2023.08.02.84
Files > Volume 8 > Vol 8 No 2 2023
Evaluation of the fungicidal activity of the aqueous extracts of some medicinal plants against Fusarium spp
1Directorate of Diyala Agriculture, Ministry of Agriculture, Iraq
2College of Science, University of Baghdad, Iraq; [email protected]
3College of Agriculture, University of Diyala, Iraq; [email protected]
4Directorate of Diyala Agriculture, Ministry of Agriculture, Iraq; [email protected]
*Correspondence: ; [email protected];
Available from: http://dx.doi.org/10.21931/RB/2023.08.02.84
ABSTRACT
To assess the performance of the aqueous extracts of Rhus coriaria, Boswellia carterii, Nigella sativa and Aloe vera. Three concentrations (5%, 10%, and 15%) for each extract were tested in vitro for their activity against three isolates of Fusarium spp. All extracts have a high inhibitory capacity against tested isolates. An inhibition percentage for selected isolates was increased with concentrations (5%, 10%, 15%). The concentration of Rhus coriaria extract 15 % resulted in a significant increase in inhibition percentage of mycelial growth of Fusarium sp.1 (63.17%), Fusarium sp2 (61.69%) and Fusarium sp3 (59.35%) compared to other concentrations, the concentration of Boswellia carterii extract 10% led to a significant increase in inhibition percentage of mycelial growth of Fusarium sp2 (82.45 %) compared to concentration 5% (73.67 %), the concentration of Nigella sativa extract 15% was recorded high inhibition percentage in Fusarium sp2 (66.15 %) compared to concentration 5% (22.21%). In contrast, concentrations 5% and 10% were recorded highest inhibition percentages in Fusarium sp3, reaching 31.73 % and 22.02%, respectively, compared to concentration 15% (4.90%).
Keywords: Rhus coriaria, Boswellia carterii, Nigella sativa, Aloe vera and Fusarium spp
INTRODUCTION
Plant diseases cause considerable damage during growth of plants, resulting in a reduction in yield and produce quality1. Fusarium species exist in the soil both in the tropical and temperate regions. They survive in the soil as chlamydospores and mycelia; they attack the roots and settle in the xylem, leading to stunting, wilting, and plant death, thus leading to severe damage 2,3. The Wilting via Fusarium is a significant disease that damages sesame plants worldwide. Excessive use of chemical fertilizers causes environmental pollution besides affecting the quality of fruit3. There is a critical need to reduce the use of chemical pesticides to limit environmental pollution and improve plant growth; it has become indispensable to choose methods that are not harmful to the environment to enhance the health and validity of crops, so botanical extracts and some microorganisms are the best alternatives to these hazardous chemicals. Rhus coriaria L., the sumac plant, belongs to the family Anacardiacea. Aqueous sumac extract includes anthocyanins, hydrolyzable tannins, flavonoids, isoflavonoids, terpenoids, butein, iridoid and derivatives of coumarin5. Boswellia carteri, commonly known as olibanum tree or frankincense, is a deciduous middle-sized tree belonging to the order Sapindales and the family Burseraceae6,7. Black cumin (Nigella sativa) belongs to the family Ranunculaceae and is commonly known as black seed. N. sativa contains tannins, phloba tannins, flavonoids, transanethole, terpenoids, apiole and thymoquinone8. Aloe vera is from the Lilaceae family that contains 200 active compounds, including minerals, enzymes, vitamins, amino acids, salicylic acid, saponin, lignin, sugar and anthraquinones, besides over 75 component nutrients9. Bio-control already exists in nature, and it is an eco-friendly approach and effective against various pathogens and pests. Besides, it can be successfully exploited in integrated disease and pest management2. Our aim of the trial was to test the effect of aqueous plant extracts of viz. Rhus coriaria, Boswellia carterii, Nigella sativa and Aloe vera against three isolations of Fusarium sp.
MATERIAL AND METHODS
Plant collection and fungus isolation
A laboratory experiment was conducted at the plant pathology Lab in the Diyala Agriculture Directorate in 2020; the plant materials such as R. coriaria, B. carterii, and N. sativa were collected from the local market of Baqubah, Diyala, the A. vera plant was obtained from a house garden. The pathogenic fungi Fusarium sp1, Fusarium sp2 and Fusarium sp3 were isolated from sesame plant roots from three regions viz Salah Elden, Baghdad and Diyala, respectively.
