2023.08.02.52
Files > Volume 8 > Vol 8 No 2 2023
The Influential Antioxidant Role Of Coenzyme Q10 and Dehydroepiandrosterone against Carbon Tetrachloride Induced Liver Damage In Male Rats
Bassim K.K. Al-Rekabi 1,*, Ali M. Hussein 2, Hatim A.J. Al-Shwilly 3, Qayssar A. Obaid 4
1 Department of Animal Production, College of Agriculture, University of Sumer
2 Department of Pathological Analysis, College of Science, University of Sumer
3 Department of Physiology, College of Medicine, University of Sumer
4 Department of Animal Production, College of Agriculture, University of Sumer
* Correspondance : [email protected]. [email protected]
Available from: http://dx.doi.org/10.21931/RB/2023.08.02.52
ABSTRACT
This study evaluated the protective role of exogenous CoQ10 and DHEA and their combination on CCl4-induced hepatotoxicity in adult male rats. Thirty adult male rats 225-250 grams, 12-14 weeks old, were used in this study and randomly divided into five equal groups, 6 animals each as in the following: Control group (G1): 6 male rats received DMSO 0.5ml/ animal/day orally, First treated group (T1): 6 male rats received daily CCl4 1ml/kg (1:1 olive oil, IP), Second treated group (T2): 6 male rats received CCl4 1ml/ kg and after 1hour injected daily with CoQ10 200 mg/kg IP, Third treated group (T3): 6 male rats received CCl4 1ml/kg and after 1hour injected daily with DHEA 25 mg/kg IP, Fourth treated group (T4): 6 male rats received CCl4 1ml/kg and after 1hour injected daily with a combination of CoQ10 200 mg/kg + DHEA 25 mg/kg IP. The experiment lasted for 28 successive days. The obtained results illustrated that male rats received CCl4 (1ml/kg) caused a significant increase in hepatic enzyme function AST, ALT and ALP, as well as MDA levels, and caused a significant decrease in antioxidant enzyme activity GPx, SOD and CAT levels. In addition, CCl4 also caused various degrees of liver damage, such as dilation and congestion of the central vein with hemorrhage, apparent fatty degeneration and infiltration of inflammatory cells compared to the control group. Whereas, the group treated with CoQ10 200 mg/kg and DHEA 25 mg/kg showed a significant decrease (P< 0.05) in serum AST, ALT and ALP as well as MDA value, and significantly increased in GPx, SOD with the decline in CAT levels compared to the group treated with CCl4 intoxication. It is also observed from the results that the combination of CoQ10 and DHEA caused a highly significant (P < 0.05) decline in AST, ALT and ALP as well as MDA levels, and a significant elevate in GPx, SOD and decline in CAT, and almost return to average level compared to control. As well as, the histopathological examination of the liver revealed that rats treated with CoQ10 and DHEA and their combination had usual central veins and hepatocytes compared to groups treated with CCl4 due to antioxidant, anti-inflammatory and anti-apoptotic properties. It has been concluded that CoQ10 and DHEA have a protective effect against liver damage induced by CCl4 through improving antioxidant enzyme activity in CCl4 treated group leading to a declined MDA level and reduced lipid peroxidation. Thus, CoQ10 and DHEA are potential therapeutic antioxidant agents on hepatotoxicity by suppressing hepatic oxidative stress.
Keywords: CoQ10, DHEA, antioxidant, CCl4, hepatic damage, male rat.
INTRODUCTION
Coenzyme Q10 (CoQ10) is an endogenous substance that acts as a vital antioxidant proposed for cellular membrane integrity by directly reacting with free radicals or regenerating another antioxidant 1. It is a lipid-soluble, vitamin-like substance required for the proper functioning of many organs and chemical reactions in the body 2. It has many beneficial effects on human and animal health, including cardiovascular disease, age-related disorders, neuromuscular and neurodegenerative disorders, autoimmune disorders, DNA damage, thyroid disorders, male infertility, cancers, diabetes, fibrosis, apoptosis, and obesity. It is an important redox and proton translocations constituent of the mitochondrial respiratory chain. It plays an essential role in mitochondrial energy production through redox activity in the electron transport chain, transporting electrons between enzymes. Thus, it plays an essential role in cellular bioenergetics and membrane stabilizer and production of ATP in the oxidative respiration process 3. 4 demonstrated that CoQ10 has anti-inflammatory properties decreasing the production of pro-inflammatory cytokines such as interleukin (IL) and tumor necrosis factor (TNF-α).
Dehydroepiandrosterone (DHEA) is one of the most abundant endogenous circulating steroid hormones with multi-functional properties; it is produced in the adrenal glands, gonads, and brain, where it functions as a metabolic intermediate in the biosynthesis of androgen and estrogen sex steroids 5. It plays a critical endogenous antioxidant and pro-oxidant activity. It can also protect against cell membrane lipid peroxidation (LPO) induced by oxidative damage 6. DHEA also has anti-inflammatory properties via suppression of pro-inflammatory cytokines secretion like IL and TNF-α and regulating the body's immune response 7. DHEA and DHEAS are products of cholesterol metabolism, with the first enzymatic reaction occurring in mitochondria and resulting from the action of cytochrome P450 8. Cholesterol transport across mitochondrial membranes requires the action of steroidogenic acute regulatory protein (STAR) and converts cholesterol to pregnenolone 9. This study aimed to evaluate the ameliorative effect of CoQ10 and DHEA and their combination on hepatotoxicity induced by CCl4 in adult male rats.
MATERIAL AND METHODS
Drugs and chemical reagents
Norfloxacin was obtained from Zwijndrecht-Holland, CoQ10 200 mg and DHEA 25mg (Sigma, St. Louis, MO, USA) and administered intraperitoneally. Dimethylsulphoxide (DMSO) was purchased from Merck, Darmstadt, Germany.
