2023.08.03.45
Files > Volume 8 > Vol 8 No 3 2023
Effect of aqueous extract of Azadirachta indica leaves on gastrointestinal nematodes in ruminants: an in vitro study.
Nelson Correa Herrera1, Marcos Edel Martínez Montero1, Omelio Cepero Rodríguez1, Manuel de Jesús Jumbo Romero4, Claudia Linares Rivero2, Dayami Fontes Marrero1, Sandra Cuello Portal3, Janet Quiñones-Galvez2, Viviana Marina Rodríguez Benalcázar5, Alva Tatiana Borja Ramos6
1 Facultad de Ciencias Agrícolas, Universidad de Ciego de Ávila Máximo Gómez Báez, Cuba.
2Centro de Bioplantas, Universidad de Ciego de Ávila Máximo Gómez Báez, Ciego de Ávila, Cuba.
3Centro Nacional de Sanidad Agropecuaria, Universidad Agraria de la Habana, Cuba
4 Universidad Laica Eloy Alfaro de Manabí, Extensión en El Carmen.
5 Laboratorio de Microbiología. Departamento de Medicina Veterinaria, Facultad de Ciencias Agropecuarias, Universidad Técnica de Ambato.
6 Laboratorio de Biología, Departamento de Medicina Veterinaria, Facultad de Ciencias Agropecuarias, Universidad Técnica de Ambato.
Correspondencia: [email protected]; Tel.: +53 55619757
Available from: http://dx.doi.org/10.21931/RB/2023.08.03.45
ABSTRACT
High rates of gastrointestinal parasite infestation lead to the frequent use of anthelmintics for their control, resulting in resistance in the main species of gastrointestinal nematodes. Natural plant extracts, particularly those from Azadirachta indica A. Juss leaves, have been described as having antiparasitic, antimicrobial, insecticidal, antioxidant, and anticorrosive activities, which are related to the diversity of their chemical composition. This research aimed to evaluate the effect of the aqueous extract of A. indica leaves on the control of gastrointestinal nematodes in ruminants. To achieve this, an in vitro test was performed using the larval development assay, with fecal samples obtained from Pelibuey sheep, Criolla goats in the development category, and Criolla calf cattle for the culture of gastrointestinal nematode larvae. Three concentrations (minimum, medium, and maximum) were evaluated for each animal species against Haemonchus placei and Cooperia sp larvae for cattle and Haemonchus contortus, Trichostrongylus sp, and Trichuris for sheep and goats. The survival percentage indicator was evaluated at 0, 24, 48, and 72 h. The research concluded that the minimum concentration was valid for sheep and goats but not for cattle, and the medium and maximum concentrations were effective against all three animal species, resulting in 100% larval mortality.
Keywords: Azadirachta, drug effects, Ruminants, Trichostrongyloidea
INTRODUCTION
Gastrointestinal strongylid infestations are a significant economic limitation to developing bovine husbandry in tropical conditions. Especially when Haemonchus spp. predominates, it can cause blood loss, resulting in a rapid drop in hematocrit values, low feed conversion, difficulties in weight gain, loss of appetite, growth retardation, and even death. Young animals are particularly susceptible, and these factors translate into economic losses for farmers1,2. Gastrointestinal helminths also affect farming systems worldwide3. These parasitic infections disrupt nutrient absorption in animals, resulting in a reduction in body weight and an increased susceptibility to secondary infections4.
An additional promising alternative for parasite control is plant extracts, based on an ethnobotanical concept that exploits the accumulated knowledge of indigenous communities in tropical America5. Using plants with anthelmintic properties has become one of the most exciting alternatives in recent years6,7. Traditional medicinal plants have proven significant as an alternative to anthelmintics in developing and developed countries. Additionally, the use of medicinal plants is a tradition in many countries8,9,10,11.
Previous findings related to traditional medicinal plants have shown that many plant species act as anthelmintics and are an alternative to conventional anthelmintics12. They can help reduce parasite influx in livestock 13,14, and are sustainable and ecologically acceptable15. Among the most commonly used species for this purpose is Neem (Azadirachta indica A. Juss. Meliaceae)16,17. Extracts from this tree have been widely used to promote health since ancient times due to its wide variety of therapeutic properties18.
