2023.08.04.32
Files > Volume 8 > Vol 8 no 4 2023
Evaluation of Calretinin and enumeration of mast cells in rectum tissue biopsies of Hirschsprung and non-Hirschsprung disease in neonate and infant
Rusul A. Abdul
Hussein 1*, Sahar A. H. AL-Sharqi 2, Nada K. Mehdi3
and Ali E. Joda 4,5
1,2 Department of
Biology, College of Science, Mustansiriyah University, Baghdad/Iraq
3 Histopathology
Specialist, Central Child Teaching Hospital, Baghdad, Iraq.
4 Pediatrics
department, College of Medicine, Mustansiriyah University, Baghdad/Iraq
5 Consultant
pediatric surgeon, Central Child Teaching Hospital, Baghdad, Iraq
* Corresponding
Author: Rusul A. Abdul Hussein, Email: [email protected]
Available from: http://dx.doi.org/10.21931/RB/2023.08.04.32
ABSTRACT
The Hirschsprung disease (HD) is a complex genetic congenital
condition characterized by the absence of ganglion cells in the myenteric and
submucosal plexuses of the colon and rectum, leading to functional intestinal
obstruction. A study was conducted from July 2022 to December 2022. The
Toluidine blue stain and calretinin immunohistochemistry were applied to 36
cases of neonates and infants who clinically presented with symptoms suspicious
of having HD, And the hematological study of cell blood counts test and compared
the result of the HD group with the non-HD group and control group. The study
showed an increase in mast cell numbers in the rectal biopsy tissue of HD
patients compared with non-HD patients using Toluidine blue stain. The Immunohistochemistry
for calretinin result displayed 27 (75%) cases as HD, while the remaining 9
(25%) cases were confirmed as non-HD and showed hypertrophied nerve fiber in HD
cases. at the same time, the complete blood count result was unrelated to HD. Some
worrying maternal risk factors were highlighted during pregnancy were the age
of the mother at conception, maternal illness, intake of drugs, type of
Childbirth, and number of previous maternal abortions; all of them show a
non-significant difference between the HD group and non-HD group, also
consanguineous marriage was detected and shows a significant difference between
the HD group and non-HD group.
Keywords: Hirschsprung,
Calretinin, Toluidine blue, CBC count
INTRODUCTION
The Hirschsprung disease (HD) is a
complex genetic congenital condition. The Latin name for HD is megacolon
congenitum 1,2. According to reports, HD has a 4:1 male-to-female ratio and impacts 1
case out of every 5,000 live births globally 3,4. The gold standard for diagnosing HD is a rectal biopsy 5. If a
patient's clinical symptoms (failure to pass meconium, abdominal distension,
constipation) and radiological findings
raise suspicion of HD, the Rectal suction biopsy is obtained 6. Calretinin is calcium signaling that involves a binding protein 7,
found in enteric neurons that project into the mucosal and submucosal layers of
the gut. This protein might be used as a marker for aganglionosis in HD; it is
a crucial component of how cells function and is expressed by the CALB2 gene 8. Mast cells (MCs) are immune cells that migrate from the bone marrow and
perform their primary functions in various peripheral tissues 9. The
proximity of MCs to nerve fibers, the possibility that they play a
physiological role in nerve fiber growth and repair, and the fact that they
produce, store, and release the nerve growth factor necessary for the growth
and repair of nerve fibers all point suggest that MCs are to blame for the
hyperplasia and hypertrophy of adrenergic and cholinergic nerve fibers, which
are typical HD symptoms. However, the precise function of MCs in HD is still
unknown. MCs can be seen thanks to the Toluidine Blue (TB) stain. The part mast
cells play in HD has recently attracted much attention 10. Because most specialist doctors find it challenging to diagnose HD, and
the different methods of diagnosis depend on clinical signs and radiological
examinations.
The study aims to establish a scientific
basis for diagnosing the disease based on the Evaluation of Histopathological
and Immunohistochemical (IHC) changes that occur in the layers of the colon,
with detecting changes that occur in blood cells and collecting, analyzing, and
studying data, to specify diagnosis fundamentals.
MATERIALS AND METHODS
Subjects
The study
included the histopathological, hematological, and IHC examination of 36 cases suspected
of having HD. The ages of cases ranged between (1day-1year) for both sexes, 9
females and 27 males; the sample was obtained from the Central Teaching
Hospital for Children and teaching laboratories at the Medical City Hospital in
Baghdad from July 2022 to December 2022.
All
participants agreed to provide the investigator with the specimens. The College
of Science, Mustansiriyah University's ethics committee approved this work. According
to the Declaration of Helsinki, informed consent was obtained from all
participants (Ref.
No.: BCSMU/0622/0012Z Appendix-1).
Collection
and preparation of the rectum tissue specimens
The tissue specimens were
obtained from the rectum of 36 neonates and infants (27 male and 9 female) who
clinically presented with symptoms suspicious of having HD after being
diagnosed by specialized doctors, the cases were rectal punch biopsies as shown
in Figure (1), the tissue samples were maintained in the fixative solution
(formalin 10%) for histopathological study.

Figure 1. Image
of cases in which patients showed stages of obtaining a biopsy from the rectum.
Tissue samples from the
rectal biopsy were prepared for histological study using Suvara 11. Each tissue sample was cut into small
fragments about 2- 3 cm long before fixation in a buffered isotonic solution of
10% formaldehyde for 24 hours. Each biopsy was processed for the dehydration
process, which was by passing them through progressive concentrations of
ethanol alcohol. They were cleared by passing them through two steps of xylene.
Then, tissues were infiltrated with paraffin wax and were embedded in a metal
template, and after that, the paraffin blocks were sectioned by rotary
microtome into sections 5 μ in thickness. After staining with TB for MC
examination, the slides were examined using a light microscope.
Enumeration of
Mast Cells in the Tissue
After sections were stained with TB, the
grading of MCs in rectal tissue was done using the following method of Amerada et al. 12: - /+: No cells or few; +: 10 cells seen per 10 high power fields; ++:
Clusters of more than 10 cells seen per 10 high power fields; +++: > 10
clusters seen in 10 per high power fields. The results were analyzed after
grading.
Immunohistochemical
study of Calretinin
The IHC stain for Calretinin
was conducted in cases that included 36 formalin-fixed, paraffin-embedded
rectal incisional biopsies from neonate and infant patients. IHC of Calretinin was
graded as A total absence of staining (negative) or presence of brown staining
(positive) according to the method of Leica Company from Germany.
Collection of Blood Samples
From the 36 patients and 20
controls, about 3 ml of venous blood was collected from a suitable vein and
withdrawn from the cases using 3 ml disposable syringes.