Experimental animals
Thirty adult male rats (Rattus norvegicus) weighing 225-250 grams, 12-14 weeks old. The rats were housed in the animal house of Basrah's College of veterinary medicine/university. They were left for 2 weeks for an adaptation previous to the experiment. Each 6 animal was housed in a cage measuring 15 × 35 × 50 cm and kept under an average temperature of 22 - 28 °C. The daily light period was 12 hours using two fluorescent lamps, and the humidity rate was about 50 %. Animals were provided with water and diet ad libitum.
Experimental Design and study strategy
After the acclimatization period, animals were randomly divided into five equal groups, 6 animals each, as in the following: Control group (G1): 6 male rats received DMSO 0.5ml/animal/day orally, First treated group (T1): 6 male rats received daily CCl4 1ml/kg (1:1 olive oil, IP), Second treated group (T2): 6 male rats received CCl4 1ml/kg and after 1hour injected daily with CoQ10 200 mg/kg IP, Third treated group (T3): 6 male rats received CCl4 1ml/kg and after 1hour injected daily with DHEA 25 mg/kg IP, Fourth treated group (T4): 6 male rats received CCl4 1ml/kg and after 1hour injected daily with a combination of CoQ10 200 mg/kg + DHEA 25 mg/kg IP. The experiment lasted for 28 successive days. All animals in the study were sacrificed at the end of the experiments. However, the rats before sacrifice were first weighed and then anesthetized by placing them in a closed beaker containing cotton sucked with chloroform for anesthesia. To take the samples, the abdominal cavity was opened through a midline abdominal incision. Blood samples were collected via cardiac puncture according to the method of 10. Then, blood samples were dropped directly from the heart using a 5 ml disposable syringe. The blood was put in the plane tube until coagulated, then centrifugated (3000 rpm for 15 minutes) to obtain the serum. The serum samples were separated into many Eppendorf tubes to avoid repeated thawing. All tubes were stored at (-4c) until they were analyzed.
Histopathological examination
Immediately were removed liver and kidneys and separated from surrounding tissues and lipids, then weighed with an electronic balance. Sorted liver fragments were collected from all groups and prepared and fixed using 10% formalin for histological examination according to 11 with the aid of the light microscope. The samples were fixated in natural buffered formalin 10 % for 24-48 hours. They were put in the rotary microtome and were sliced by the microtome, steel blade into sections 5 micrometers thick. Sections were floated on a water bath (50-55o C ) and then transferred into glass slides coated with Mayers albumin as an adhesive substance and left to dry. The histological section of organs was stained with Hematoxylin-Eosin stain and photomicrographs were taken at 40X magnifications.
Statistical analysis
In this study, ANOVA Analysis and LSD tests are used according to (IBM SPSS, version 20) program at (P≤0.05) to find the means for all treatments (IBM SPSS, 2011).
RESULTS
Biochemical assessment
The results in Table 1 demonstrate that male rats who received CCl4 (1ml/kg) caused a significant increase (P< 0.05) in serum AST, ALT and ALP levels compared to the control group. Whereas the groups treated with CoQ10 200 mg/kg and DHEA 25mg/kg showed a significant decrease (P< 0.05) in serum AST, ALT and ALP levels compared to groups treated with CCl4 intoxication, but they were still high significantly (P< 0.05) compared to the control value. It is also clear from Table (1) that combination of CoQ10 and DHEA caused a highly significant declined in AST, ALT and ALP levels and almost returned to their normal level compared with the control value. However, results in Table (2) pointed out that male rats who received CCl4 showed a sharp, significant decrease (P< 0.05) in serum GPx and SOD value compared to the control group. Whereas the groups treated with CoQ10, DHEA, and a combination of CoQ10 and DHEA showed a significant increase (P< 0.05) in serum GPx and SOD levels compared to groups treated with CCl4 intoxication, and they seem they reached statistically to GPx and SOD normal value compared to the control value.
In contrast, Table (2) also showed that male rats who received CCl4 showed significant elevation (P< 0.05) in serum CAT value compared to the control group. Whereas the groups treated with CoQ10, DHEA and a combination of CoQ10 and DHEA showed a significant sharp decline (P< 0.05) in serum CAT values compared to groups treated with CCl4. However, they returned statistically to CAT normal value compared to the control value.
Lipid peroxidation assessment
According to the results obtained in Table (2), serum MDA concentration increased significantly (P<0.05) in male rats that received CCl4 compared to the control group. Whereas the group treated with CoQ10, DHEA and a combination of CoQ10 and DHEA showed a significant decrease (P< 0.05) in serum MDA value compared to the group treated with CCl4, but they still high significantly (P< 0.05) compared with control value.