Different studies report the antiparasitic effect of A. indica leaves in sheep19,20,21, with variable results. These could be due to different reasons, such as the type of extract, method of material collection used, as well as the phenological state of the plant, which could modify the type and concentration of active compounds22. Cruz et al.21 demonstrated a significant reduction in the excretion of gastrointestinal nematode eggs after administering 0.8 g/kg of aqueous extract of A. indica leaves. On the other hand, previous studies have evaluated the anthelmintic effect of A. indica leaves in Haemonchus contortus in goats23,24,25,26 and bovine strongylosis27.
Due to the growing challenge of antihelmintic resistance against common drugs, there is a great need to explore natural resources that can replace these compounds due to their therapeutic action against gastrointestinal nematodes. In this sense, this research aimed to evaluate the effect of an aqueous extract of A. indica leaves on controlling gastrointestinal nematodes in ruminants.
MATERIALES Y MÉTODOS
This study was conducted at the microbiology laboratory and the Juan Tomás Roig Experimental Station of the University of Ciego de Ávila. The Department of Natural Products facilities at the Bioplants Center in Cuba were also used.
Preparation of the extract
To obtain the powdered extract from Neem (Azadirachta indica A. Juss) leaves, they were manually harvested in February 2022 from plants established at the Experimental Station. Immature fruits were removed, and the leaves were washed with running water and then sun-dried on racks for 24 hours, as recommended by Schmutterer 28, to prevent the decomposition of active principles. The dried leaves were collected in a nylon bag and placed in an oven at 60°C for 72 h until they were crispy and crumbled when touched19.
The dried leaves were ground to obtain a light green powder with particles of 1 mm and a moisture content of 8 to 10%19. The aqueous extract was obtained using the method developed by Toledo et al.29. The aqueous extraction process was carried out in the afternoon before the day of the animal treatment to avoid fermentation of the sugars contained in Neem, which leads to the loss of its properties19. For the development of this method, the powdered extract was mixed with distilled water at a temperature of 45°C in an electric mixer until the mixture was homogeneous (Figure 1).
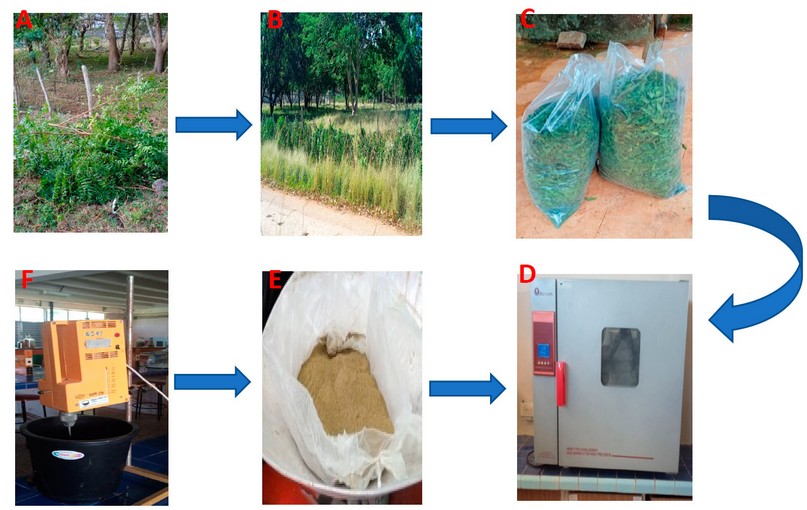
Figure 1. Scheme for obtaining the aqueous extract from Neem (Azadirachta indica A. Juss) leaves. A (Leaf harvesting), B (Sun-drying), C (Leaf collection), D (Oven-drying), E (Grinding), F (Mixture).
Determination of extraction yield.
To determine the extraction yield, aliquots of 1 ml (in triplicate) were taken from the obtained extracts and concentrated to dryness at 60°C in a Speed Vac SC100 Savant, Russia. The resulting product was weighed, and the extraction yield was determined in mg of dry extract per ml. The three concentrations to be evaluated were obtained by diluting the extract in water based on the yield calculation.