The blood (3 ml) was then
collected in a tube containing ethylene diamine tetra acidic acid (EDTA) as an
anticoagulant with a slow mix for a hematological investigation.
Some Complete
Blood Count (CBC)
In this test, 3 ml of the non-hemolyzed blood
is anti-coagulated with EDTA at the collection and was examined by using an
automated system Sysmex XP300 hematology analyzer, which is a computerized,
highly specialized machine that counts the number of total WBCs and different
types of cells such as neutrophil, lymphocyte, RBCs, HCT, and platelets in a
blood sample.
Statistical analysis Statistical Package
for Social Sciences (SPSS) version 21 is used to interpret the data. The
information is given as a mean, standard deviation, and ranges. Frequencies and
percentages are used to display categorical data. ANOVA was used to compare the
tested mean Data expressed as mean± SD. Values of p>0.05 were considered
statically non-significant, while p≤0.05 considered significant results.
RESULTS AND DISCUSSION
Enumeration
of mast cells in the rectal biopsy tissue
Enumeration of MCs in the rectal biopsies tissue of non-HD and HD cases by using a TB
special stain, table (1) represents the MCs for each HD case and non-HD case, which is divided into four categories
that are (Occasional cells, Few natural cells, Moderate number cells, and
clumps). In the HD case, the highest grade registered for a Moderate number of
cells, while the lowest was for Occasional cells. In contrast, in the non-HD
case, the highest grade was reported for Occasional cells, while the lowest was
for a Moderate number of cells. That could be because MC is observed in significant
amounts in the digestive tract.
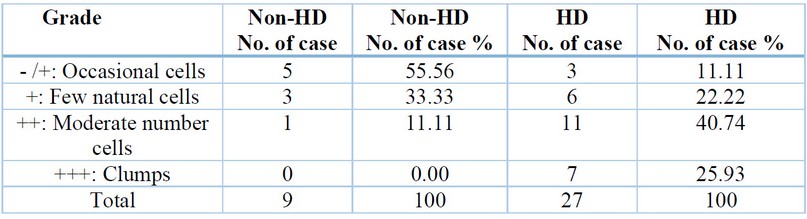
Table 1.
Enumeration of mast cells in the rectal biopsies tissue of non-HD and HD cases
Results are expressed
as percentage - /+: No cells or few; +: 10 cells seen per 10 high
power fields; ++: Clusters of more than 10 cells seen per 10 high power fields;
+++: > 10 clusters seen in 10 per high power fields In this study
mast cells were seen in the submucosa and
showed clump of mast cell in the muscular layer (Figure 2).
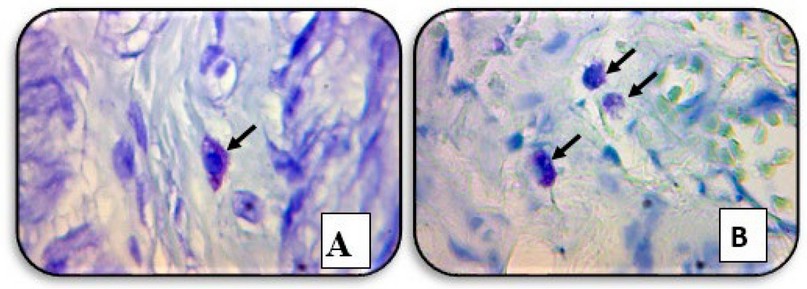
Figure 2. A cross-section of the patient's
rectal biopsy tissue showed (A) a mast cell in the submucosa. (B) a clump of
mast cells in the muscular layer (black arrows) (TB staining, X40).
Recently, there has been a
lot of interest in the role of MCs in HD, where TB
stain highlights MCs, according to 13..
Their description of the transmural distribution of these cells in HD
cases, particularly around nerve fibers and perivascular, is consistent with
the findings of our study. This may be because MCs secrete a wide range of
biologically active substances. MC synthesizes, stores, and releases nerve
growth factor, essential for nerve fiber growth and repair 14. A
similar finding was mentioned by 15. This could result from the MCs
potentially having a significant impact on the regeneration and differentiation
of the intestinal neural system. MCs are more prevalent in HD patients 16.
However, Hermanowicz 17 carried
out a study that found no statistically significant difference existed between
the number of MCs in the submucosa of the HD group compared to the number of
MCs in the other investigations and when comparing the mean number of MCs in
the submucosa of the non-HD group in their study with a mean number of MCs in
earlier 16. This study proved that the number of MCs in the submucosa
of HD and non-HD groups did not differ statistically significantly. It may be
caused by the MC's reaction to
allergens or pathogenic pathogens rather than by their relationship with
aganglionosis 15.18. Other gastrointestinal conditions like acute
appendicitis, ulcerative colitis, celiac disease, and gluten enteropathy have
also been linked to an increase in MCs. In the case of suspected HD, this poses a problem for interpreting a
rectal biopsy 19. However, one study done by 20 reported
an increase in MCs in the mucosa of HD but no statistically significant change
in the number of MCs in the
submucosa, muscularis propria, and serosa; it has been discovered that numerous
inflammatory illnesses at this site are connected with an increase in MCs, which are near
arteries and peripheral nerves, which may be because the MCs are primarily
present in the gastrointestinal tract 21, Given that many of our
patients have several comorbid conditions, this may be explained by the rising
number of Mcs, which may not necessarily be related to HD but may instead be
caused by these conditions.22
Immunohistochemistry
of Calretinin
Calretinin IHC was applied
to all 36 studied cases, and 27 (75%) of the cases were identified as HD, while
the remaining 9 (25%) cases were identified as non-HD Table (2). Figure (3)
showed positive expression in the testis as (positive control) of IHC staining
of Calretinin.

Figure 3. The immunohistochemical
staining method detected Calretinin in the testis as (positive control),
showing positive expression
(X10).

Table
2. Expression of Calretinin in the non-HD and HD cases.
The IHC for Calretinin
was applied to all 36 cases after the application of Calretinin IHC; out of 36
cases, 27 (75%) cases were confirmed as HD while the remaining 9 (25%) cases
were confirmed as non-HD; hence, all suspicious HD cases had been confirmed and
categorized in HD and non-HD. In our study, strong calretinin immunoreactivity
was observed in all ganglionic segments (non-HD cases), figure (4), showing positive expression between the two muscularis layers and positive
expression in the submucosa layer.

Figure 4. Immunohistochemical
staining method detection of Calretinin in the rectal biopsies of non-HD case
(A) showing positive expression
between the two layers of muscularis (B) showing
positive expression in the submucosa (Large figure: X10, small figure: X40)
Whereas any immunoreactivity
was not observed in almost all aganglionic segments (HD cases), (Figure 5) shows
a negative expression of Calretinin in the two layers of the muscularis layer
while showing a complete absence of staining expression of Calretinin in the
mucosa and submucosa layers.