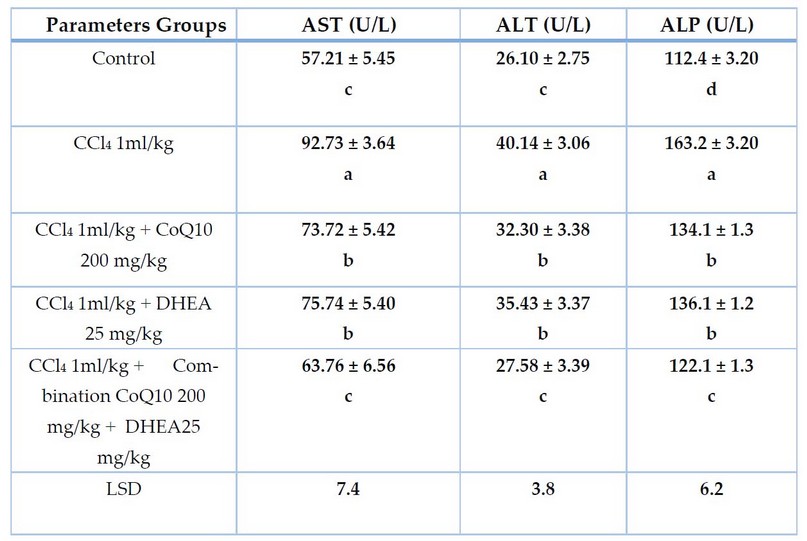
Table 1. The effect of CCl4 and the protective role of CoQ10, DHEA, and their combination on liver function enzymes of male rats for 28 days of exposure. Mean ± SD
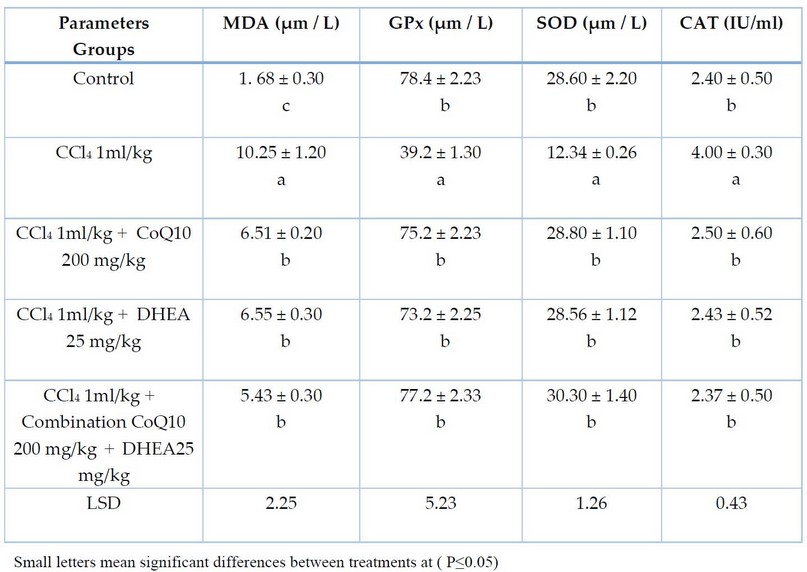
Table 2. The effect of CCl4 and the protective role of CoQ10, DHEA, and their combination on antioxidant enzyme activities of male rats for 28 days of exposure. Mean± SD
Histopathological studies
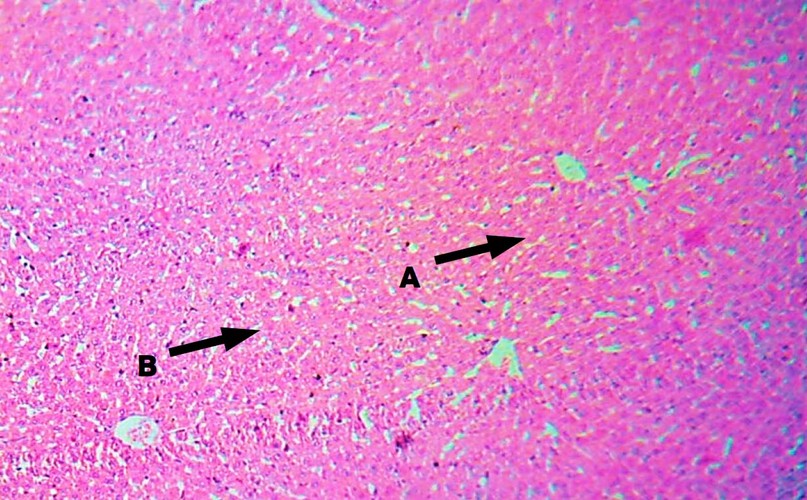
Figure 1. Liver of male rats treated with 0.5 DMSO as a control (H&E Stain, 100 X) observe normal central vein (A), and normal hepatocytes (B).

Figure 2. The liver of male rats treated with CCl4 (T1) for 28 days (H&E Stain, 400X) observed dilation and congestion of the central vein (A), evident fatty degeneration (B), and infiltration of inflammatory cells (C).

Figure 3. The liver of male rats treated with 1m/kg CCl4 + 200 mg/kg CoQ10 for 28 days (H&E stain, 100X) observe usual central vein (A), and normal hepatocytes (B).

Figure 4. The liver of male rats treated with 1m/kg CCl4 + 25 mg/kg DHEA for 28 days (H&E stain, 100X) observed normal central vein (A), and normal hepatocytes (B).
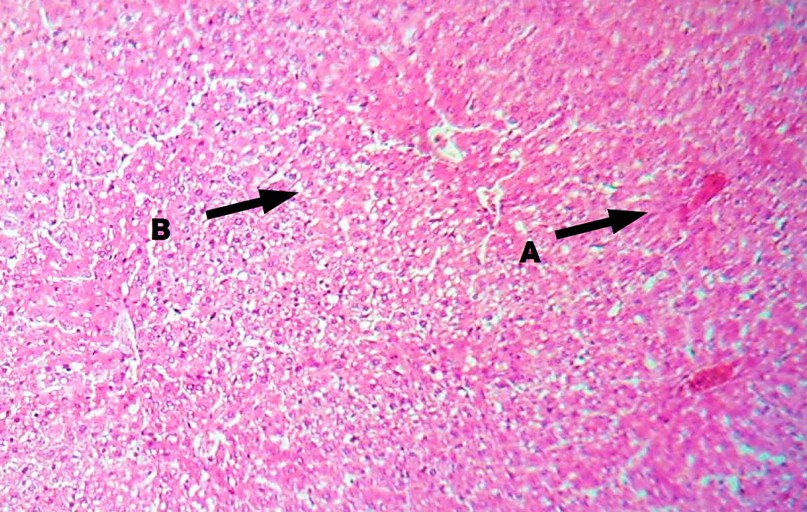
Figure 5. Liver of male rats treated with 1mg/kg CCl4 and a combination of 200 mg/kg CoQ10 + 25 mg/kg DHEA for 28 days (H&E Stain, 100X) observe usual central vein (A), and normal hepatocytes (B).
