socioeconomic status and the percentage of smokers were similar between the two groups, with no significant differences. On the other hand, 25.53% of patients without comorbidities compared to 58.14% of the control group with a significant difference. In particular, the incidence of diabetes was higher in patients versus the control group (29.79% versus 13.95%)(Table 1).
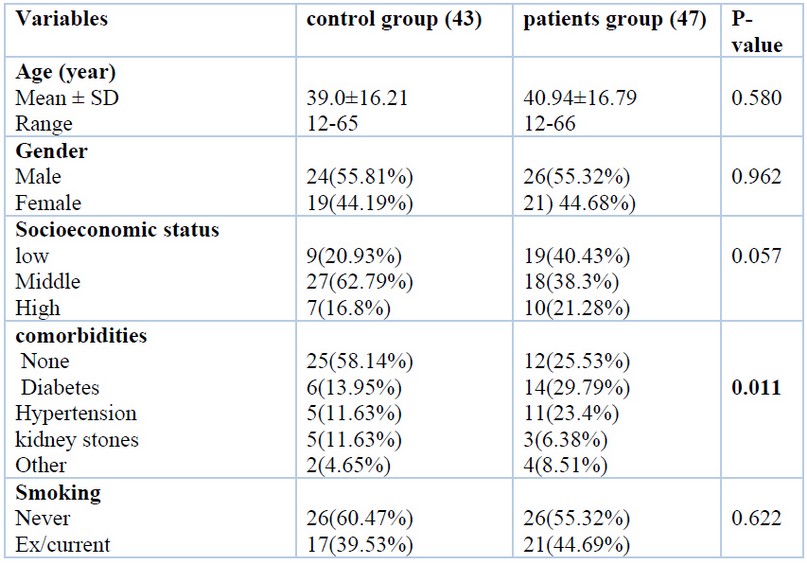
Table 1. Demographic characteristics of the study population
Serum level of soluble ATG5 and PD-1
Data regarding soluble ATG5 and PD-1 levels were found to be non-normally distributed. Accordingly, they expressed as median and range and analyzed with Mann Whitney U test. The median value of ATG5 level in patients was 2.08 µg/ml (range 1.1-4.06 µg/ml), which was higher than that of the control group (median = 0.93 µg/ml, range = 0.45-5.26 µg/ml) with a highly significant difference (Figure 2).
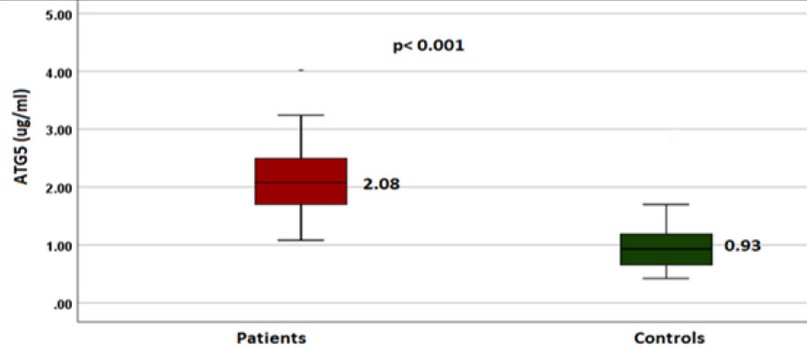
Figure 2. The Median serum level of soluble ATG5 in patients and controls
Similarly, the median value of PD-1 level in patients was 259.4 ng/ml (range 107.76-924.25 ng/ml) which is higher than that of the control (median = 146.4 ng/ml, range = 50.32-1117.1 ng/ml) and with a highly significant difference (Figure 3).