Preparation of aqueous extracts
Each powder of R. coriaria, B. carterii, and N. sativa at a rate of 50 g was soaked in 200 ml distilled water for 24 hours; each of them and 50 g of A. vera gel were blended in the blender for homogeneity and mixing, then filtered through two layers of clothes, then placed in the centrifuge at a speed of 3000 RPM for 3-10 minutes and the supernatants were preserved in the refrigerator until use.
Poisoned food test
To assess the performance of extracts against the tested fungi, the technique of poisoned food using three concentrations of each extract 5, 10, and 15 % were prepared with PDA medium (Potato Dextrose Agar), then poured separately into petri plates (9 cm), the control treatment included only PDA medium, after the medium became solid, agar discs (6 mm) of F. sp1, F.sp2 and F. sp3 from the seven-day-old culture were transferred to a petri plates, then incubated at 25±2 Co for seven days. The tests were performed in three replications for each concentration against all of the fungi, and the diameters mean of fungus growth was measured. The inhibition ratio was calculated according to the following formula.
% Inhibition = (1- control colony/ treatment colony) X 100
Statistical analysis
The Statistical Analysis System (SAS (2012) program was used to detect the effect of different factors on study parameters. The least significant difference –LSD test (Analysis of Variation-ANOVA) was used to compare between means in this study 10 significantly.