DISCUSSION
It is clear from the results that CCl4 intoxication caused a significant increase in serum levels of liver function enzyme AST, ALT and ALP activities in male rats may be clearly due to increased serum levels of MDA in this study, where increased lipid peroxidation led to depression in antioxidant and liver enzymes activity compared to the control group. This pointed to detrimental results of CCl4 on the liver, evidenced by an increase of AST, ALT and ALP, which indicate liver injury. Also, 12 have reported damaged liver after streptozotocin treatment, showing a decrease in serum AST, ALT and ALP activity. The CCl4 hepatotoxicity may be affected in two ways, firstly by the incidence of inflammatory conditions and secondly by the direct toxic action of CCl4 on liver cells. The utilization of CoQ10 significantly decreased levels of these enzymes compared to control and CCl4 intoxicated groups. Similar results were reported by 13, who established the role of CoQ10 in hepatotoxicity induced by sodium arsenite in male rats; it also shows that hepatic enzymes are mainly responsive biomarkers directly concerned with existing cellular damage and toxicity due to they are located in the cytoplasm and are released into circulation after cellular damage. It has been known that the increase in AST, ALT and ALP are good indicators of impaired liver function. However, the hepatotoxicity induced by CCl4 may be affected in two ways, firstly by the occurrence of inflammatory condition, and secondly by direct toxic action of CCl4 on the liver cell through trichloromethyl- peroxyl free radicals leading to induce lipid peroxidation and destruction of Ca2+ homeostasis, finally results in cell death 14. Other authors have shown that CoQ10 can elevate the level of AST, ALT and ALP after intoxication; this may be attributed to CoQ10, which stabilizes hepatocytes' plasma membrane and prevent delivery of AST, ALT and ALP to extracellular fluid 15. In contrast, Co-administration of CoQ10 with CCl4 re-establish the AST, ALT and ALP activity in male rats as a sign of protecting the effect CoQ10 against liver injury induced by CCl4 intoxication. (16) reported that CoQ10 characterized the liver as a target organ by distributing CoQ10 into different rat tissues. This effect may be related to powerful CoQ10 effectiveness in suppressing increased lipid peroxidation and the hepatocyte membrane's destruction in regenerating liver cells. Furthermore, CoQ10 shows signs of defense against hepatotoxicity by lower levels of TBARs and alanine release, leading to decreased hepatic enzyme activities 17. This is agreed with 18, who mentioned that CoQ10 has a prophylactic effect against aflatoxin-B1-induced hepatotoxicity. Prophylactic effects of CoQ10 on metabolic stress by inhibition of apoptosis in hepatocytes 19. However, 20, 21, and 22 mentioned that CoQ10 protects against metabolic stress-induced hepatotoxicity induced by acute acetaminophen, mainly probably through its antioxidant, anti-inflammatory, and anti-apoptosis effects. Moreover, CoQ10 shows the anti-inflammatory and antigenotoxic effect on 1,2-dimethyl hydrazine (DMH) induced leukocytic DNA damage in blood cells by direct inhibition and modulating gene expression of cyclooxygenase-2 activity (COX-2) and inducible nitric oxide synthase (iNOS) in the colonic mucosa of male rats 23. In contrast, present results in at similar Table show that supplementation of DHEA with CCl4 reduced serum levels of hepatic enzymes ALT, AST and ALP activities compared to control and CCl4 intoxicated groups. This pointed to a potential protective effect of DHEA against liver injury in adult male rats. While it is stated previously the supplementation with DHEA protects against hepatotoxicity in rats, it may be through its antioxidant, anti-inflammatory, and anti-apoptosis effects 24. Similar results were given by 25. According to 26, the antioxidant role of DHEA may be associated with its active metabolites (DHEA-S), exposing cell membranes more resistant to being attacked by ROS. Moreover, liver tissue characterized the target organ for DHEA on considering circulation of DHEA into various rat tissues as reported previously by 27 after demonstrating copper-induced oxidative lipid peroxidation. These results may be associated with the efficiency of DHEA in suppressing the elevated lipid peroxidation and damage of cell membranes in restored liver cells of rats. The administration of CoQ10 by itself did not lead to a significant decrease in liver damage induced by CCl4 intoxication. It seems from the above Table that the ameliorating effect of DHEA may be attributed to their antioxidant properties that would reduce lipid peroxidation by protecting cellular GSH content.
The results also clearly show that CCl4 caused a significant decrease in the antioxidant enzymes GPx, SOD, and CAT activity in male rats compared to the control group. These results showed that CCl4 causes clear changes in enzymatic GPx, SOD, and CAT constituents of antioxidants due to increased lipid peroxidation, which leads to depression in antioxidant enzyme activity. These results agree with 28 stated that SOD and CAT antioxidants enzymes act as protection agents from oxidative damage by scavenging ROS. 29 who showed that the effect of CCl4 on cellular antioxidant defense system is the second mechanism for CCl4 induced oxidative stress by changes in antioxidant activities by inhibiting function SH groups in the SOD, CAT and GPx enzymes which generally protect against free radical toxicity. However, SOD is a first line of defense against oxygen free radicals and catalyzes superoxide anion radical (O2־˙) into less toxic H2O2 and O2, whereas CAT reduces H2O2 to non-toxic H2O and O2. The CCl4 causes widespread oxidation leading to depletion of GSH, and decreased GSH and GPx activity are led to elevate oxidative damage to DNA, lipids and proteins. Generally, the decline of endogenous antioxidants by exposure to CCl4 could be because CCl4 has a very high affinity for glutathione. Therefore, exposure to CCl4 decreases GSH levels due to either increased use of GSH by cells to act as scavengers of free radicals caused by toxic chemical agents or enhanced utilization of GSH by GPX under oxidative stress which results in increased lipid peroxidation. Also, exposure to CCl4 decreases nitric oxide (NO) generation, and inhibition of NO synthesis leads to an apparent decrease in GSH synthesis through the down-regulation of the rate-limiting enzyme 30.
