2023.08.02.46
Files > Volume 8 > Vol 8 No 2 2023
Evaluation of serum Selenium level as a risk factor for Colorectal cancer
Hussam H. Hassan 1, Ali A. H. Albakaa 2 and Khwam R Hussein 2,*
1 Southern Technical University, Faculty of Graduate Studies, Iraq 1; [email protected] .
2 Southern Technical University, Al-Nasiriyah Technical Institute, Iraq 2; [email protected].
* Correspondence: [email protected]; 07810412664.
Available from: http://dx.doi.org/10.21931/RB/2023.08.02.46
ABSTRACT
Selenium, an antioxidant enzyme component, has been shown to protect against colorectal cancer risk. A diet is the primary source of these antioxidants, and selenium level is inversely related to colorectal cancer risk and may be responsible for around 50% of colorectal cancer risk. The study aims to evaluate selenium levels as a marker for colorectal cancer risk. The participants in this study were 180 individuals, comprising patients and healthy people, separated into two distinct groups: The first comprised 90 cases, 47 of them were men, and 43 were female patients. The second group had 90 healthy individuals, including 60 men and 30 women. All individuals were subjected to blood sampling to determine serum selenium by using Flame Atomic Absorption Spectrometer. The mean serum selenium concentration in the colorectal cancer group was significantly lower (P< 0.01) than in healthy control people. The result shows a strong association between low levels of selenium and the risk of colorectal cancer.
Keywords: Selenium, Colorectal cancer
INTRODUCTION
Cancer is a disease characterized by the unregulated proliferation of aberrant cells. When this kind of tumor arises in the colon or rectum, it is termed colorectal cancer or bowel cancer. It's a malignant tumor in the colon or rectum, accounting for around two-thirds and one-third of all diagnosed individuals, respectively. About 95% of them are adenocarcinomas of the glandular epithelium 1,2. Selenium (Se) is a trace element essential for human health. Diet is the primary source of Se, and intake is determined by the concentration of dietary sources and the quantity ingested. The daily-recommended requirement for selenium is 55 mg for females and 70 mg for males 3.
Selenium is a constituent of selenoproteins, one of which is an antioxidant. The human genome has 25 selenoprotein genes. Glutathione peroxidases (GPx) are selenoprotein enzymes that help protect cells from reactive oxygen species 4,5. Selenium is present in animals as selenocysteine and selenomethionine. The amount of selenium in the diet varies depending on where plants grow, and animals thrive. The primary source of selenium is inorganic selenates found in blackcurrant, onion, common walnut, and pistachio, which are then transformed into organic forms 6. Most of the selenium compounds we eat are absorbed from the duodenum, with a smaller amount in the jejunum and ileum 7.
Human health is linked to excess and deficit selenium intake 8. Low selenium levels in the soil in the affected region likely resulted in low selenium levels in local wheat crops. Selenium insufficiency has been proposed as a probable cause of Keshan's illness, cardiovascular disease, infertility, degenerative disorders, and cognitive decline 5. According to the studies, there is a strong relationship between low selenium levels and the risk of colorectal cancer, and it may protect against CRC progression through anti-oxidative actions 9,10. Human selenium poisoning (selenosis) has been recorded in areas with high selenium in the soil. Chronic intoxication causes emaciation, hair and hoof loss, joint erosion, heart atrophy, cirrhosis, and anemia 11. A more significant selenium (Se) level is linked to a decreased risk of CRC, especially in areas with low Se concentration in soils 12. Several studies have shown that Se status affects gene and protein expression in the antioxidant reactions, immunological role, inflammatory pathways, cell growth and death, and cellular mobility 13. In addition, selenoproteins contribute to better CRC survival by regulating programmed cell death and inhibiting angiogenesis. As a result of these features, it has been suggested that Se compounds may be helpful in cancer therapy 14. The study aims to evaluate selenium levels as a marker for colorectal cancer risk.