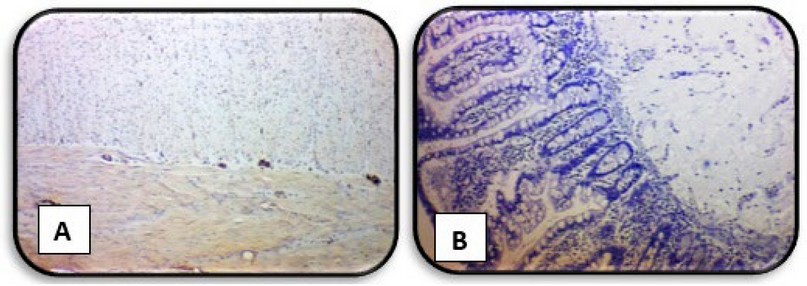
Figure 5. Immunohistochemical
staining method detection of Calretinin in the rectal biopsies of HD case (A) showing negative expression of Calretinin in the two layers of
muscularis layer (B)
showing negative expression of Calretinin in the mucosa and
submucosa layers (X4)
For HD to be pathologically
diagnosed, the colonic neural plexus must be devoid of ganglion cells. Identifying
tiny immature ganglion cells is made more accessible by IHC labeling of Calretinin,
which causes strong ganglia staining 23. IHC expression in this
study found that the calretinin IHC approach is less complicated to use, easier
to interpret, and requires fewer serial sections of the microscopic rectal
biopsy to detect and identify small immature ganglion cells 24. Aganglionic
and ganglionic regions differed significantly from one another. Using Calretinin
it was successful in detecting the presence of ganglions. The current results showed
that Calretinin IHC has good diagnostic value and that Calretinin is an
extremely valuable, sensitive, and specific marker for detecting aganglionosis
in patients who are believed to have HD 25. This outcome is
consistent with the research by 26. Barshack et al. were the
first authors to report that expression of Calretinin was not observed in
aganglionic areas in HD, but it was kept in ganglionic areas. They also
concluded that aganglionic segments showed negative calretinin expression while
positive in all rectal biopsies with ganglionic cells.
Various research has reported that Calretinin is a good marker in displaying ganglia in HD,
as Musa ZA et al. in Iraq revealed in 2017 23, Calretinin is
a perfect and trustworthy diagnostic aid to histological examination of HD,
where claimed sensitivity and specificity are 100%.
Various research have reported that Calretinin is a good marker for
displaying HD ganglia. The presence of hypertrophic submucosal nerve bundles is
a beneficial positive finding because HD is diagnosed based on the absence of a
histological characteristic, namely the ganglion cells 24. many large nerves are usually present in Submucosal nerve hypertrophy, shown
in the aganglionic rectal submucosa of a patient with HD cases in (Figure 6).

Figure 6. The immunohistochemical
staining method detection of Calretinin in the rectal biopsies of the HD case
showed hypertrophied nerve fiber (brown color) and no ganglion cells in the submucosa layers (X10).
According to 27, aberrant nerves and aganglionosis are linked
to hypertrophied submucosal nerve trunks. Nine (32.1%) of the 28 patients in
their study who lacked ganglionic cells had hypertrophied nerve trunks. The
diagnosis can be made without additional testing in a state where no ganglion
cells are seen, and overt submucosal nerve hypertrophy is present.
Unfortunately, some biopsies lack this unmistakable diagnostic evidence or
exhibit aberrant traits that require further investigation. The presence of hypertrophic submucosal nerve bundles is a
beneficial positive finding for HD because HD is diagnosed based on the absence
of a histological characteristic, namely the GCs24. When GCs are absent, the affected part of the
colon cannot contract and relax in a coordinated manner. As a response, the
nerve fibers present in the affected part of the colon increase in size and
number. This increase, known as hypertrophy, is a compensatory mechanism. The
purpose is to circumvent the dysfunctional or missing GCs and stimulate the
contraction of the intestinal muscles.
The Hematological Study
Our study is the first to address the complete blood
cell (CBC) count in HD and compare HD patients with non-HD patients and control,
as shown in (table 3).

Table 3. CBC counts in HD, non-HD and control cases.
The result above showed a significant difference (p
< 0.05) in Mean ± SE of WBC total, platelets, eosinophil and monocyte
between HD patients, non-HD patients and the control group. Meanwhile, the
results of neutrophil, lymphocyte, basophil, RBC, and HGB revealed no
significant difference between HD patients, non-HD, and control groups. CBC
test is usually obtained to ensure the preoperative hematocrit and platelet
count are suitable for surgery 28. In most cases, values were within
the reference ranges. Usually, it is a standard or nonspecific finding, but the
surgeon specialist completes a blood cell (CBC) count if enterocolitis is
suspected. Elevating the white blood cell (WBC) count or a pandemic should
raise concern for enterocolitis 29. According to disease stage, patient
condition, and complications of HD, our results find Leukocytosis; the current
findings generally agree with earlier research that demonstrated a substantial
difference in WBC total, the primary blood indication of overall inflammation 30,31.
The result also showed a significant difference (p < 0.05) in Mean ± SE of
blood eosinophil and monocyte percentage. The multifunctional leukocytes known
as eosinophils are crucial to the beginning and controlling inflammation 32,
and increases in monocytes indicate that the body is working to combat certain
diseases 33. 33. High
circulating monocyte levels are thought to facilitate the fast migration of
many cells into damaged tissues in response to inflammatory signals 34. Monocytes rapidly undergo phenotypic cell
changes as they enter peripheral tissue after migrating from the circulation 35.
According to the current finding, HD patients' platelet counts significantly
increased (p > 0.05) compared to a non-HD group. In addition to their
critical functions in hemostasis and thrombosis, platelets also play key roles
in microbial host defense, wound healing, and angiogenesis 36, and
their involvement in inflammatory pathways, which has been shown in several
studies, as well as their involvement in inflammatory illnesses. 37 38.
The 39 found a cute inflammatory disorders, chronic infections, and
inflammatory diseases, such as connective tissue disorders and inflammatory
bowel disease, both result in changes in platelet indices. As a consequence of
the disease coupled with enterocolitis, failure to diagnose early HD can also
result in iron deficiency anemia, hypoproteinemia, and hypoalbuminemia due to
protein-losing enteropathy; this may also be the cause of the change in CBC 40.
Additionally, viral diseases like rotavirus and cytomegalovirus can cause
hematological changes 41 In extremely few instances, intractable
anemia may be detected on a patient's CBC as one of HD's clinical symptoms 42,
consistent with the recent findings. While the results of lymphocytes,
neutrophils, basophils, RBC count, HGB, and HCT in the blood did not differ
significantly (p > 0.05) between HD patients, non-HD patients, and the
control group, it was clear that the disease had no impact on these cells
because there was no change in their counts.