On the other hand, CoQ10 supplementation significantly increases endogenous antioxidant enzymes GPX, SOD and CAT, which may be due to its direct free radical scavenging activity and decrease in lipid peroxidation as compared to CCl4 intoxicated groups. These results agreed with results obtained by 31, who stated that pretreatment with CoQ10 is related with preserving against isoproterenol induced cardiac hypertrophy in rats heart through decreased myocardial injury by protecting endogenous antioxidant and decreased lipid peroxidation. Whereas 32 concluded that CoQ10 is a powerful antioxidant either directly or indirectly against CCl4 toxicity by suppressing oxidative stress, scavenging oxygen radicals, and inhibiting lipid peroxidation, thus increasing glutathione, GPX, SOD and CAT activities. The current study results agreed with results obtained by many researchers, such as 33 in their study on CoQ10 in adult males with fructose-induced metabolic syndrome, 34 in adult male albino rats fed on high cholesterol diet in the cerebellar cortex. Ameliorating effect of CoQ10 on antioxidant enzymes in the present study may be attributed mainly to antioxidant action, which is known to supplement GSH and antioxidant enzyme levels and scavenge lipid peroxides.
The results of CCl4 caused a significant increase in serum malondialdehyde (MDA) levels. The study results came in agreement with the results of other articles, such as 35,36 and 37, which studied intraperitoneally administration of CCl4 and toxicity effects on lipid peroxidation of male rats. The CCl4 metabolism in the liver results in the generation of reactive metabolites like trichloromethyl (CCl3·) and trichloromethyl peroxy (CCl3OO·) radicals which initiate membrane peroxidation of unsaturated fatty acids and causes fatty liver, fibrosis and cell necrosis. 38 reported that the increase in oxidative stress and lipid peroxidation might be attributed to the depletion and reduction in hepatic GSH content, which has an essential and protective role against oxidative stress. Therefore, CCl4 begins producing free radicals and induces lipid peroxidation in subcellular membrane structures and accumulation of ROS to inhibit the electron transfer respiratory chain in the mitochondria 39. However, 40 concluded that CCl4-induced oxidative stress might be attributed to CCl4-inducing inflammation in tissue with the production of inflammatory mediators, which stimulates the generation of free radicals in tissue. It arises when hydroxyl radicals like oxygen react with membranes' unsaturated lipids resulting in lipid peroxide radicals (ROO•), lipid hydroperoxide (ROOH) generation, and disintegration generates, such as malondialdehyde 41. Thus, increased levels of MDA in our study are a good indicator of oxidative stress and lipid peroxidation caused by CCl4 intoxication. Co-administration of CoQ10 with CCl4 resulted in a significant reduction in the malondialdehyde level. These results indicate that CoQ10 has potent antioxidant activity, as obviously by malondialdehyde reduction. The protective role of CoQ10 against CCl4-induced oxidative stress may be attributed to its potent free radical scavenger activity and inhibit lipid peroxidation or to the regulation of oxidative phosphorylation and prevention of lipid peroxidation. Also, it can normalize endothelial function by combining oxidative phosphorylation in mitochondria and endothelial nitric oxide synthesis activity 42. Furthermore, 43 and 44 demonstrate that CoQ10 decreased the production of ROS, which changes redox balance in cells toward oxidative stress under inflammatory conditions due to its anti-inflammatory and immuno-modulatory action through suppression release and generation of pro-inflammatory cytokine from the cell and increase of anti-inflammatory cytokine mediators such as TNF-α and IL-10. The results also showed that DHEA supplementation reduced the oxidative stress and lipid peroxidation induced by CCl4 toxicity. This was detected by potent antioxidant actions by decreasing MDA levels. 45 demonstrate that reduced MDA concentration may be related to the antioxidant activity of DHEA to inhibit definite enzymes occupied in free radicals formation. Moreover, the ameliorating effect of DHEA against oxidative stress may be attributed to antioxidant properties that would reduce lipid peroxidation by protecting cellular GSH content 46. The antioxidant role of DHEA may be associated with its active metabolites (DHEA-S), exposing cell membranes more resistant to being attacked by ROS. However, DHEA inhibits NADPH level, a substrate essential for NADPH oxidase reaction to generate O•- from O• by inhibiting glucose-6-phosphate dehydrogenase (G-6-PDH). 47 demonstrate that DHEA exerts antioxidant and anti-inflammatory role by reducing tissue susceptibility to the oxidation of both lipid and protein and improving endothelial function, which changes redox balance in cells toward oxidative stress under inflammatory condition due to it has anti-inflammatory and immune modulation properties through suppression release and generation of pro-inflammatory cytokine from cell and increase of anti-inflammatory cytokine mediators such as TNF-α and IL- 6 and IL-10.
It is clear from the results that CCl4 toxicity caused various degrees of liver injury in treated male rats, such as dilation and congestion of the central vein, apparent fatty degeneration of hepatocytes, and infiltration of inflammatory cells after 28 days of exposure compared to the control group. These results followed those obtained by many researchers, such as 48,49,50,51,52,53,54, who studied intraperitoneally administration of CCl4-induced liver damage and hepatotoxicity. The metabolism of CCl4 in the liver results in the generation of reactive metabolites like trichloromethyl (CCl3·) and trichloromethyl peroxy (CCl3OO·) radicals which initiate membrane peroxidation of unsaturated fatty acids and causes fatty liver fibrosis and cell necrosis 55. Conversely, supplementation of CoQ10, DHEA and a combination of CoQ10 and DHEA caused reduced changes. It restructured reliability with the standard architecture of the liver, which may be attributed to antioxidant, anti-inflammatory, and anti-apoptotic activities compared to CCl4 intoxicated groups. The pretreatment with CoQ10 and DHEA improved of degeneration effect with inhibited inflammatory infiltration and nearly restored them to normal architecture, possibly due to prevented CCl4 conversion into its reactive metabolites like trichloromethyl (CCl3·) and trichloromethyl peroxy (CCl3OO·) radicals, reduced oxidative stress with scavenging its free radical, and protected hepatic antioxidant enzymes activities 56.