MATERIALS AND METHODS
This study was conducted at Tumors Oncology/ Al-Habooby Teaching Hospital and Department Surgery/ Al-Hussein Teaching Hospital in Thi-Qar province, south of Iraq. One hundred eighty participants in a case-control study, comprising patients and healthy people, were separated into two groups: the first group included 90 patients comprising 47 males and 43 females. The second group involved 90 healthy individuals, 60 of whom were men and 30 were women. A blood sample (5 milliliters) was taken from the subjects, where it was allowed to clot before being centrifuged at 3000 revolutions per minute for 10 minutes. All tube samples were kept at (-20°C) deep-freezing until they were analyzed. Serum Selenium levels were measured by Flame Atomic Absorption Spectrometer (FAAS). Laboratory tests were completed in the Department of Chemistry, College of Science at the University of Thi-Qar.
Consent was obtained from each patient participating in this study to fulfill the international research ethical criteria. The study's data were analyzed using IBM Statistical Package for Social Sciences (SPSS) Statistics software, version 27. All statistical comparisons were made using independent t-tests, and one-way ANOVA and numeric variables were reported as mean and Standard deviation. In contrast, Chi-square (X2) test was used to compare the frequency with a P-value ≤0.05 considered statistically significant.
RESULTS
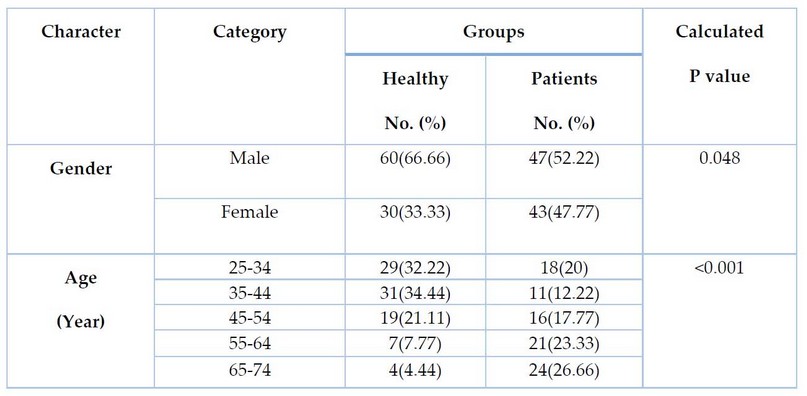
Table 1. Demographic Characteristics of study groups (patients and controls)
Table 1 shows the statistical distribution (frequency & percentage) of study groups by sex and age. The descriptive statistics and differences of study groups by sex were significant differences between patients and control groups (P-value = 0.048). The same table revealed a highly significant (p <0.001) difference in the age of the patients' group as compared to the control group, and the highest percentage of the age subgroup was (65-74 years).
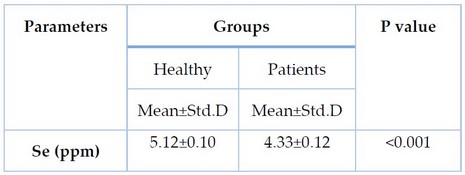
Table 2. Serum Selenium levels between CRC Patient and control group
Table 2 exhibits the differences in the measurement of serum selenium between CRC patients and the control group. The mean of the selenium concentration in the CRC group was significantly lower (p < 0.001) than that of the healthy group.
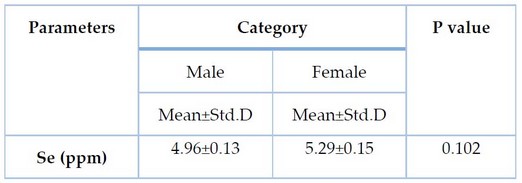
Table 3. Serum Selenium levels according to the gender among CRC patients
Table 3 reveals no significant differences (P-value = 0.102) in serum selenium levels between men and women among CRC patients.