RESULTS
The recorded results in Table (1) and Fig (1) revealed that R. coriaria concentrations led to a significant increase in the inhibition percentage of mycelial growth, where a concentration of 15 % was significantly superior in the inhibition percentage of F.sp1, F.sp2 and F.sp3, which reached 63.17%, 61.69% and 59.35%) respectively. In comparison, a concentration of 10 % led to a significant increase in the inhibition percentage of mycelial growth of F.sp1 (46.41%) compared to a concentration of 5 % (32.45%), whereas no significant differences between concentrations 5 and 10% for both F. sp2 and F. sp3.
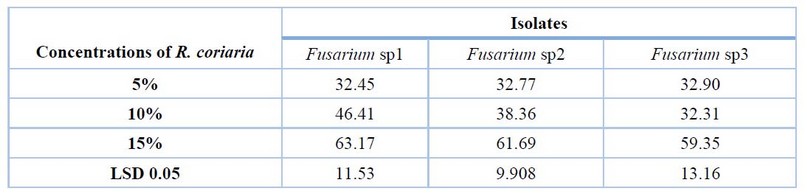
Table 1. Effect of various concentrations of R. coriaria in vitro on growth inhibition of Fusarium sp1, Fusarium sp2 and Fusarium sp3
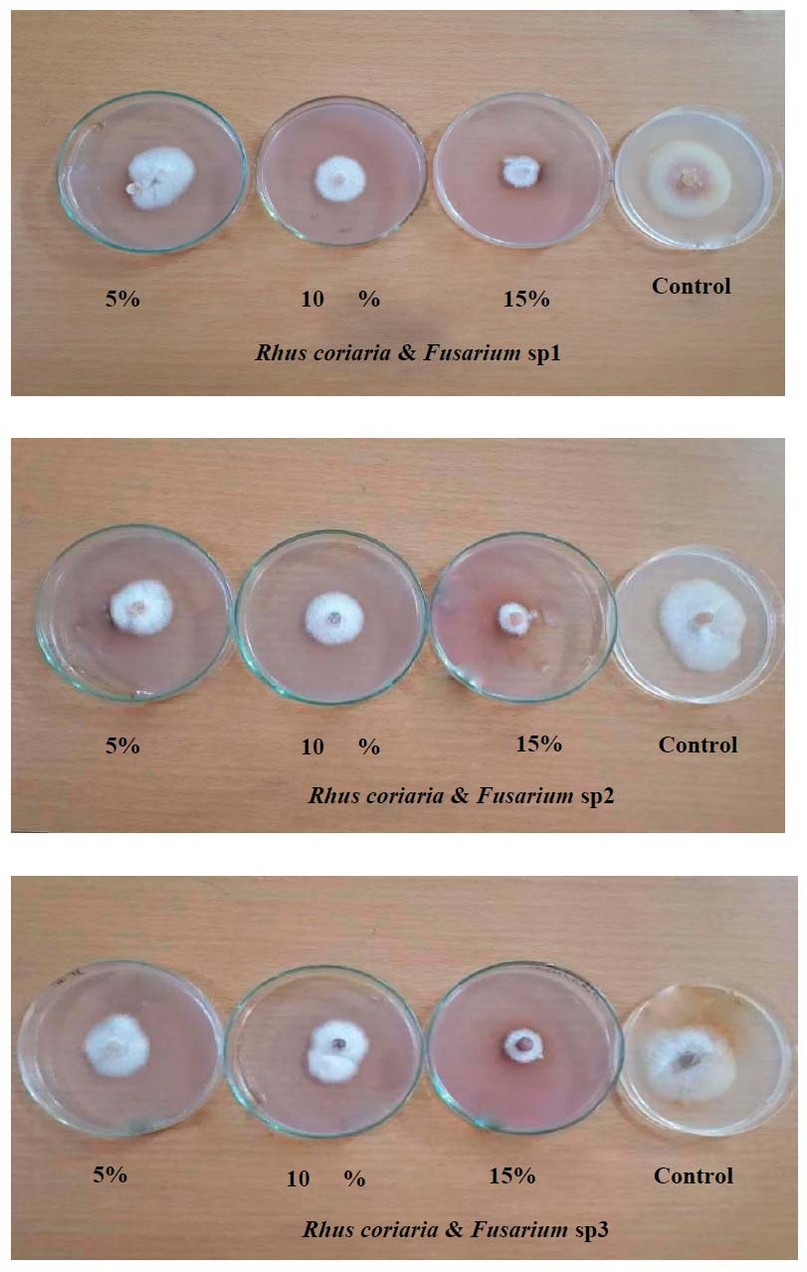
Figure 1. Effect of various concentrations of R. coriaria in vitro on growth inhibition of Fusarium sp1, Fusarium sp2 and Fusarium sp3
Table (2) and Fig (2) shows that a concentration of 10% of B. carterii resulted in a significant increase in the inhibition percentage of mycelial growth of F.sp2 (82.45 %) compared to the concentration of 5% (73.67 %). In contrast, there were no significant differences between all concentrations in inhibition percentage of mycelial growth of F.sp1 and F.sp3.