Prenatal causes
In a recent study, some
worrying maternal risk factors were highlighted during pregnancy were the age
of the mother at conception, shown in Table (4), maternal illness, intake of
drugs, type of Childbirth, and number of previous maternal abortions, all of
them offer a non-significant difference (p>0.05) between the HD group and
non-HD group. Still, consanguineous marriage was detected and showed a
significant difference (p<0.05) between the HD group and non-HD group shown
in table (5).
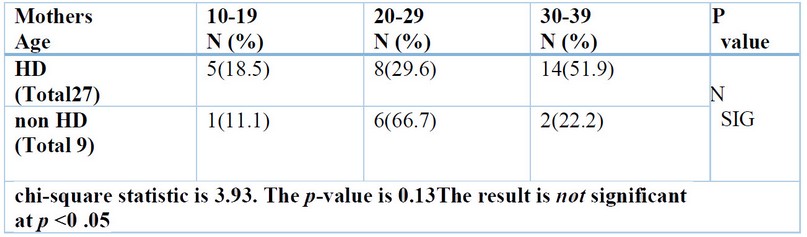
Table 4. Mother's age of the studied groups.
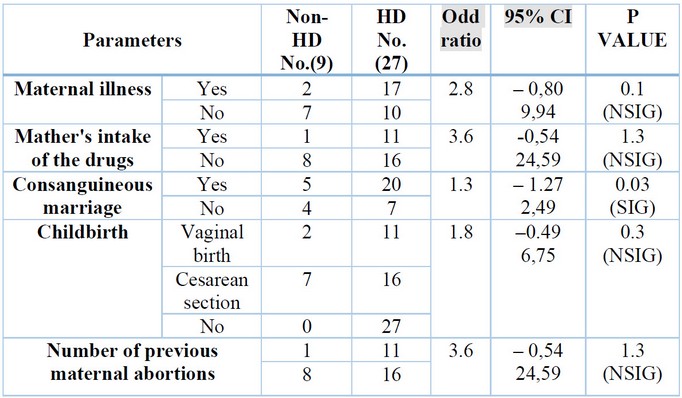
Table 5.
Mother's signs of the studied groups.
The result above found
non-significant associations between the mother's age at Childbirth and the
development of HD; this result is consistent with the results
of a study by 43. Although not statistically significant, some
studies associated maternal age with HD frequency; these studies found HD was
more common in infants with mothers aged ≥30 years at Childbirth. However, 44
found no significant association between mothers' age and HD frequency.
Numerous studies have found
that the risk of congenital disabilities rises with maternal age, especially in
moms older than 35 years. At the same time, 45 could not discover a
connection between congenital abnormalities and maternal age in their
investigation. The development of HD is influenced by several risk factors,
including Down syndrome (trisomy 21). They anticipated that longer maternal
ages would result in a more significant proportion of newborns with HD since
pregnant women over the age of 35 have a higher probability of having a baby
with trisomy 21 46. Aganglionosis of the colon diagnosed during
pregnancy is highly uncommon; just two cases have been documented in the
literature, and both of them had polyhydramnios and dilated bowel loops. 47,48.Where the mother-to-be displayed several symptoms related
to her prenatal illnesses, such as Polyhydramnios. Apart from noninvasive
ultrasound, prenatal diagnosis of HD has been attempted, but with limited
success, by measuring amniotic fluid disaccharides activity in the amniotic
fluid 49. The study of 50 antenatal ultrasounds of a term
male newborn at 33 weeks revealed decreased growth and Polyhydramnios.
Following delivery, testing revealed the infant had HD and other abnormalities.
Some authors say Polyhydramnios appears to be a little more common (9% in their
cohort vs. 1% in the general population). Additionally, the mothers in the current
study, in some instances, had Covid-19, Toxoplasmosis, Rubella, Asthma, Anemia,
Diabetes and Hypertension, where numerous writers have provided evidence
suggesting that viruses may have long-lasting adverse effects on the fetus.
High levels of maternal inflammation during viral infection can affect all
facets of fetal brain development and result in extensive neurological
aftereffects 51,52.
Concerns about spreading the
virus and damaging fetuses by vertical transmission 53 exist. According to
other investigators, the danger of vertical transmission was minimal, and
neither antenatal nor postnatal evaluations revealed any congenital disabilities
54. Panahi 55 findings do not point to a significant
increase in the probability of abortion or genetic abnormalities in newborns in
COVID-19-infected mothers. According to 56, SARS infection has been
linked to a higher incidence of intrauterine development retardation but not
SARS-CoV-2 infection. Since the infection is novel, monitoring
COVID-19-positive pregnant women is therefore essential to prevent negative
maternal and fetal consequences 54. Congenital abnormalities,
stillbirth, and increased fetal growth remain the most severe adverse
consequences of diabetic pregnancy, according to a prior study 57. Congenital
anomalies were described by 58. It is undeniably connected between more
significant risks of congenital abnormalities and poor glycemic control at the
time of conception and during the first trimester. The 59
investigated the impact of chronic hypertension on fetal growth and concluded
that it increased the likelihood of intrauterine growth restriction. No,
HD-causing prenatal illness exists. According to the findings of the study 60,
9% and 6% of the population, respectively, had gestational hypertension and
diabetes. Similar rates (10% and 4%, respectively) have been recorded for the
general population. In their article, 61 describes how maternal
asthma during pregnancy affects fetal growth and development, which may impair
the future health of the offspring. Although many expectant mothers abstain
from taking any medications while they are pregnant, frequent asthma attacks
may lower the amount of oxygen in the mother's blood, which in turn reduces the
amount of oxygen in the fetus and causes issues with the fetus's development. When
using the asthma medications as directed.
According to a recent
systematic analysis, iron deficiency during the first and second trimesters
increases maternal morbidity and the likelihood of unfavorable pregnancy
outcomes, such as low birth weight preterm, or intrauterine growth restriction 62.