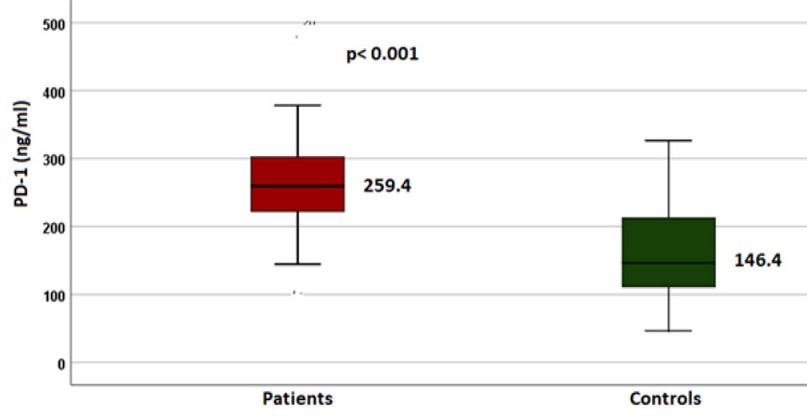
Figure 3. The Median serum level of soluble ATG5 in patients and controls
Diagnostic value of ATG5 and PD-1
The receiver operating characteristic curve was used to determine the diagnostic value of ATG5 and PD-1 in discriminating between patients and control. For ATG5, the area under the curve was 0.921, 95% CI = 0.984-0.857, p < 0.001. The sensitivity and specificity of the test at the cut-off value for ATG5 1.32 µg/mL were 89% and 84%, respectively. For PD-1, the area under the curve was 0.797, 95% confidence interval = 0.898-0.695, P < 0.001 The sensitivity and specificity of the test at the cut-off value of PD-1 = 205.4 ng/ml were 80% and 74 % respectively (Figure 4).
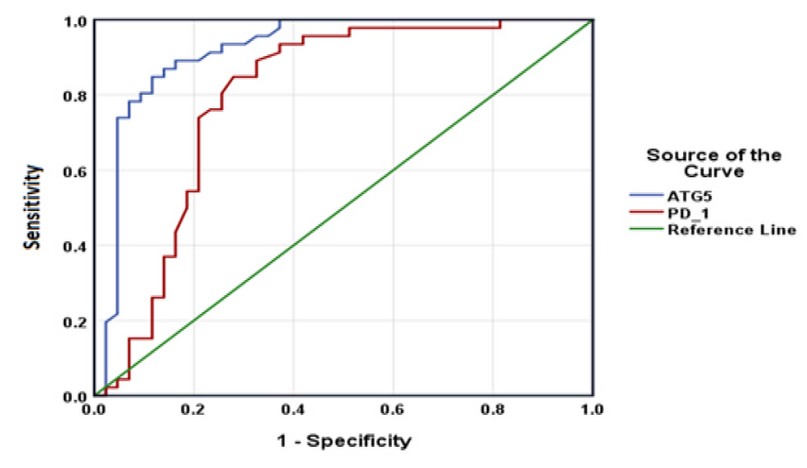
Figure 4. Receptor operating characteristic curve for ATG5 and PD-1 to distinguish between TB patients and controls.
DISCUSSION
When the host exposes to Mtb, and autophagy mechanism is induced. This results in fusion of lysosomal compartments with mycobacterial phagosome and triggers the generation of neo-antimicrobial peptides, essential for mycobacterial killing 14. Moreover, autophagy may play an indirect role in Mtb phagocytosis through the regulation of scavenger receptors expression 15. In vivo experiments have documented that autophagy protein ATG5 in macrophages is required to restrain Mtb infection in laboratory animals 16,17. Moreover, using autophagy inhibitors enhances mycobacterial load in zebrafish embryo model 18. This reflects the importance of autophagy proteins, particularly ATG5, in host defense against TB. Thus, it is reasonable to elevate serum levels of ATG5 protein in TB patients because this protein may reflect the gene expression of membranous ATG5.
In laboratory animals, PD-1 expression was found to be upregulated in severe TB disease infection 19. Mtb infection of PD-1−/− mice leads to enhanced susceptibility to TB disease characterized by increased bacterial loads, increased inflammatory and necrotic responses in the lungs, and reduced survival 20,21. These studies in PD-1-deficient mice demonstrate an essential role for PD-1 in limiting excessive IFN-γ production by CD4 T cells, which has been associated with exacerbated disease in murine models of TB 22. Increasing evidence suggests PD-1 expression is upregulated on innate and adaptive immune cells in the setting of Mtb infection and disease in humans 23. Although all these studies focused on membranous PD-1, soluble PD-1 may also reflect the host's immune status.