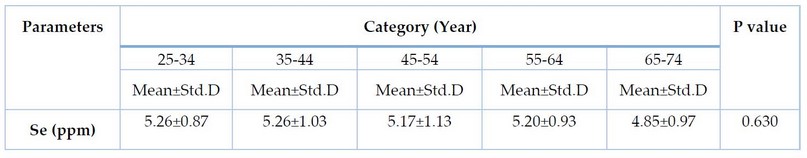
Table 4. Levels of Selenium according to the age among CRC patients
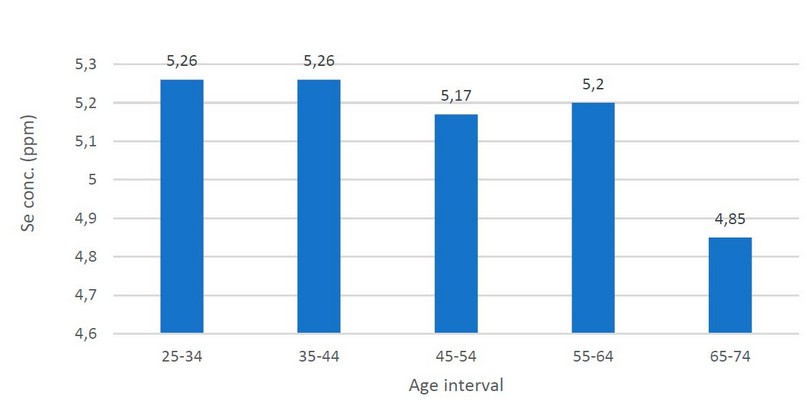
Figure 1. Bar chart for statistical distribution (Mean) of selenium according to the age among CRC patients
Table 4 shows no significant differences (P-value = 0.630) in serum selenium levels according to age among CRC patients. Figure 1 shows the statistical distribution (Mean) of selenium according to the age among CRC patients.
DISCUSSION
The descriptive statistics and differences in study groups by sex were significant differences between patients and control groups (P-value = 0.048). Females have a low incidence of colorectal cancer, and colonoscopy studies reveal fewer colorectal adenomas than males. Colon lesions vary by sex, with right-sided malignancies common in women associated with a high diet of carbohydrates and fat, which are more challenging to identify in screening 15,16,17. On the other hand, left-sided lesions are more prevalent in males and individuals with familial adenomatous polyposis, corresponding with high protein consumption 18,19. The Nutritional Prevention of Cancer Trial discovered that selenium supplementation had an inverse relationship with overall cancer incidence only in males 20. Gender effect modification may be plausible owing to possible sex variations in selenium metabolism; women have been shown to have more excellent excretion rates than males 21. In one earlier observational study, Knekt 22 identified an inverse relationship between selenium and CRC incidence in males.
In contrast, another found a positive relationship in women only 23. However, two studies found an inverse correlation between men and women 24,25. To solve this issue, more research with appropriate numbers is required.
According to the present findings, the most significant proportion of patients was among the age group 65–74 years. The current investigation supported the findings of Wong, 26, who found a link between CRC risk and advanced age. On the other hand, the results disagreed with Gondran et al. 27, who claimed that the age-specific incidence of CRC rose dramatically among those aged 35-64 years compared with ≥65 years. Similarly, Singh et al. 28 have observed that most CRC patients were males aged 40–60 years, with 30% being under 40 years old.
Regarding Se, the study results disagree with those of Bizerea-Moga 29, who evaluated serum selenium concentrations in healthy people and found non-normal selenium distribution in the overall population and a significant difference in selenium levels among age groups. The median selenium levels in participants over the age of 66 were up to 31% higher than in younger participants, and they were more unevenly distributed. The two groups must have the same age distribution to compare selenium levels in healthy people with those in CRC patients. Therefore, the selenium levels among CRC patients were compared, and it was found that individuals over 65 years had lower selenium levels than others. The study also found that CRC incidence rose significantly among individuals over 65. As a result, lower selenium levels in the elderly may be linked to an increased risk of CRC cancer.
The outcome of the current research was consistent with the findings of previous studies 30,31,32, which indicated that the concentrations of Se have significantly been lowered in patients with CRC—selenium, whose function is partly mediated by its incorporation into a family of selenoproteins (SePs). As several SePs perform antioxidant activities, they may protect against CRC progression via their anti-oxidative properties. A greater Se level has been related to a decreased risk for CRC. However, the role of selenium in survival following colorectal cancer diagnosis hasn't been thoroughly investigated. The cohort study evaluated prediagnostic circulating Se level correlations with overall and colorectal cancer-specific mortality. It concluded a high prediagnostic Se concentration at an optimum (100–150 µg/L) might be linked to a better prognosis in CRC patients 12. Dietary components, such as vitamin D, are thought to impact the progression and development of CRC 33,34.