CONCLUSIONS
The group treated with CoQ10 200 mg/kg and DHEA 25 mg/kg showed a significant decrease (P< 0.05) in serum AST, ALT and ALP as well as MDA value and a significantly increased in GPx, SOD with a decline in CAT levels compared to the group treated with CCl4 intoxication. It is also observed from the results that the combination of CoQ10 and DHEA caused a highly significant (P < 0.05) decline in AST, ALT and ALP as well as MDA levels, and a significant elevate in GPx, SOD and decline in CAT, and almost return to average level compared to control. As well as, the histopathological examination of the liver revealed that rats treated with CoQ10 and DHEA and their combination had normal central veins and hepatocytes compared to groups treated with CCl4 due to antioxidant, anti-inflammatory and anti-apoptotic properties. It has been concluded that CoQ10 and DHEA have a protective effect against liver damage induced by CCl4 through improving antioxidant enzyme activity in CCl4 treated group leading to a declined MDA level and reduced lipid peroxidation. Thus, CoQ10 and DHEA are potential therapeutic antioxidant agents on hepatotoxicity by suppressing hepatic oxidative stress.
REFERENCES
1 Ali, S.A.; Faddah, L.; Abdel-Baky, A. and Bayoumi, A.: Protective effect of L-carnitine and coenzyme Q10 on CCl4-induced liver injury in Rats. Sci. Pharm. 2010; 78(4): 881-896.
2 Tran, M.T.; Mitchell, T.M.; Kennedy, D.T.and Giles, J.T.: Role of coenzyme Q10 in chronic heart failure, angina, and hypertension. Pharmacotherapy., 2001; 21(7):797-806.
3 López, L., Quinzii, C., Area, E., Naini, A., Rahman, S., Schuelke, M., Salviati, L., Dimauro, S . and Hirano, M.: Treatment of CoQ10 deficient, fibroblasts with Ubiquinone, CoQ analogs, and vitamin C: time- and compound-dependent effects. PLOS One, 2010; 5(7): 897-903.
4 Sayed-Saleh, A.B.; Shahin, M.I. and Kelada, N.A.: Hepato- protective effect of taurine and coenzyme Q10 and their combination against acrylamide-induced oxidative stress in rats. Tropical Journal of Pharmaceuti- cal Research August., 2017; 16 (8): 1849-1855.
5 Prough, R.A.; Clark, B.J. and Klinge, C.M.: Novel mechanisms for DHEA action. J Mol Endocrinol., 2016; 56(1): 139-155.
6 Kim, B.M.; Yim, S.H.; Jeong, S.J.; Choi, Y.S.; Nam, Y.S.; Jeong, J.H.; Yun, S.W.; Do, JH; Lim, H.M. and Park, E.S.: Pro-oxidantive effect of dehydroepiandrosterone on indomethacin induced acute gastritis in rats. Biomolecules and Therapeutics., 2009; 17(1): 57-61.
7 Du, M.C.; Khalil, W. and Sriram, S.: Administration of Dehydroepiandrosterone suppresses experimental allergic encephalomyelitis in SJL/J Mice. J.immunology ., 2017;167:7094-7101.
8 Miller, W.L. and Auchus, R.J.: Molecular Biology, Biochemistry, and Physiology of Human Steroidogenesis and Its Disorders. Endocr Rev., 2011; 32(1): 81–151.
9 Yehya, W. A. . Seasonal Monumental Insects Accompanying Euphrates Poplar Leaves. Journal of Life Science and Applied Research.2020, 1, 45-53..
10 Hoff, J. and Rlatg, L. Methods of blood collection in the mouse. J. Lab. Anim., 2000; 29: 47-45.
11 Mescher , A. L. Junqueira,s basic histology text and atlas. 12th edition, 2010 : 1 -5 .
12 Aragno, M.; Tamagno, E.; Gatto, V.; Brignardello, E.; Parola, S.; Danni, O. and Boccuzzi, G. Dehydroepiandrosterone protects tissues of streptozotocin treated rats against oxidative stress. Free Radic Biol Med., 1999; 26:1467-1474.
13 Adil, M.; Visnagri, A.; Shiva Kumar, V.; Kandhare, A.M.; Ghosh, P. and Bodhankar S.L. The protective effect of naringin on sodium arsenite induced testicular toxicity via modulation of biochemical and perturbation in experimental rats. DOI,10.5567, 2014; pharmacologia. 222-234.
14 Johnson, D.E. and Kruening, C. Ethanol feeding stimulates trichloromethyl radical formation from CCL4 in cultural rat hepatocytes. Pharmacol. Toxicol., 1998; 83: 231-239.
15 Okuyama, Y.; Shinzawa, H.; Ukai, K.; Ono, k. and Yamada, N. Changes of Cu, Zn-SOD and PLO in the regenerating liver after partial hepatoctomy-effect of coenzymeQ10 administration. J. Jap. Soci .Gastrol., 1991; 88 : 1208-15.
16 Scalori, V.; Alessandri, MG; Giovannini, L. and Bertelli, A. Plasma and tissue concentrations of coenzymeQ10 in the rat after intravenous, oral and topical administration.Int.J.Tiss.Read., 1990; 12:149-154.
17 Zamora, R., Hidalgo, F.J. and Tappel, A.L. Comparative antioxidant effectiveness of dietary B-carotene, vitamin E, selenium and coenzymeQ10 in rat erthyocytes and plasma. J.Nutr., 1991; 121:50-6.
18 Kheir-Eldin, A.A.; Motawi, T.M and Sadik, N.A. Effect of some natural antioxidant on aflatoxin-B1 induced hepatic toxicity. EXCLI Journal., 2008; 7:119-131.
19 Vasiliev, A.V., Martinova, E.A., Sharanova, N.V. and Gapparov, M.M. Effects of coenzyme Q10 on rat liver cells under conditions of metabolic stress. Bull Exp Biol Med., 2011; 150(4):416-9.