In a Chinese study, Li et al. 24 showed that the concentration of soluble PD-1 in the serum of patients with cystic echinococcosis was elevated compared with the healthy control subjects or patients that received treatment. The authors explained this elevation in terms of an increase in soluble PD-1 expression inhibits the PD-1/PD-L1 signaling pathway in T cells through negative feedback, which reduces the inhibition of T cell activation and increases the activity of the immune system in managing the infection. This supports the findings of Wang et al., which suggest that sPD-1 blocks the membrane PD-1 binding site on activated T-cells, thereby attenuating the PD-1 signaling pathway and increasing the immune response 25.
CONCLUSION
Serum levels of soluble PD-1 and ATG5 protein are increased in tuberculosis patients compared with controls. The soluble ATG5 protein has an outstanding diagnostic value in discrimination between patients with TB and controls and could be used as additional diagnostic tools in suspicious cases of TB.
Author Contributions: Suhaib Waad Oklah Musa: sample and data collection; manuscript writing; Reyam F. Saleha: statistical analysis and manuscript editing.
REFERENCE
2. Tabong, P. T. N., Akweongo, P., & Adongo, P. B. Community beliefs about tuberculosis in Ghana: Implications for the end tuberculosis global agenda. Cogent Med 2021;8(1), 1870069.
3. Liu, L., Zhai, K., Chen, Y., Chen, X., Wang, G., & Wu, L. Effect and mechanism of Mycobacterium tuberculosis lipoprotein LpqH in NLRP3 inflammasome activation in mouse Ana-1 macrophage. BioMed Research International, 2021; Article ID 8239135
4. Hmama, Z., Pena-Diaz, S., Joseph, S., and Av-Gay, Y. Immunoevasion and immunosuppression of the macrophage by Mycobacterium tuberculosis. Immunol. Rev. 2015; 264, 220–232
5. Weiss, G., and Schaible, U. E. Macrophage defense mechanisms against intracellular bacteria. Immunol. Rev. 2015;264, 182–203.
6. Lamb, C. A., Yoshimori, T., and Tooze, S. A. The autophagosome: origins unknown, biogenesis complex. Nat. Rev. Mol. Cell Biol. 2013;14, 759–774.
7. Deretic, V. Autophagy as an innate immunity paradigm: expanding the scope and repertoire of pattern recognition receptors. Curr. Opin. Immunol. 2012;24, 21–31.
8. Richetta, C., and Faure, M. (2013). Autophagy in antiviral innate immunity. Cell. Microbiol. 15, 368–376.
9. Huang, J., and Brumell, J. H. (2014). Bacteria-autophagy interplay: a battle for survival. Nat. Rev. Microbiol. 12, 101–114.
10. Yamane, H., Isozaki, H., Takeyama, M., Ochi, N., Kudo, K., Honda, Y., Yamagishi, T., Kubo, T., Kiura, K., & Takigawa, N. Programmed cell death protein 1 and programmed death-ligand 1 are expressed on the surface of some small-cell lung cancer lines. Am J Cancer Res. 2015; 5(4), 1553–1557.
11. Alvarez IB, Pasquinelli V, Jurado JO, Abbate E, Musella RM, de la Barrera SS, et al. Role played by the programmed death-1-programmed death ligand pathway during innate immunity against Mycobacterium tuberculosis. J Infect Dis. 2010;202:524–32.
12. . Shen L, Gao Y, Liu Y, Zhang B, Liu Q, Wu J, et al. PD-1/PD-L pathway inhibits M.tb-specific CD4(+) T-cell functions and phagocytosis of macrophages in active tuberculosis. Sci Rep. 2016;6:38362
13. Espert L, Beaumelle B, Vergne I. Autophagy in Mycobacterium tuberculosis and HIV infections. Front Cell Infect Microbiol. 2015;2;5:49.