Later researchers found that dietary selenium intakes per capita were likewise adversely connected with death rates from other cancer forms, including CRC, at recommended daily consumption levels of 55 mg/day 35,36,37. Although specific research found that selenium supplementation lowers CRC incidence, this relationship was not generally seen 31. Conversely, Se supplementation was linked to an increased risk of type 2 diabetes (T2D). These investigations were not the first to relate elevated blood Se to an increased risk of T2D in Se-deficient people 38. As a result, extensive selenium supplementation is discouraged since it may have negative consequences.
Additionally, the bioavailability of Se may be influenced by the different food formulations utilized in these distinct clinical trials. The bioavailability of selenium as selenomethionine is roughly double that of selenium as selenite. As a result, the relationship between Se supplementation and the development of CRC may be unclear, necessitating additional clinical investigation 39.
CONCLUSIONS
There was a highly significant relation between Se levels and CRC but a non-significant association between these levels and the age and sex of the patients.
Funding: This research received no external funding
REFERENCES
1 Firth M.;Blackmore T.; Chepulis L.; Keenan R.; Stokes T.; Elwood M.; Weller D.; Emery J.; Lawrenson R. Why does New Zealand have such poor outcomes from colorectal cancer?: the importance of the prediagnostic period. Journal of Primary Health Care. 2021 Mar 31;13(1):15-26. doi:10.1071/HC20049.
2 Degett T, H. Thea Helene Degett Short-term outcome after acute colorectal cancer surgery – risk factors and prediction Supervisors. PhD Thesis : Ismail Gögenur Lene Hjerrild Iversen. Published online 2019.
3 Zachara B, A. Selenium in Complicated Pregnancy. A Review. Vol 86. Elsevier Ltd; 2018. doi:10.1016/bs.acc.2018.05.004
4 Phiri F, P.; Ander E, L.; Lark R, M.; Bailey E, H.; Chilima B.; Gondwe J.; Joy E, J.; Kalimbira A, A.; Phuka J, C.; Suchdev P,S.; Middleton D, R. Urine selenium concentration is a useful biomarker for assessing population level selenium status. Environment International. 2020 Jan 1;134:10518. doi:10.1016/j.envint.105218
5 Rattanachaiwong S.; Singer P. Diets and Diet Therapy: Trace Elements. Elsevier; 2018. doi:10.1016/B978-0-08-100596-5.21941-0
6 Kieliszek M. Selenium–fascinating microelement, properties and sources in food. Molecules. 2019;24(7). doi:10.3390/molecules24071298
7 Hall J, O. Sulfur. Third Edit. Elsevier Inc.; 2018. doi:10.1016/B978-0-12-811410-0.000350
8 Yim S, H.; Clish C, B.; Gladyshev V, N. Selenium Deficiency Is Associated with Pro-longevity Mechanisms. Cell Rep. 2019;27(9):2785-2797.e3. doi:10.1016/j.celrep.2019.05.001
9 Lener M, R.; Gupta S.; Scott R, J. Can selenium levels act as a marker of colorectal cancer risk? BMC Cancer. 2013;13. doi:10.1186/1471-2407-13-214
10 Luo H.; Fang Y, J.; Zhang X. Association between Dietary Zinc and Selenium Intake, Oxidative Stress-Related Gene Polymorphism, and Colorectal Cancer Risk in Chinese Population - A Case-Control Study. Nutr Cancer. 2021;73(9):1621-1630. doi:10.1080/01635581.2020.1804950
11 Lahoop Q, R. Estimation of Some Minerals and Trace Elements Levels in Patients Infected by Hereditary Hemoglobinopatheic in Basrah and Thi-Qar Governorates / Iraq. Thesis. Southern Technical University. Published online 2020.