Fetal hazards result from low fetal iron levels, which can be caused by
maternal anemia or pregnancy complications that impair the transfer of fetal
iron from the mother 63. Several findings state that there is no
connection between maternal Hb levels and growth retardation 64. Furthermore,
65 found a link between low maternal Hb levels and unfavorable
pregnancy outcomes, including preterm, low birth weight, fetal death, and other
medical anomalies. According to the study's questionnaire, some pregnant mothers did not take any medications other than some vitamins and were under
medical supervision. Still, some of them admitted to taking medications for
diseases they experienced during pregnancy, some of which were taken under
medical supervision, and others were self-medication. Because it is simple to
obtain medicines from pharmacies or drug stores without a prescription, time
savings, the lengthy wait times for medical services, the perception that their
sickness was not severe, and the lower cost of self-medication may also be
contributing factors 66. They concluded that taking a medication
that is contraindicated during pregnancy does not necessarily represent a
high-risk situation; however, other authors supported the idea that taking
drugs that are illegal while pregnant poses a risk to both the mother and the fetus
because drug exposure during this time is likely to result in congenital
malformations 67. Others consumed herbs. There are no studies or
studies that are similar to this topic. Still, there are studies on the effects
of using herbs indiscriminately while pregnant, which puts the mother and fetus
at risk because these herbs contain substances that may be hazardous to the
mother and fetus 68, and this may be a valid reason for their
children to have many diseases and congenital malformations.
Additionally, the results of
the recent study did not discover a connection between congenital disabilities
and the kind of pregnancy or prior abortions. This differs from other studies 69,70.
Previous research in Iraq has linked prior abortions to congenital
abnormalities 71. In our study, from the maternal risk factors, only
Consanguinity shows a significant difference (p<0.05) between the HD group
and non-HD group. Consanguinity and HD: The prevalence of consanguineous
marriages—marriages between close biological relatives—varies greatly worldwide,
from less than 1% in North America and most of Europe to over 50% in some areas
of Asia and the Middle East. The prevalence of HD in the offspring of
consanguineous parents has not been widely recorded in these places. However, Consanguinity
between parents is frequent in the Middle East and several nations in Asia 72.
According to a study looking at HD in Oman 73, parents of HD
patients had a consanguinity rate of 75% compared to 33% in the general
community. According to a more recent study from Bangladesh, parents of HD
babies had a 16% consanguinity rate compared to a 10% rate in the general
community. Twelve percent (15/129) of the families were consanguineous,
according to a more recent study from China 74. It is advised to
avoid consanguineous marriage because it appears to be a risk factor for HD. It
has been shown that Consanguinity increases the likelihood that a husband and
wife may have a gene that originated from a common ancestor. Numerous studies
have shown that children of such a marriage are more likely to be homozygous
for a dangerous gene and subsequently have autosomal recessive genetic diseases
75-77. Other researchers, however, examined seven (25%) children
with consanguineous parents who had HD (P = 1.000) and found no evidence of a
connection between the two.
Additionally, Consanguinity
between parents is quite common in the Middle East, and consanguineous marriage
is also quite common there. As a result, a future study that includes cases
from all regional communities could be conducted to look into the genetic
predisposition of HD and the significance of consanguinity 78. Nevertheless,
these publications provide compelling evidence that a maternal risk factor harms
fetal growth and pregnancy outcomes. However, referring to some risk variables
as potentially dangerous rather than as an adequate appraisal clearly
demonstrating a harmful effect on the fetus would be preferable. Furthermore,
it is crucial to note that risk factors are frequently linked to other
pathologic disorders, making it difficult to determine if a risk factor for the
mother directly causes or even contributes to an increase in HD.
CONCLUSION
The current results showed that TB stain can quickly
be done for diagnosis of HD; the MCs count in the
submucosa is a significant criterion in HD and inflammation. Calretinin immunostaining
is a reliable and beneficial test for the diagnostic of HD, aid in
histopathological examination of suspected HD, and detect hypertrophic nerve
bundles, which may aid in the diagnosis of HD. Submucosal nerve bundle
hypertrophy is considered an adjuvant histological criterion for detecting HD. Finally,
the result of the CBC count was not related to HD.
Acknowledgments: We thank
the Department of Biology, College of Science, Mustansiriyah University
(http://uomustansiriyah.edu.iq/), and Baghdad for advice and support.
REFERENCES
1.
Klein
M, Varga I. Hirschsprung's disease-recent understanding of embryonic aspects,
etiopathogenesis and future treatment avenues. Medicina (Kaunas) Internet.
2020; 56(11):611. Available from: http://dx.doi.org/10.3390/medicina56110611.
2. Gustafson E, Larsson T, Danielson J. Controlled outcome of
Hirschsprung's disease beyond adolescence: a single center experience. Pediatr
Surg Int Internet. 2019;35(2):181–5. Available from: http://dx.doi.org/10.1007/s00383-018-4391-5.
3. Holschneider AM, Meier-Ruge W, Ure BM. Hirschsprung's
disease and allied disorders--a review. Eur J Pediatr Surg Internet.
1994;4(5):260–6. Available from: http://dx.doi.org/10.1055/s-2008-1066115.
4. Gorbatyuk OM. Current approaches to diagnosis and treatment
of Hirschsprung disease in newborns and infants (literature review and
first-hand experience). Wiad Lek Internet. 2022;75(4):1026–30.
Available from: http://dx.doi.org/10.36740/wlek20220420120.
5. Bahrami A, Joodi M, Moetamani-Ahmadi M, Maftouh M, Hassanian
SM, Ferns GA, et al. Genetic background of hirschsprung disease: A bridge
between basic science and clinical application. J Cell Biochem Internet.
2018;119(1):28–33. Available from: http://dx.doi.org/10.1002/jcb.26149.
6. Kapur RP, Ambartsumyan L, Smith C. Are we underdiagnosing
hirschsprung disease? Pediatric and Developmental Pathology. Pediatric and
Developmental Pathology: The Official Journal of the Society for Pediatric
Pathology and the Paediatric Pathology Society Internet.
2020;23(1):60–71. Available from: http://dx.doi.org/10.1177/1093526619889434.
7. Lim KH, Wan WK, Lim TKH, Loh AHL, Nah SA, Chang KTE. Primary
diagnosis of Hirschsprung disease-Calretinin immunohistochemistry in rectal
suction biopsies, with emphasis on diagnostic pitfalls. World Journal of
Pathology. 2014;3(3).
8. Rytting H, Dureau ZJ, Vega JV, Rogers BB, Yin H. Autopsy
study of calretinin immunohistochemistry in the anorectal canal in young
infants and potential implications for rectal biopsy approach in the neonatal
period. Pediatr Dev Pathol Internet. 2021;24(6):542–50. Available
from: http://dx.doi.org/10.1177/10935266211030172.
9. Ravanbakhsh N, Kesavan A. The role of mast cells in
pediatric gastrointestinal disease. Ann Gastroenterol Internet.
2019;32(4):338–45. Available from: http://dx.doi.org/10.20524/aog.2019.0378.
10. Yasseen HA. Toluidine blue stain and crystal violet stain
versus H and E stain in the diagnosis of Hirschsprung's disease: A study in
Sulaimani city in Kurdistan/Iraq. Ann Pathol Lab Med. 2015;2:A54-61.