14. Ponpuak, M., Davis, A. S., Roberts, E. A., Delgado, M. A., Dinkins, C., Zhao, Z., et al. Delivery of cytosolic components by autophagic adaptor protein p62 endows autophagosomes with unique antimicrobial properties. Immunity 2010;32, 329–341.
15. Bonilla, D. L., Bhattacharya, A., Sha, Y., Xu, Y., Xiang, Q., Kan, A., et al. Autophagy regulates phagocytosis by modulating the expression of scavenger receptors. Immunity 2013;39, 537–547.
16. Castillo, E. F., Dekonenko, A., Arko-Mensah, J., Mandell, M. A., Dupont, N., Jiang, S., et al. Autophagy protects against active tuberculosis by suppressing bacterial burden and inflammation. Proc. Natl. Acad. Sci. U.S.A. 2012; 109, E3168–E3176.
17. Watson, R. O., Manzanillo, P. S., and Cox, J. S. Extracellular M. tuberculosis DNA targets bacteria for autophagy by activating the host DNA-sensing pathway. Cell 2012;150, 803–815.
18. Van Der Vaart, M., Korbee, C. J., Lamers, G. E., Tengeler, A. C., Hosseini, R., Haks, M. C., et al. The DNA damage-regulated autophagy modulator DRAM1 links mycobacterial recognition via TLP-MYD88 to authophagic defense. Cell Host Microbe 2014;15, 753–767.
19. Day CL, Abrahams DA, Bunjun R, Stone L, de Kock M, Walzl G, et al. PD-1 Expression on Mycobacterium tuberculosis-specific CD4 T cells is associated with bacterial load in human tuberculosis. Front Immunol. 2018; 9, 1995.
20. Lazar-Molnar E, Chen B, Sweeney KA, Wang EJ, Liu W, Lin J, et al. Programmed death-1 (PD-1)-deficient mice are extraordinarily sensitive to tuberculosis. Proc Natl Acad Sci USA. 2010;107:13402–7.
21. Tousif S, Singh Y, Prasad DV, Sharma P, Van Kaer L, Das G. T cells from programmed death-1 deficient mice respond poorly to Mycobacterium tuberculosis infection. PLoS ONE. 2011;6:e19864.
22. Sakai S, Kauffman KD, Sallin MA, Sharpe AH, Young HA, Ganusov VV, et al. CD4 T Cell-Derived IFN-gamma plays a minimal role in control of pulmonary Mycobacterium tuberculosis infection and must be actively repressed by PD-1 to prevent lethal disease. PLoS Pathog 2016;12:e1005667.
23. Luo Y, Xue Y, Cai Y, Lin Q, Tang G, Song H, Liu W, Mao L, Yuan X, et al. Lymphocyte non-specific function detection facilitating the stratification of Mycobacterium tuberculosis infection. Front Immunol, 2021;19;12:641378
24. Li Y, Xiao Y, Su M, Zhang R, Ding J, Hao X and Ma Y. Role of soluble programmed death-1 (sPD-1) and sPD-ligand 1 in patients with cystic echinococcosis. Exp Ther Med. 2016;11: 251-256.
25. Wang D, Zhou D, Du Q, Liang Q, Wang Q, Fang L, Wang G, Fan Q, Liu B, Zhou J, et al. Aberrant production of soluble inducible T-cell co-stimulator (sICOS) and soluble programmed cell death protein 1 (sPD-1) in patients with chronic hepatitis C. Mol Med Rep. 2013;7:1197–1202.
Received: 15 September2021 / Accepted: 29 December 2021 / Published:15 May 2022
Citation: Oklah Musa S , Gaidan A. M., Saleh R. F. Soluble (PD-1) and Autophagy protein (ATG-5) in patients with tuberculosis. Revis Bionatura 2022;7(2) 8. http://dx.doi.org/10.21931/RB/2022.07.02.9