From Table (3) and Fig (3), N. sativa concentrations showed an increment in the inhibition percentage of mycelial growth of all fungi; a concentration 15% was recorded with a high inhibition percentage in F.sp2 reached 66.15 %) compared to a concentration 5% (22.21%). In comparison, concentrations 5% and 10% were recorded as the highest inhibition percentage in F.sp3, reaching 31.73 % and 22.02%, respectively, compared to concentration 15% (4.90%), whereas no significant differences between all concentrations in F.sp1.
A. vera concentrations showed an increment in inhibition percentage of mycelial growth of all fungi, with no significant differences between all concentrations (Table 4 and Fig 4).
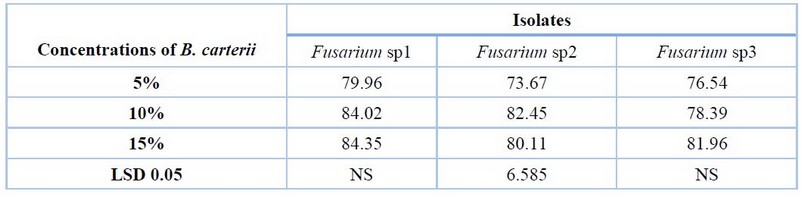
Table 2. Effect of various concentrations of B. carterii in vitro on growth inhibition of Fusarium sp1, Fusarium sp2 and Fusarium sp3
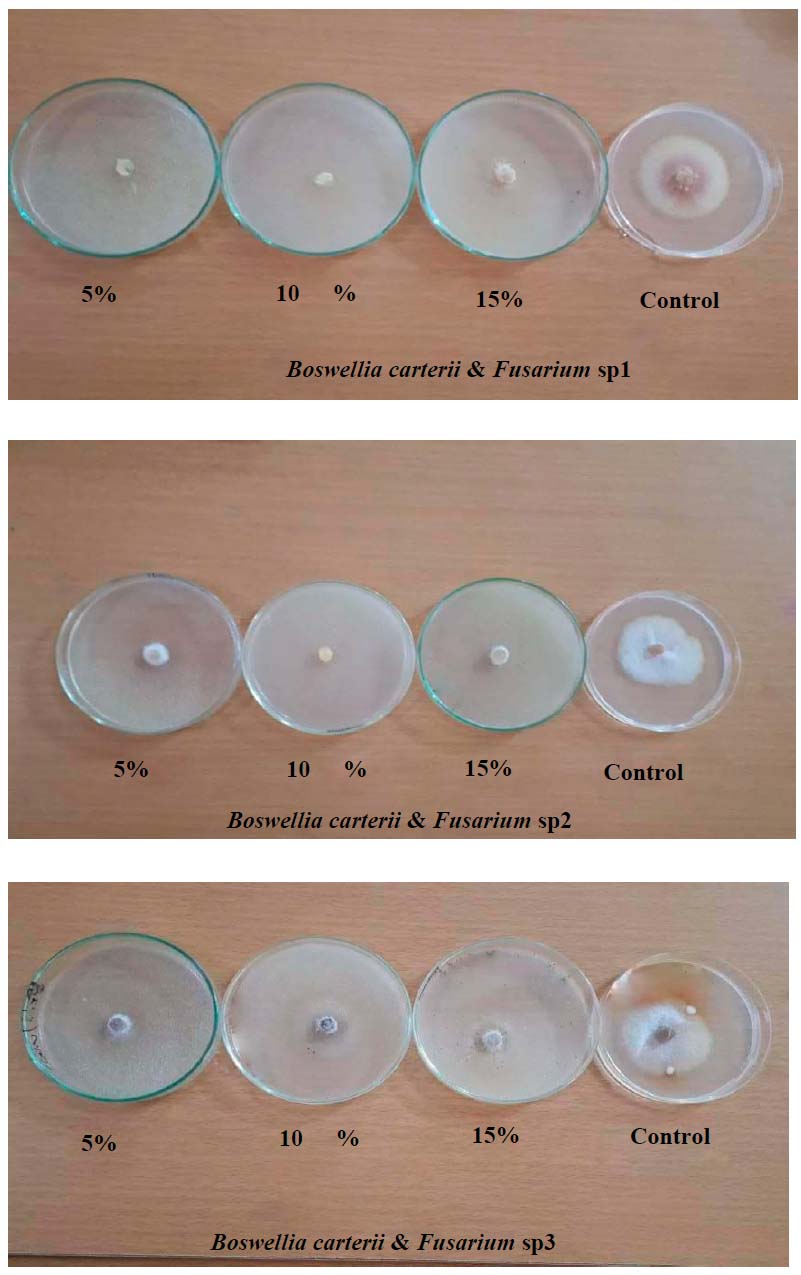
Figure 2. Effect of various concentrations of B. carterii in vitro on growth inhibition of Fusarium sp1, Fusarium sp2 and Fusarium sp3
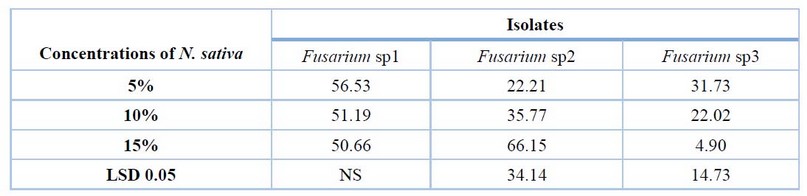
Table 3. Effect of various concentrations of N. sativa in vitro on growth inhibition of Fusarium sp1, Fusarium sp2 and Fusarium sp3
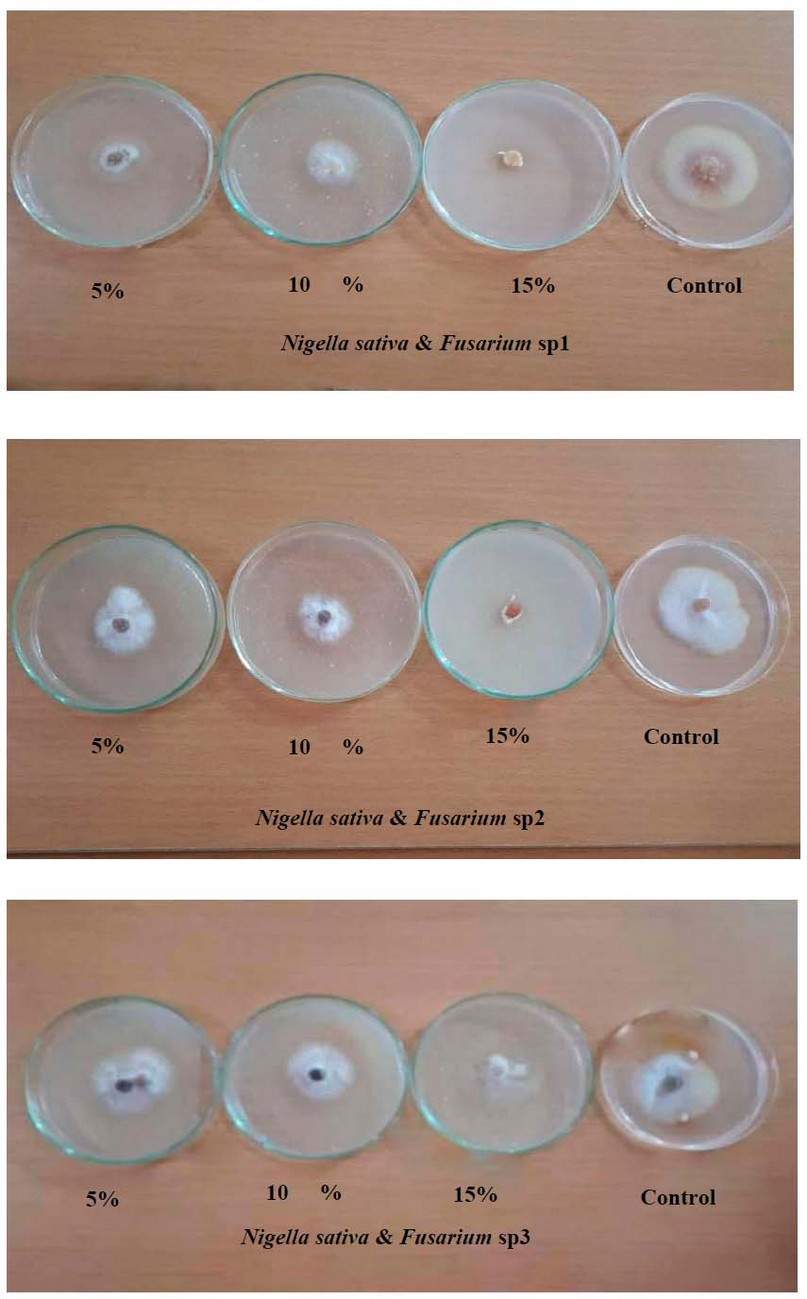