12 Baker J, R.; Umesh S.; Jenab M.; Schomburg L.; Tjønneland A.; Olsen A.; Boutron-Ruault MC, Rothwell J, A.; Severi G.; Katzke V.; Johnson T. Prediagnostic Blood Selenium Status and Mortality among Patients with Colorectal Cancer in Western European Populations. Biomedicines. 2021 Nov;9(11):1521. doi.10.3390
13 Peters K, M.; Carlson B, A.; Gladyshev V, N.; Tsuji P, A. Selenoproteins in colon cancer. Free Radic Biol Med. 2018;127:14-25. doi:10.1016/j.freeradbiomed.2018.05.075
14 Bhattacharjee A.; Basu A.; Biswas J.; Sen T.; Bhattacharya S. Chemoprotective and chemosensitizing properties of selenium nanoparticle (Nano-Se) during adjuvant therapy with cyclophosphamide in tumor-bearing mice. Mol Cell Biochem. 2017;424(1-2):13-33. doi:10.1007/s11010-016-2839-2
15 Secretan B, L.; Vilahur N.; Bianchini F. Special Report The IARC Perspective on Colorectal Cancer Screening. Published online 2018. doi: 10.1056
16 White A.; Ironmonger L.; Steele R.; Ormiston-smith N.; Crawford C.; Seims A. A review of sex-related differences in colorectal cancer incidence , screening uptake , routes to diagnosis , cancer stage and survival in the UK. Published online 2018:1-11. doi:10.1186
17 Selby K.; Levine E, H.; Doan C.; Gies A.; Brenner H.; Quesenberry C.; Lee J, K.; Corley D, A. Effect of sex, age, and positivity threshold on fecal immunochemical test accuracy: a systematic review and meta-analysis. Gastroenterology. 2019 Dec 1;157(6):1494-505. doi:10.1053
18 Grobbee E, J.; Wieten E.; Hansen B, E.; Stoop E, M.; Wijkerslooth T, R.; Lansdorp-Vogelaar I.; Bossuyt P, M.; Dekker E.; Kuipers E, J.; Spaander M, C. Fecal immunochemical test-based colorectal cancer screening: The gender dilemma. United European gastroenterology journal. 2017 Apr;5(3):448-54. Doi:10.1177/2050640616659998
19 Hultcrantz R. Aspects of colorectal cancer screening , methods , age and gender. BMC Cancer. 2020. doi:10.1111/joim.13171
20 Duffield-Lillico A, J.; Reid M, E.; Turnbull B,W.; Combs G, F.; Slate E, H.; Fischbach L,A.; Marshall J,R.; Clark L, C. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiology and Prevention Biomarkers. 2002 Jul 1;11(7):630-9.
21 Waters D, J.; Chiang E, C.; Cooley D, M.; Morris J, S.; Making sense of sex and supplements: differences in the anticarcinogenic effects of selenium in men and women. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2004 Jul 13;551(1-2):91-107. doi:10.1016/j.mrfmmm
22 Knekt P.; Aromaa A.; Maatela J. Serum selenium and subsequent risk of cancer among finnish men and women. J Natl Cancer Inst. 1990;82(10):864-868. doi:10.1093/jnci/82.10.864
23 Garland M.; Morris J, S.; Stampfer M, J.; Colditz G, A.; Spate V, L.; Baskett C, K.; Rosner B.; Speizer F, E.; Willett W, C.; Hunter D, J. Prospective study of toenail selenium levels and cancer among women. J Natl Cancer Inst. 1995;87(7):497-505. doi:10.1093/jnci/87.7.497
24 Ghadirian P.; Maisonneuve P.; Perret C. A case-control study of toenail selenium and cancer of the breast, colon, and prostate. Cancer Detection and Prevention. 2000 ;24(4):305-313. PMID: 11059562.
25 Jacobs E, T.; Jiang R.; Alberts D, S.; Greenberg E, R.; Gunter E, W.; Karagas M, R.; Lanza E.; Ratnasinghe L.; Reid M, E.; Schatzkin A.; Smith-Warner S, A. Selenium and colorectal adenoma: Results of a pooled analysis. J Natl Cancer Inst. 2004;96(22):1669-1675. doi:10.1093/jnci/djh310
26 Abdulateef SM, Atalla OK, L-Ani QA, Mohammed TH, Abdulateef FM, Abdulmajeed OM. Impact of the electric shock on the embryonic development and physiological traits in chick's embryo. Indian Journal of Animal Sciences. 2021;90(11):1541-5.