In vitro test
An in vitro test was performed using the larval development inhibition test in a 96-well plate to evaluate the effect of the crude aqueous extract. Samples of fecal matter were obtained from 18 female animals, including 6 Pelibuey sheep, 6 Criollo goats, and 6 Criollo breed calves.
Larvae identification
The larvae identified in the experiment belong to the order Strongylida, of the superfamily Trichostrongylidae, for cattle (Haemonchus placei and Cooperia sp.) and sheep-goat (Haemonchus contortus, Trichostrongylus sp, and Trichuris).
Experimental design and statistical analysis
The experimental design consisted of three treatments and two control groups: positive control (Ivermectin 100 mg/ml) and negative control (Distilled water). The treatments consisted of three concentrations (0.06 mg/ml, 0.07 mg/ml, and 0.08 mg/ml of crude extract of A. indica leaves) for each animal species, with 6 wells per triplicate for 100 larvae per well, divided into three assays. The survival percentage was determined at 0, 24, 48, and 72 h after adding the extract. The statistical analysis was performed using IBM SPSS Statistics version 23, and a two-factor ANOVA was conducted.
RESULTS
Trial 1 (Sheep).
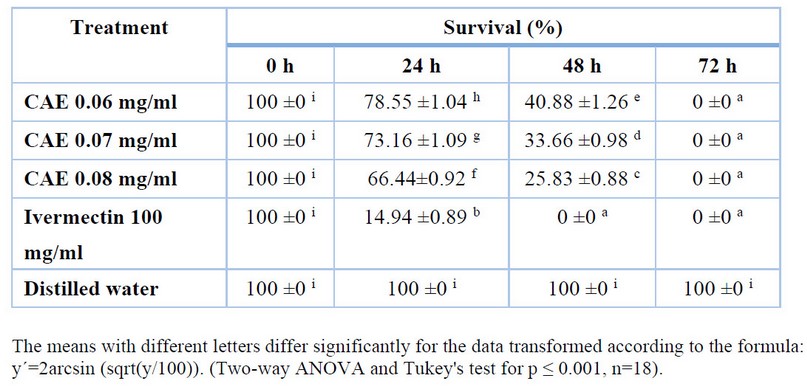
Table 1. Shows the larval survival percentages for sheep. It was demonstrated that there are statistically significant differences in the effect of exposure time and concentration of the aqueous extract of A. indica leaves on larval survival.
Table1. In vitro larvicidal effect of crude aqueous extract (CAE) from Azadirachta indica leaves on sheep gastrointestinal nematodes: impact of exposure time and concentration.
The means with different letters differ significantly for the data transformed according to the formula:
y´=2arcsin (sqrt(y/100)). (Two-way ANOVA and Tukey's test for p ≤ 0.001, n=18).
The highest concentration (0.08 mg/ml) demonstrated better effectiveness at 24 and 48 h, with the lowest percentages of larval survival, below that of Ivermectin but higher than the other concentrations. All concentrations tested showed effectiveness at 72 h, with 0% larval survival (100% larval mortality), showing a similar behavior to Ivermectin.
Trial 2 (Goat).
Table 2 displays the percentage of larval survival for all concentrations tested at each time point for goat species. The results showed that at 24 and 48 h, the highest concentration of the crude aqueous extract from A. indica leaves (0.08 mg/ml) demonstrated the most excellent effectiveness, with the lowest percentages of larval survival among all the concentrations tested. However, the larval mortality rate was still slightly below that of Ivermectin.
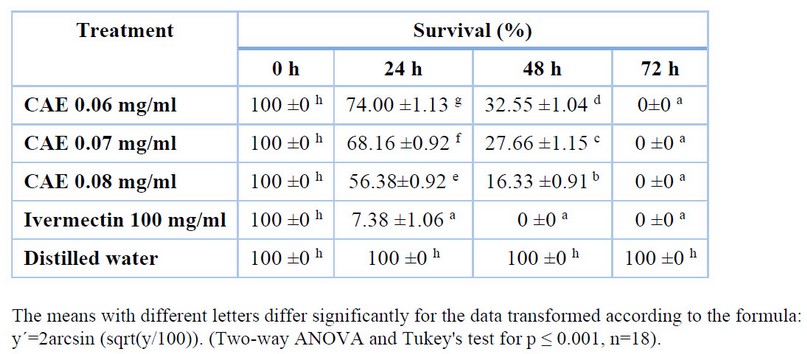
Table 2. In vitro larvicidal effect of crude aqueous extract (CAE) from A. indica leaves on goat gastrointestinal nematodes: impact of exposure time and concentration.