Figure 3. Effect of various concentrations of N. sativa in vitro on growth inhibition of Fusarium sp1, Fusarium sp2 and Fusarium sp3
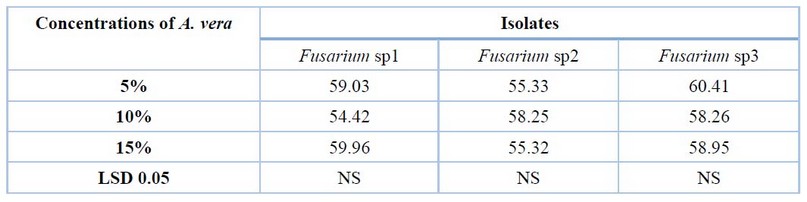
Table 4. Effect of various concentrations of A. vera in vitro on growth inhibition of Fusarium sp1, Fusarium sp2 and Fusarium sp3
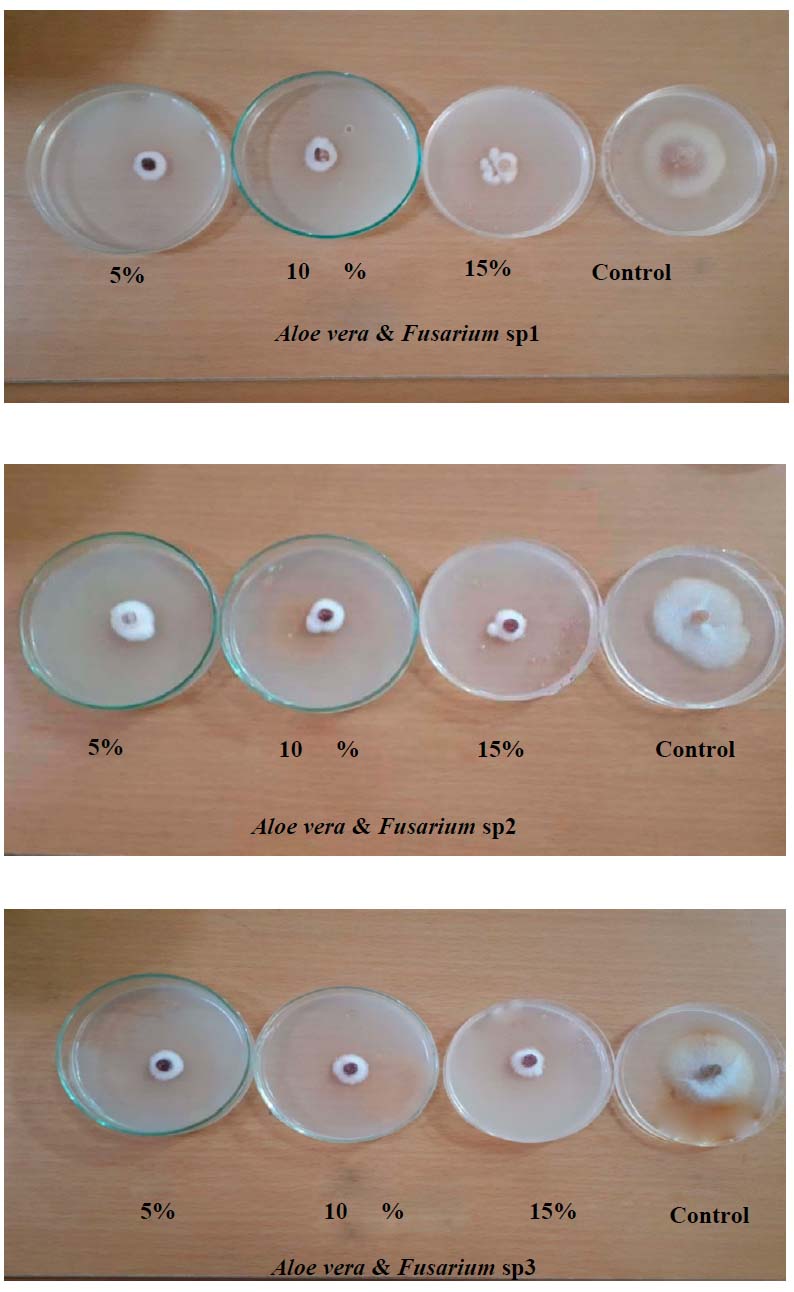
Figure 4. Effect of various concentrations of A. vera in vitro on growth inhibition of Fusarium sp1, Fusarium sp2 and Fusarium sp3
DISCUSSION
The methanolic extract of Rhus coriaria showed the highest inhibition in the fungi Aspergillus flavus, Candida albicans and Penicillium citrinin 11.12 reported that Rhus coriaria extract inhibited the growth of Penicillium citrinum, Aspergillus flavus and Candida albicans . 13 stated the superior inhibitory activity of Rhus Mueller against Fusarium oxysporum f. sp. lycopersici. The antimicrobial activity of R. coriaria extract against food pathogens has been proved in numerous of vitro studies14. Rhus coriaria extract has more effective antifungal activity than the standard antifungal Nystatin15. The presence of phenolic and flavonoid constituents in R. coriaria suppresses enzyme actions and cellular membrane functions of pathogenic fungi 16.