11. Suvara K. Bancrofts theory and practic of histological
techniques. Eight Edtion. Churchill Livingstone Elsevier; 2019.
12. Anuradha GP, Anita A, Seemant SK, Pratima S. t cell profile
in appendicitis. Indian Journal of Pathology and Oncology. 2017;4(4):555–9.
13. Kobayashi H, Yamataka A, Fujimoto T, Lane GJ, Miyano T. Mast
cells and gut nerve development: implications for Hirschsprung's disease and
intestinal neuronal dysplasia. J Pediatr Surg Internet.
1999;34(4):543–8. Available from: http://dx.doi.org/10.1016/s0022-3468(99)90069-6.
14. Li W-T, Luo Q-Q, Wang B, Chen X, Yan X-J, Qiu H-Y, et al.
Bile acids induce visceral hypersensitivity via mucosal mast cell-to-nociceptor
signaling that involves the farnesoid X receptor/nerve growth factor/transient
receptor potential vanilloid 1 axis. FASEB J Internet.
2019;33(2):2435–50. Available from: http://dx.doi.org/10.1096/fj.201800935RR.
15. Yadav AK, Mishra K, Mohta A, Agarwal S. Hirschsprung's
disease: Is there a relationship between mast cells and nerve fibers? World J
Gastroenterol Internet. 2009;15(12):1493. Available from: http://dx.doi.org/10.3748/wjg.15.1493.
16. Demirbilek S, Ozardali HI, Aydm G. Mast-cells distribution
and colonic mucin composition in Hirschsprung's disease and intestinal neuronal
dysplasia. Pediatr Surg Int Internet. 2001;17(2–3):136–9. Available
from: http://dx.doi.org/10.1007/s003830000467.
17. Hermanowicz A, Debek W, Dzienis-Koronkiewicz E, Chyczewski
L. Topography and morphometry of intestinal mast cells in children with
Hirschsprung's disease. Folia Histochem Cytobiol Internet.
2008;46(1):65–8. Available from: http://dx.doi.org/10.2478/v10042-008-0008-5.
18. Do Carmo Neto JR, Braga YLL, Da Costa AWF, Lucio FH, Do
Nascimento TC, Reis MA, et al. Biomarkers and their possible functions in the
intestinal microenvironment of chagasic megacolon: an overview of the (neuro)
inflammatory process. Journal of Immunology Research. 2021.
19. Nanagas VC, Kovalszki A. Gastrointestinal manifestations of
hypereosinophilic syndromes and mast cell disorders: A comprehensive review.
Clin Rev Allergy Immunol Internet. 2019;57(2):194–212. Available
from: http://dx.doi.org/10.1007/s12016-018-8695-y
20. Kini U. Pathology of the gut motility disorders:
Hirschsprung's disease. In: Surgical Pathology of the Gastrointestinal System.
Singapore: Springer Singapore; 2022. p. 339–74.
21. Singh SK, Rajoria K. Ayurvedic management of chronic
constipation in Hirschsprung disease-A case study. Journal of Ayurveda and
Integrative Medicine. 2018;9(2):131–5.
22. Olivera A, Beaven MA, Metcalfe DD. Mast cells signal their
importance in health and disease. J Allergy Clin Immunol Internet.
2018; Available from: http://dx.doi.org/10.1016/j.jaci.2018.01.034.
23. Musa ZA, Qasim BJ, Ghazi HF, Al Shaikhly AWAK. Diagnostic
roles of Calretinin in hirschsprung disease: A comparison to neuron-specific
enolase. Saudi J Gastroenterol Internet. 2017;23(1):60–6. Available
from: http://dx.doi.org/10.4103/1319-3767.199118.
24. Green N, Smith CA, Bradford MC, Ambartsumyan L, Kapur RP.
Rectal suction biopsy versus incisional rectal biopsy in the diagnosis of
Hirschsprung disease. Pediatr Surg Int Internet.
2022;38(12):1989–96. Available from: http://dx.doi.org/10.1007/s00383-022-05246-4.
25. Naimi A, Shegeft E. Evaluation of the Diagnostic Value of
Calretinin Immunohistochemistry Assay in the Superficial Rectal Biopsy of
Children Suspected of Hirschsprung's Disease. Iranian Journal of Neonatology.
2022;13(1).
26. Barshack I, Fridman E, Goldberg I, Chowers Y, Kopolovic J.
The loss of calretinin expression indicates aganglionosis in Hirschsprung's
disease. J Clin Pathol Internet. 2004;57(7):712–6. Available from: http://dx.doi.org/10.1136/jcp.2004.016030.
27. Alizai NK, Batcup G, Dixon MF, Stringer MD. Rectal biopsy
for Hirschsprung's disease: what is the optimum method? Pediatr Surg Int Internet.
1998;13(2–3):121–4. Available from: http://dx.doi.org/10.1007/s003830050264.
28. Umesh G, Bhaskar SB, Harsoor SS, Dongare PA, Garg R, Kannan
S, et al. Preoperative investigations: Practice guidelines from the Indian
society of anaesthesiologists. Indian J Anaesth Internet.
2022;66(5):319–43. Available from: http://dx.doi.org/10.4103/ija.ija_335_22.
29. Frykman PK, Kim S, Wester T, Nordenskjöld A, Kawaguchi A,
Hui TT. & HAEC Collaborative Research Group. Critical evaluation of the
Hirschsprungassociated enterocolitis (HAEC) score: a multicenter study of 116
children with Hirschsprung disease. Journal of pediatric surgery.
2018;53(4):708–17.
30. Alkarzae M, Alsanosi A, Alharbi M, Altamimi F, Alzendi N.
Role of infection in post-tonsillectomy secondary haemorrhage-an experience at
King Abdulaziz University Hospital. Glob J Otolaryngol. 2017;6.
31. Lefta AS, Daway HG, Jouda J. Red Blood Cells detecting
depending on binary conversion at multi threshold values. Al-Mustansiriyah J
Sci Internet. 2022;33(1):69–76. Available from: http://dx.doi.org/10.23851/mjs.v33i1.1079.
32. Diny NL, Rose NR, Čiháková D. Eosinophils in autoimmune
diseases. Front Immunol Internet. 2017; 8:484. Available from: http://dx.doi.org/10.3389/fimmu.2017.00484.
33. Lichtman MA, Beatler E, Kipps TJ, Seligsohn U, Prchal JT.
Williams Hematology. The McGrawHill Companies. 2010;
34. Arol C, Yona S. Origins and tissuecontext dependent fates of
blood monocytes. Immunology and cell biology. 2009;87(1):30–8.
35. Al-Sarray ZA, Hussein RH, Al-Hafidh AH, Al-Rayahi IA.
Vitamin D deficiency associates with disease severity in rheumatoid arthritis
patients. Al-Mustansiriyah J Sci Internet. 2023;33(5):33–8.