20 Fouad, AA and Jresat, I. Hepatoprotective effect of coenzyme Q10 in rats with acetaminophen toxicity. Environ Toxicol Pharmacol. 2012; 33(2):158-67.
21 Esfahani, S.A.; Bagheri, F.; Emami, Y.; Esmaeilzadeh, E.; Azarpira, N.; Hassanabadi, N.; Keshtkar, M.; Farjam,M.; Koohi-Hosseinabadi, O. and Noorafshan, A. Protective effects of coenzyme Q10 on thioacetamide induced acute liver damage and its correlation with behavioral, biochemical, and pathological factors. Iran Red Crescent Med J., 2016; 18(8): 29166.
22 Tarry-Adkins, J.L.; Fernandez-Twinn, D.S.; Hargreaves, I.P.; Neergheen, V.; Aiken, C.E.; Martin-Gronert, M.S.; McConnell, J.M. and Ozanne, S.E. Coenzyme Q10 prevents hepatic fibrosis, inflammation, and oxidative stress in a male rat model of poor maternal nutrition and accelerated postnatal growth. Am J Clin Nutr., 2016;103:579-88.
23 Kim, J,M. and Park, E. Coenzyme Q10 attenuated DMH-induced precancerous lesions in SD rats. J Nutr Sci Vitamino., 2010; 56(2):139-44.
24 Ding, X.; Yu, L.; Ge, C. and Ma, H. Protective effect of DHEA on hydrogen peroxide-induced oxidative damage and apoptosis in primary rat Leydig cells. Oncotarget., 2017; 8 ( 10): 16158-16169.
25 Heibashy, M.I.A. and Mazen, G.M.A. Role of dehydroepiandr- osterone on oxidative stress biomarker in CCl4 induced acute liver injury in rats. Isotope and Rad. Res., 2011; 43(4): 891-902.
26 Aragno, M., Parola, S., Brignardello, E., Mauro, A., Tamagno, E., Manti, R., Danni, O. and Boccuzzi, G. Dehydroepiandrosterone prevents oxidative injury induced by transient ischemia/reperfusion in the brain of diabetic rats. Diabetes., 2000; 49:1924-1931.
27 Boccuzzi, G.; Aragno, M.; Seccia, M.; Brignardello, E.; Tamagno, E.; Albano, E.; Danni, O. and Bellomo, G. Protective effect of dehydroepiandrosterone against copper-induced lipid peroxidation in the rat. Free Radic Biol Med., 1997; 22:1289-1294.
28 Bakan, N.; Taysi, S.; Yilmaz, O.; Bakan, E.; Kuskay, S.; Uzun, N.; and Gundogdu, M. Glutathione peroxidase, glutathione reductase, Cu–Zn superoxide dismutase activities, glutathione, nitric oxide, and malondialdehyde concentrations in serum of patients with chronic lymphocytic leukemia. Clin. Chim. Acta, 2003; 338, 143–149.
29 Karthikeyan, R., Anantharaman, P., Chidambaram, N., Balasubramanian, T. and Somasundaram, S.T. Padina boergessenii ameliorates carbon tetrachloride induced nephrotoxicity in Wistar rats. Journal of King Saud University – Science., 2012; 24, 227–232.
30 Karadeniz, A.; Yildirim , A.; Karakoc, A.; Kalkan, Y. and Celebi, F. Protective effect of Panax ginseng on carbon tetrachloride induced liver, heart and kidney injury in rats. Revue Méd. Vét., 2009;160 (5):237-243.
31 Ghule , A.E.; Kulkami , C.P.; Bodhankar , S.L . and Pandit , V.A. Effect of pretreatment with Co- enzyme Q10 on isoproterenol- induced cardiotoxicity and cardiac hyper trophy in rats. J. Current therapeutic Research ., 2009; 70(6): 460-471.
32 Ognjanovic, B.I.; Markovic, S.D.; Pavlovic, S.Z.; Zikic, R.V., Stajn, A.and Saicic, Z.S. Combined effects of coenzyme Q10 and vitamin E in cadmium induced alterations of antioxidant defense system in the rat heart.Environ Toxicol Pharmacol., 2006; 22: 219-224.
33 Mansour, S. M.; Zaki, H.F. and El-Denshary, E. S. Beneficial effects of CoQ10 and rosiglitazone in fructose-induced metabolic syndrome in rats. Bulletin of Faculty of Pharmacy, Cairo University., 2013; 51: 13-21.
34 Abd El-Haleem, M.R.; Yassen, QY and Raafat, N. Protective role of coenzyme Q 10 against high cholesterol diet induced histological and biochemical changes in cerebellar cortex of adult albino rats. IOSR Journal of Dental and Medical Sciences., 2014; 13(5): 50-61.
35 Al-Seeni, M.N., El Rabey, H.A., Zamzami, M.A. and Alnefayee, A.M. The hepatoprotective activity of olive oil and Nigella sativa oil against CCl4 induced hepatotoxicity in male rats. BMC Complementary and Alternative Medicine., 2016; 16,438:2-14.
36 Laouar, A.; Klibet, F.; Bourogaa, E.; Benamara, A.; Boumendjel, A.; Chefrour, A. and Messarah, M. Potential antioxidant properties and hepatoprotective effects of Juniperusphoenicea berries against CCl4 induced hepatic damage in rats. Asian Pacific Journal of Tropical Medicine., 2017; 10(3):263-269.
37 Kokhdan, E.P., Ahmadi, K., Sadeghi, H., Sadeghi, H., Dadgary, F., Danaei, N. and Aghamaali, M.R. Hepatoprotective effect of Stachys pilifera ethanol extract in carbon tetrachloride induced hepatotoxicity in rats. Pharmaceutical Biology., 2017; 55 (1): 1389-1393.