The B. carteri extract demonstrated effectiveness against Fusarium solani, Helminthosporium rostratum and Alternaria alternate in vitro, where the scanning electron microscopy proved the existence of ultra-structural variations in a treated mycelia with extract of B. carterii comparison with mycelia which untreated 17. An oil of Boswellia serrata led to inhibition of the following fungi such as Fusarium verticillioides, Fusarium equiseti, F. oxysporum, Fusarium udum, Fusarium lateritium, Penicillium citrinum, Aspergillus ochraceous, Aspergillus geophila, Aspergillus brassicola, Aspergillus fumigatus, Aspergillus terreus, Curvularia tetramera, Penicillium expansum and Penicillium citrinum 18. The antifungal activity of the essential oil of B. carter against Candida albicans, Aspergillus niger and Cryptococcus neoformansi was the result of the presence of a-copaene, p-cymene, d-cadinene, b-caryophyllene and limonene with at various ratios 19.
It can use N. sativa as an eco-friendly fungi-toxicant against Macrophomina phaseolina and Fusarium oxysporum because it has considerable potential as fungicidal20. Antifungal activity of N. sativa oil was determined against eight fungi viz., Fusarium oxysporum, Fusarium moniliforme, Fusarium semitectum, Fusarium nivale, Alternaria alternata, Aspergillus niger, Aspergillus flavus, Drechslera hawiensis21. Proteins of N. sativa have a significant effect on the fungal cell permeability22. N. sativa oil showed more efficacies in sporulating and reducing mycelial growth of Aspergillus flavus 23.