27 Gondran G.; Fauchais A, L.; Lambert M.; Ly K.; Launay D.; Queyrel V.; Benazahari H.; Liozon E.; Loustaud-Ratti V.; Hachulla E.; Jauberteau M, O.; Hatron P. Y.; Vidal E. Primary Sjögren’s syndrome in men. Scandinavian Journal of Rheumatology. 2018; 37(4), 300–305. https://doi:10.1080/03009740802001426
28 Singh S.; Kumar R.; Kumar U.; Kumari R. Clinical Significance and Role of TK1, CEA, CA 19-9 and CA 72–4 levels in Diagnosis of Colorectal Cancers. Asian Pacific J Cancer Prev. 2020;21(11):3133-3136. doi:10.31557/APJCP.2020.21.11.3133
29 Bizerea-moga T, O.; Pitulice L.; Bizerea-spiridon O.; Moga T, V. Evaluation of serum selenium status by age and gender: A retrospective observational cohort study in western Romania. Nutrients. 2021;13(5). doi:10.3390/nu13051497
30 Short S, P.; Pilat J, M.; Williams C, S. Roles for selenium and selenoprotein P in the development, progression, and prevention of intestinal disease. Free Radic Biol Med. 2018;127:26-35. doi:10.1016/j.freeradbiomed.2018.05.066
31 Luo H.; Fang Y, J.; Zhang X.; Feng X, L.; Zhang N, Q.; Abulimiti A.; Huang C, Y.; Zhang C, X. Association between Dietary Zinc and Selenium Intake, Oxidative Stress-Related Gene Polymorphism, and Colorectal Cancer Risk in Chinese Population - A Case-Control Study. Nutr Cancer. doi:10.1080/01635581.2020.1804950
32 Ulrike Peters.; Yumie Takata. Selenium and the Prevention of Prostate and Colorectal Cancer. Bone. 2011;23(1):1-7. doi:10.1002/mnfr.200800103
33 Muzny D, M.; Bainbridge M, N.; Chang K.; Dinh H, H.; Drummond J, A.; Fowler G.; Kovar C, L.; Lewis L, R.; Morgan M, B. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330-337. doi:10.1038/nature11252
34 Ali, A. F. .; Mohammed, T. T. .; Al-Bandar, L. K. . The role of optifeed®, vêo® premium, and oleobiotec® in diets for improvement of the production performance of male broilers in heat stress. JLSAR 2022, 3, 32-36.
35 Kuria A.; Fang X.; Li M.; Han H.; He J.; Aaseth J, O.; Cao Y. Does dietary intake of selenium protect against cancer? A systematic review and meta-analysis of population-based prospective studies. Crit Rev Food Sci Nutr. 2020;60(4):684-694. doi:10.1080/10408398.2018.1548427
36 Najim, Y. S. .; Mohammed, T. T. .; Hussain, F. M. . The Effect Of The Use Of Different Levels Of Azolla To Male Broilers Diets In The Productive And Physiological Performance. JLSAR 2022, 3, 37-41.
37 Albakaa A, A, H.; Auda M, A. Association Between Levels Of Vitamin E And Selenium To Detection For Prostate Cancer In Thi-Qar Province. Biochem Cell Arch. 2020;20(1):2107-2113. doi:10.35124/bca.2020.20.1.2107
38 Ogawa-Wong A, N.; Berry M, J.; Seale LA. Selenium and metabolic disorders: An emphasis on type 2 diabetes risk. Nutrients. 2016;8(2). doi:10.3390/nu8020080
39 Krehl S.; Loewinger M.; Florian S.; Kipp A, P.; Banning A.; Wessjohann L, A.; Brauer M, N.; Iori R.; Esworthy R, S.; Chu F, F.; Brigelius-Flohé R. Glutathione peroxidase-2 and selenium decreased inflammation and tumors in a mouse model of inflammation-associated carcinogenesis whereas sulforaphane effects differed with selenium supply. Carcinogenesis. 2012;33(3):620-628. doi:10.1093/carcin/bgr288
Received: 15 May 2023/ Accepted: June 10 2023 / Published:15 June 2023
Citation: Hassan H, Albakaa A, Hussein K. Evaluation of serum Selenium level as a risk factor for Colorectal cancer. Revis Bionatura 2023;8 (2) 46. http://dx.doi.org/10.21931/RB/2023.08.02.46