38 Kodai, S., Takemura, S., Minamiyama,Y., Hai, S., Yamamoto, S., Kubo, S., Yoshida,Y., Niki, E., Okada, S., Hirohashi, K. and Suehiro, S. S-allyl cysteine prevents CCl4-induced acute liver injury in rats. Journal Free Radical Research, 2007; 41(4): 31-22.
39 Kamel, H.H.; Azza, H. A.; Walaa, M.S. A. and Amira, H. M. Protective Effect of some Antioxidants against Ccl4-Induced Toxicity in Liver Cells from BRL3A Cell Line. Journal of American Science., 2010; 6(10). 992 -1003.
40 Ma, JQ, Li, Z., Xie, W.R., Liu, C.M. and Liu, S.S. Quercetin protects mouse liver against CCl4 induced inflammation by the TLR2/4 and MAPK/NF-kappaB pathway. Int Immunopharmacol., 2015; 28(1):531-539.
41 Pandey, G., Jain, G.C. and Mathur, N. Therapeutic potential of metals in managing diabetes mellitus: A review. J. Mol. Pathophysiol., 2012; 1: 63-76.
42 Scialò, F.; Fernández-Ayala, D.J. and Sanz, A. Role of Mitochondrial Reverse Electron Transport in ROS Signaling: Potential Roles in Health and Disease. Frontiers in Physiology., 2017;8(428):1-7.
43 Weber, C. Dietary intake and absorption of coenzyme Q. In, Kagan V, Quinn P(eds): Coenzyme Q: Molecular Mechanisms in Health and Disease. Boca Raton, FL: CRC Press., 2001; 209-215.
44 Jung, H.J.; Park, E.H. and Lim, C.J. Evaluation of anti-angiogenic, anti-inflammtory and antinociceptive activity of coenzymeQ10 in experimental animals. J.Pharm.Pharmacol., 2009; 61:1391-1395.
45 Luchetti, C.G.; Paz, D.A. and Motta, A.B. Metformin prevents the increase of nitric oxide and lipid peroxidation induced by DHEA in early pregnant mice. The Open Reproductive Science Journal., 2013; 5: 14-22.
46 Gümüş, M., Celebi, F., Böyük, A., Gürsan, N. and Akçay, F. Dehydroepiandrosterone ameliorates hepatocellular damage in obstructive jaundice. Cell Biochem Funct. 2010; 28(6): 515-520.
47 Pélissier, M.A., Muller, C., Hill, M. and Morfin, R. Protection against dextran sodium sulfate-induced colitis by dehydroepiandrosterone and 7α-hydroxy-dehydroepiandrosterone in the rat. Steroids., 2006; 71(3):240-248.
48 Antony, B.; G.; Santhakumari, G.; Merina, B.; Sheeba, V. and Mukkadan, J. Hepatoprotective effect of Centella asiatica (L) in carbon tetrachloride induced liver injury in rats. Indian. J .Pharmacology. Sci ., 2006; 68( 6) :772-776.
49 Lee, C.; Park, S.; Kim, Y.S.; Kang, S.S.; Jeong, A.H.; Kim, J.A.; Lee, S.H. and Lee, S. Protective mechanism of glycyrrhizin on acute liver injury induced by carbon tetrachloride in mice .Biol. Pharm. Bull., 2007; 30(10) :1898-1904.
50 Noorani, A.A Mulla, K. and Patil. SD Hepatoprotective effect of Cocculus hirsutus linn. against carbon tetrachoride induced liver damage in albino wistar rats. IJPI's J. of Pharmacol. and Toxicol ., 2010; 1(1):1-7.
51 Gurusamy, K.; Kokilavani, R. and Arumuasamy, K. Hepatoprotective activity of polyherbal formulation against CCl4 induced hepatotoxicity in rats .Afr J. of Biotechnol., 2010; 9 (49) : 8429-8434.
52 Khan, M.R., Khan, G.N. and Ahmed, D. Evaluation of antioxidant and fertility effects of Digera muricata in male rats. African Journal of Pharmacy and Pharmacology., 2011; 5(6): 688-699.
53 Ben Hsouna, A., Saoudi, M., Trigui, M., Jamoussi, K., Boudawara, T. and Jaoua, S. Characterization of bioactive compounds and ameliorative effects of Ceratonia siliqua leaf extract against CCl4 induced hepatic oxidative damage and renal failure in rats. Food Chem Toxicol., 2011; 49 (12): 3183-3191.
54 Kale, I., Khan, M.A., Irfan, Y. and Goud, VA Hepatoprotective potential of ethanolic and aqueous extract of flowers of Sesbania grandiflora (Linn) induced by CCl4. Asian Pac J Trop Biomed., 2012; 2(2): 670-S679.
55 Mustafa, H.N., El Awdan, S.A.; Gehan A. Hegazy,G.A. and Abdel Jaleel, GA Prophylactic role of coenzyme Q10 and Cynara scolymus L on doxorubicin induced toxicity in rats: Biochemical and immunehisto- chemical study. Indian J Pharmacology., 2015; 47 (6): 649-656.
56 Yubero, D.; Montero, R.; Martín, MA; Montoya, J.; Ribes, A.; Grazina, M.; Trevisson, E.; Rodriguez-Aguilera, J.C.; Hargreaves, I.P. and Salviati, L. CoQ deficiency study group. Secondary coenzyme Q10 deficiencies in oxidative phosphorylation (OXPHOS) and non-OXPHOS disorders. Mitochondrion., 2016; 30: 51-58.
Received: May 15, 2023/ Accepted: June 10, 2023 / Published: June 15, 2023
Citation: Al-Rekabi , B.K.; Hussein ,A.M.; Al-Shwilly ,H.A.J.; Obaid ,Q.A. The Influential Antioxidant Role Of Coenzyme Q10 And Dehydroepiandrosterone Against Carbontetracholride Induced Liver Damage In Male Rats. Revis Bionatura 2023;8 (2) 52. http://dx.doi.org/10.21931/RB/2023.08.02.52