Aloe vera extract significantly affected the inhibition of mycelial growth of Fusarium udum, reaching 55.9% 24. Aloe vera extract has antifungal activity against Fusarium oxysporum, Penicillium gladioli, Botrytis gladiolus and Heterosporium prune 25. 26 found that the Aloe vera is effective against Fusarium oxysporum, Collectotrichum coccodes and Rhizoctonia solani. Aloe vera extract can inhibit the growth of fungi such as Alternaria alternata, Alternaria citri and Alternaria tenuissima 27. Aloe vera extract had an antifungal effect on Aspergillus glaucus, Aspergillus flavus, Candida albicans, Candida tropicalis, Trichophyton rubrun and Trichophyton mentagrophytes28. Aloe vera gel caused a significant reduction in the growth of pathogenic fungi viz., Penicillium digitatum, Alternaria alternata, Aspergillus flavus, Aspergillus niger and Drechslera hawaiensis (Sitara et al. 2011).
CONCLUSION
The present investigation suggests that aqueous extracts such as R. coriaria, B. carterii, N. sativa and A. vera have great potential as a natural fungicide, but further studies are required.
REFERENCES
1. Salim H.A., Simon S. and Lal A.A. Integrated diseases management (IDM) against tomato (Lycopersicon esculentum L.) Fusarium wilt. J Environ Agric Sci , 2017, 11: 29-34
2. Salim H.A. and Simon S. Effect of carbendazim and solarized soil with Pseudomonas fluorescens spent mushroom compost against Fusarium oxysporum f.sp. lycopersici in Tomato, European academic research, 2015, vol. II, Issue 12,p15997-16010
3. Salim H.A., Salman I.S., Jasim B.N. IPM Approach for the management of wilt disease caused by Fusarium oxysporum f. sp. lycopersici on tomato (Lycopersicon esculentum). Journal of Experimental Biology and Agricultural Sciences, 2016, 4: 742–747.
4. Wasniker A.R., Deshmukh M.R. and Sharma S.M. Toxicity of fungal metabolites on germination and growth of sesame. Current Res-UAS Bangalore, 1991, 23:185–186 .
5. Abu-Reidah I.M., Ali-Shtayeh M.S., Jamous R.M., Arráez-Román D., Segura-Carretero A.. HPLC–DAD–ESI–MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem, 2015, 166, 179–191.
6. Thulin M.. “Boswellia sacra in IUCN 2012”. IUCN Red List of Threatened Species. Version 2012.2. International Union for Conservation of Nature and Natural Resources 1998.
7. Niebler J. and Buettner A. Identification of odorants in frankincense (Boswellia sacra Flueck.) by aroma extract dilution analysis and two-dimensional gas chromatography–mass spectrometry/ olfactometry. Phytochemistry 109: 66–75 (2015).
8. Aftab A, Yousaf Z, Javaid A, Rabbani A, Ahmed S, and Khan F. Nigella sativa from traditional to contemporary medicine- a review. Intl. J. Biol. Biotechnol, 2018, 15: 237‒254.
9. Park YI and Jo TH. The perspective of industrial application of Aloe vera. In: New Perspective on Aloe. (Eds.): Y.I. Park and S.K. Lee. Springer Verlag, New York, USA, 2006, pp: 191-200. ISBN: 0387317996.
10. SAS. Statistical Analysis System, User's Guide. Statistical. Version 9.1th ed. SAS. Inst. Inc. Cary. N.C. USA ,2012.
11. Singh S. Genesis and development of DPPH method of antioxidant assay. J. Food Sci.Technol. 2011, 48, 412–422 .
12. Onkar S, Mohammed A, Nida A. New antifungal aromatic compounds from the seeds of Rhus coriaria L. Int. Res. J. Pharm , 2011, 2, 188–194 .
13. de Rodríguez D.J., Trejo-González F.A., Rodríguez-García R., Díaz-Jimenez M.LV., Sáenz-Galindo A., Hernández-Castillo F.D., Peña-Ramos F.M. et al. Antifungal activity in vitro of Rhus muelleri against Fusarium oxysporum f. sp. Lycopersici. Ind. Crops Prod, 2015, 75, 150–158 .