Available from: http://dx.doi.org/10.23851/mjs.v33i5.1310.
36. Golebiewska EM, Poole AW. Platelet secretion: From
haemostasis to wound healing and beyond. Blood Rev Internet.
2015;29(3):153–62. Available from: http://dx.doi.org/10.1016/j.blre.2014.10.003.
37. Kostakis ID, Angelidou M, Kambouri K, Gardikis S, Cholidou
GK, Gioka T, et al. Hematological diagnostic markers of acute appendicitis in
children. Hell Cheirourgike Internet. 2018;90(3):127–36. Available
from: http://dx.doi.org/10.1007/s13126-018-0457-z.
38. Semple JW, Freedman J. Platelets and innate immunity. Cell
Mol Life Sci Internet. 2010;67(4):499–511. Available from: http://dx.doi.org/10.1007/s00018-009-0205-1.
39. Schafer AI. Thrombocytosis and thrombocythemia. Blood Rev Internet.
2001;15(4):159–66. Available from: http://dx.doi.org/10.1054/blre.2001.0162.
40. Sun X, Chu J, Li C, Deng Z. Hirschsprung's disease
presenting as intractable anemia: a report of two cases and review of the
literature. BMC Pediatr Internet. 2020;20(1):525. Available from: http://dx.doi.org/10.1186/s12887-020-02423-z
41. Urrechaga E, Aguirre U, España PP, De Guadiana LG. Complete
blood counts and cell population data from Sysmex XN analyser in the detection
of SARSCoV-2 infection. Clinical Chemistry and Laboratory Medicine (CCLM).
2021;59(2):e57–60.
42. Al-Shamaileh T, Hashem H, Farhoud E, Al-Edwan A, Alomari MS,
Levitt MA. MDelayed diagnosis of Hirschsprung disease presenting initially as
anemia: A case report. Journal of Pediatric Surgery Case Reports. 2023.
43. Sukarelawanto AVR, Ritana A, Balela N, Putri WJK, Sirait DN,
Paramita VMW. & Makhmudi, A. Postoperative enterocolitis assessment using
two different cut-off values in the HAEC score in Hirschsprung patients
undergoing Duhamel and Soave pull-through. BMC pediatrics. 2020;20(1):1–6.
44. Granström L, Svenningsson A, Hagel A. Maternal risk factors
and perinatal characteristics for Hirschsprung disease. Pediatrics. 2016;138.
45. Ajao AE, Adeoye IA. Prevalence, risk factors and outcome of
congenital anomalies among neonatal admissions in OGBOMOSO, Nigeria. BMC
Pediatr Internet. 2019;19(1):88. Available from: http://dx.doi.org/10.1186/s12887-019-1471-1.
46. Demehri FR, Halaweish IF, Coran AG.
Hirschsprungassociated enterocolitis: pathogenesis, treatment and prevention.
Pediatr Surg Int. 2013; 29:873–81.
47. Wrobleski D, Wesselhoeft C. Ultrasonic diagnosis of prenatal
intestinal obstruction. J Pediatr Surg Internet. 1979;14(5):598–600.
Available from: http://dx.doi.org/10.1016/s0022-3468(79)80146-3.
48. Vermesh M, Mayden KL, Confino E, Giglia RV, Gleicher N.
Prenatal sonographic diagnosis of Hirschsprung's disease. J Ultrasound Med Internet.
1986;5(1):37–9. Available from: http://dx.doi.org/10.7863/jum.1986.5.1.37.
49. Aldaffaa M, Mahfouz A, Alaqeel S, Alakeel HA, Al Naamshan M.
Hirschsprung's disease in a genetically diagnosed Cri-du-chat syndrome baby. J
Pediatr Surg Case Rep Internet. 2023;91(102600):102600. Available
from: http://dx.doi.org/10.1016/j.epsc.2023.102600.
50. Broch A, Trang H, Montalva L, Berrebi D, Dauger S, Bonnard
A. Congenital central hypoventilation syndrome and Hirschsprung disease: A
retrospective review of the French National Registry Center on 33 cases. J
Pediatr Surg Internet. 2019;54(11):2325–30. Available from: http://dx.doi.org/10.1016/j.jpedsurg.2019.02.014.
51. Cornish EF, Filipovic I, Åsenius F, Williams DJ, McDonnell
T. Innate immune responses to acute viral infection during pregnancy. Front
Immunol Internet. 2020; 11:572567. Available from: http://dx.doi.org/10.3389/fimmu.2020.572567.
52. Leung KKY, Hon KL, Yeung A, Leung AKC, Man E. Congenital
infections in Hong Kong: an overview of TORCH. Hong Kong Med J Internet.
2020;26(2):127–38. Available from: http://dx.doi.org/10.12809/hkmj198287.
53. Lebel C, MacKinnon A, Bagshawe M, Tomfohr-Madsen L,
Giesbrecht G. Elevated depression and anxiety symptoms among pregnant
individuals during the COVID-19 pandemic. J Affect Disord Internet.
2020; 277:5–13. Available from: http://dx.doi.org/10.1016/j.jad.2020.07.126.
54. Mascio D, Sen C, Saccone G, Galindo A, Grünebaum A,
Yoshimatsu J, et al. Risk factors associated with adverse fetal outcomes in
pregnancies affected by Coronavirus disease (COVID-19): a secondary analysis of
the WAPM study on COVID19. Journal of perinatal medicine. 2019;48(9):950–8.
55. Panahi L, Amiri M, Pouy S. Risks of novel Coronavirus
disease (COVID-19) in pregnancy; A narrative review. Arch Acad Emerg Med.
2020;8(1):e34.
56. Salem D, Katranji F, Bakdash T. COVID-19 infection in
pregnant women: Review of maternal and fetal outcomes. Int J Gynaecol Obstet Internet.
2021;152(3):291–8. Available from: http://dx.doi.org/10.1002/ijgo.13533.
57. Schaefer-Graf U, the Diabetic Pregnancy Study Group, Napoli
A, Nolan CJ. Diabetes in pregnancy: a new decade of challenges ahead.
Diabetologia Internet. 2018; Available from: http://dx.doi.org/10.1007/s00125-018-4545-y.
58. Zabihi S, Loeken MR. Understanding diabetic teratogenesis:
where are we now and where are we going? Molecular Causes of Diabetic
Teratogenesis. Birth Defects Res A Clin Mol Teratol Internet.
2010;88(10):779–90. Available from: http://dx.doi.org/10.1002/bdra.20704.
59. Haelterman E, Breart G, Paris-Liado J, Dramaix M,
Tchobrousky C. Effect of uncomplicated chronic hypertension on the risk of
smallforgestational age birth. Am J Epidemiol. 1997;145:689–95.