14. Nasar-Abbas S.M and Halkman A.K.Antimicrobial effect of water extract of sumac (Rhus coriaria L.) on the growth of some food borne bacteria including pathogens. Int. J. Food Microbiol; 2004, 97: 63-69 .
15. Ertürk O. Antibacterial and antifungal effects of alcoholic extracts of 41 medicinal plants growing in Turkey. Czech J. Food Sci , 2010, 28, 53–60.
16. Gabr S. A. and Alghadir A. H. Phytochemical Analysis and In vitro Antifungal Activities of Bioactive Fractions from Leaves of Rhus coriaria (SUMAC), Journal of Pure and Applied Microbiology, 2015, Vol. 9(Spl. Edn. 1), p. 559-565.
17. Al-Otibi F., Alharbi R. I, Albasher G., Almeer R. and Alsaggabi N. S.Antifungal Potential of Aqueous Extract of Boswellia carteri, Fatimah et al. J Pure Appl Microbiol , 2019, 13(4), 2375-2381 .
18. Venkatesh HN, Sudharshana T.N, Abhishek R.U, et al. Antifungal and antimycotoxigenic properties of chemically characterized essential oil of Boswellia serrata Roxb. ex Colebr. Int. J. Food Prop 20(sup2):, 2017, 1856-68.
19. Powers C.N, Osier J.L, McFeeters R.L. et al.Antifungal and cytotoxic activities of sixty commercially-available essential oils. Molecules , 2018, 23(7). https://doi. org/10.3390/molecules23071549 .
20. Aftab A., Yousaf Z., Javaid A., Riaz N., Younas A., Rashid M., Shamsheer H.B. and Chahel A.A. Antifungal activity of methanolic extracts of Nigella sativa against Fusarium oxysporum and Macrophomina phaseolina and its phytochemical profiling by GCMS analysis. Intl. J. Agric. Biol, 2019, 21: 569‒576 .
21. Sitara U, Niaz I, Naseem J and Sultana N.Antifungal effect of essential oils on in vitro growth of pathogenic fungi, Pak. J. Bot., 2008, 40(1): 409-414.
22. Shokri H.. A review on the inhibitory potential of Nigella sativa against pathogenic and toxigenic fungi. Avicena J. Phytomed 6: 2016, 21‒33 .
23. Amrouche A, Benmehdi H, Moussaoui A, Mebarki K, Chaoufi A, Saneba A, Lazouni A, Chabane H. and Sari D.. Evaluation of the antifungal activity of some oils from Algerian medicinal plants against Aspergillus flavus strain produced aflatoxins, Journal of Applied Pharmaceutical Science, 2011, 01 (08): 48-53.
24. Devi T. R. and Chhetry G.K.N. Evaluation of antifungal activities of certain plant against Fusarium udum Butler causing wilt in pigeonpea (Cajanus cajan (L.) Millsp.), International Journal of Scientific and Research Publications, 2012, Volume 2, Issue 6 :1-4 .
25. Cassian O.R, Parvu M, Vlase L. and Tamas M. Antifungal activity of Aloe vera leaves. Fitoterapia , 2007,78(3): 219-222 .
26. Jasso de Rodriguez D, Hernandez-Castillo D, Rodriguez-Gracia R and Angulo-Sanchez JL. Antifungal activity In vitro of Aloe vera pulp and liquid fraction against plant pathogenic fungi. Industrial Crops and Products, 2005, 21(1): 81-87 .
27. Bajwa R and Shafique S. Appraisal of antifungal activity of Aloe vera. Mycopath, 2007 , 5(1): 5-9 .
28. Coopoosamy R.M. and Magwa M. L. Traditional use, antibacterial activity and antifungal activity of crude extract of Aloe excelsa. African Journal of Biotechnology, 2007, 6(20): 2406-2410.
29. Sitara U, Hassan N. and Naseem J. Antifungal activity of Aloe vera gel against plant pathogenic fungi, Pak. J. Bot., 2011, 43(4): 2231-2233 .
Received: May 15, 2023/ Accepted: June 10, 2023 / Published: June 15, 2023
Citation: Salim H, Alsaady M, Al-zuhairi A, Kassoub F. Evaluation of the fungicidal activity of the aqueous extracts of some medicinal plants against Fusarium spp. Revis Bionatura 2023;8 (2) 84. http://dx.doi.org/10.21931/RB/2023.08.02.84