60. Broch A, Trang H, Montalva L, Berrebi D, Dauger S, Bonnard
A. Congenital central hypoventilation syndrome and Hirschsprung disease: A
retrospective review of the French National Registry Center on 33 cases. J
Pediatr Surg Internet. 2019;54(11):2325–30. Available from: http://dx.doi.org/10.1016/j.jpedsurg.2019.02.014.
61. Meakin AS, Saif Z, Seedat N, Clifton VL. The impact of
maternal asthma during pregnancy on fetal growth and development: a review.
Expert Rev Respir Med Internet. 2020;14(12):1207–16. Available from:
http://dx.doi.org/10.1080/17476348.2020.1814148
62. Ru Y, Pressman EK, Guillet R, Katzman PJ, Vermeylen F, O'Brien
KO. Umbilical cord hepcidin concentrations are positively associated with the
variance in iron status among multiple birth neonates. J Nutr Internet.
2018;148(11):1716–22. Available from: http://dx.doi.org/10.1093/jn/nxy151.
63. Monk C, Georgieff MK, Xu D, Hao X, Bansal R, Gustafsson H,
et al. Maternal prenatal iron status and tissue organization in the neonatal
brain. Pediatr Res Internet. 2016;79(3):482–8. Available from: http://dx.doi.org/10.1038/pr.2015.248.
64. Kaltreider DF, Kohl S. Epidemiology of preterm delivery.
Clin Obstet Gynecol Internet. 1980;23(1):17–31. Available from: http://dx.doi.org/10.1097/00003081-198003000-00005.
65. Garn SM, Ridella SA, Petzold AS, Falkner F. Maternal
hematologic levels and pregnancy outcomes. Semin Perinatol. 1981;5(2):155–62.
66. Hoeltzenbein M, Slimi S, Fietz A-K, Stegherr R, Onken M,
Beyersmann J, et al. Increasing use of newer antiseizure medication during
pregnancy: An observational study with special focus on lacosamide. Seizure Internet.
2023; 107:107–13. Available from: http://dx.doi.org/10.1016/j.seizure.2023.02.015.
67. Black RA, Hill DA. Over-the-counter medications in
pregnancy. Am Fam Physician. 2003;67(12):2517–24.
68. Bruno LO, Simoes RS, de Jesus Simoes M, Girão MJBC,
Grundmann O. Pregnancy and herbal medicines: An unnecessary risk for women's
health-A narrative review. Phytother Res Internet.
2018;32(5):796–810. Available from: http://dx.doi.org/10.1002/ptr.6020.
69. Ronya R, Gupta D, Ghosh SK, Narang R, Jain KB. Spectrum of
congenital surgical malformations in newborns. J Indian Med Assoc.
2002;100(9):565–6.
70. Cherian AG, Jamkhandi D, George K, Bose A, Prasad J, Minz S.
Prevalence of congenital anomalies in a secondary care hospital in South India:
A cross-sectional study. J Trop Pediatr Internet. 2016;62(5):361–7.
Available from: http://dx.doi.org/10.1093/tropej/fmw019.
71. Ameen SK, Alalaf SK, Shabila NP. Pattern of congenital
anomalies at birth and their correlations with maternal characteristics in the
maternity teaching hospital, Erbil city, Iraq. BMC Pregnancy Childbirth Internet.
2018;18(1):501. Available from: http://dx.doi.org/10.1186/s12884-018-2141-2.
72. Puri P, Nakamura H. Familial Hirschsprung's Disease. In:
Hirschsprung's Disease and Allied Disorders. Cham: Springer International
Publishing; 2019. p. 115–9.
73. Rajab A, Freeman NV, Patton MA. Hirschsprung's disease in
Oman. J Pediatr Surg Internet. 1997;32(5):724–7. Available from: http://dx.doi.org/10.1016/s0022-3468(97)90015-4.
74. Xiao J, Hao L-W, Wang J, Yu X-S, You J-Y, Li Z-J, et al.
Comprehensive characterization of the genetic landscape of familial
Hirschsprung's disease. World J
75. Mobarak AM, Chaudhry T, Brown J, Zelenska T, Khan MN,
Chaudry S, et al. Estimating the health and socioeconomic effects of cousin
marriage in south Asia. J Biosoc Sci Internet. 2019;51(3):418–35.
Available from: http://dx.doi.org/10.1017/S0021932018000275.
76. Al-Hamed MH, Alsahan N, Tulbah M, Kurdi W, Ali W, Sayer JA,
et al. Fetal anomalies associated with novel pathogenic variants in TMEM94.
Genes (Basel) Internet. 2020;11(9):967. Available from: http://dx.doi.org/10.3390/genes11090967.
77. Najafi K, Mehrjoo Z, Ardalani F, Ghaderi-Sohi S, Kariminejad
A, Kariminejad R, et al. Identifying the causes of recurrent pregnancy loss in
consanguineous couples using whole exome sequencing on the products of
miscarriage with no chromosomal abnormalities. Sci Rep Internet.
2021;11(1):6952. Available from: http://dx.doi.org/10.1038/s41598-021-86309-9.
78. Al-Taher R, Daradkeh HT, Hadadin H, Obiedat A, Hijazein Y,
Hijazein L, et al. Children with Hirschsprung disease in a developing country:
A cohort study of the predictors of a positive rectal biopsy result. Medicine
(Baltimore) Internet. 2022;101(46):e31601. Available from: http://dx.doi.org/10.1097/MD.0000000000031601.
Received: 28 September
2023/ Accepted: 15 November 2023 / Published:15 December 2023
Citation: Abdul Hussein R A., H. AL-Sharqi S A, Mehdi N K., Joda A E.
Evaluation of Calretinin and enumeration of mast cells in rectum tissue
biopsies of Hirschsprung and non-Hirschsprung disease in neonate and infant. Revis Bionatura 2023;8 (4) 32. http://dx.doi.org/10.21931/RB/2023.08.04.32
Additional
information Correspondence should be addressed to [email protected]
Peer
review information. Bionatura thanks anonymous
reviewer(s) for their contribution to the peer review of this work using https://reviewerlocator.webofscience.com/
All articles published
by Bionatura Journal are made freely and permanently accessible online
immediately upon publication, without subscription charges or registration
barriers.
Bionatura
ISSN. 13909355. Scopus coverage years: from 2016 to the Present
Publisher's
Note: Bionatura stays neutral concerning jurisdictional claims in published maps
and institutional affiliations.
Copyright:
© 2023 by the authors. They were submitted for
possible open-access publication under the terms and conditions of the Creative
Commons Attribution (CC BY) license
(https://creativecommons.org/licenses/by/4.0/).