The means with different letters differ significantly for the data transformed according to the formula:
y´=2arcsin (sqrt(y/100)). (Two-way ANOVA and Tukey's test for p ≤ 0.001, n=18).
For the goat species, the crude aqueous extract from A. indica leaves was effective for all three concentrations, showing the lowest larval survival percentages (0%) at 72 hours, with no differences between the treatments and Ivermectin. All extract concentrations showed effectiveness at 72 hours, with 0% larval survival, indicating 100% larval mortality. This result is similar to Ivermectin, commonly used as a parasiticide in veterinary medicine.
The results of the second experiment (goat trial) showed that the behavior of the concentrations of the aqueous extract from A. indica leaves on larval survival during the experimental times was similar to the first experiment (sheep trial). This is because the larvae used in both experiments belonged to the same taxonomic family and genus for both sheep and goat species. As a result, the aqueous extract from A. indica leaves was effective for all three concentrations tested in the goat species, with the lowest larval survival percentages (0%) at 72 hours, similar to the results observed in the sheep species.
These findings demonstrate that the aqueous extract from A. indica leaves has a larvicidal effect on gastrointestinal nematodes in both sheep and goat species, reducing the number of larvae and their subsequent death during the experimental times.
Trial 3 (Bovine).
The results of the third experiment on the effect of exposure time of concentrations of the aqueous extract from A. indica leaves on the survival of larvae from the bovine species showed differences between the concentrations tested, as indicated in Table 3. However, there were no significant differences between the minimum concentration (0.06 mg/ml) and the control group (distilled water). These findings suggest that for the Haemonchus placei and Cooperia sp larvae of the bovine species evaluated in this experiment, the concentration of 0.06 mg/ml is not adequate. Therefore, for in vivo tests in this animal species to validate this in vitro experiment, concentrations starting from 0.07 mg/ml or higher should be used.
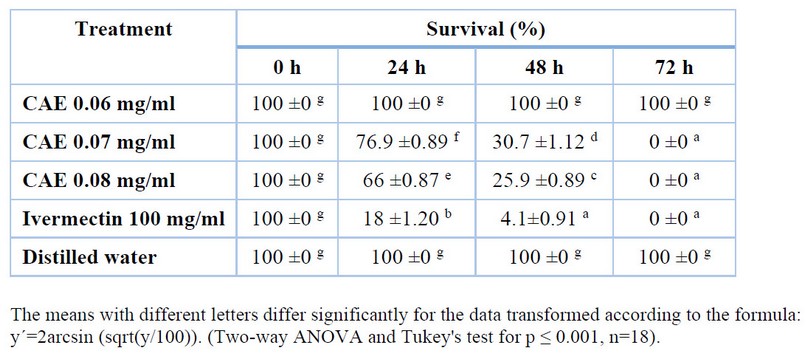
Tabla 3. In vitro larvicidal effect of crude aqueous extract (CAE) from A. indica leaves on bovine gastrointestinal nematodes: impact of exposure time and concentration.
The means with different letters differ significantly for the data transformed according to the formula:
y´=2arcsin (sqrt(y/100)). (Two-way ANOVA and Tukey's test for p ≤ 0.001, n=18).
In the third experiment, only the medium (0.07 mg/ml) and the highest (0.08 mg/ml) concentrations of the crude aqueous extract from A. indica leaves showed effectiveness at 72 h, with 0% larval survival (100% larval mortality), which is similar to the results observed for Ivermectin. However, at the start of the experiment (0 h), all larvae showed the highest percentage of survival (100%), indicating active motility.
The highest concentration (0.08 mg/ml) demonstrated better effectiveness at 24 and 48 h, with the lowest percentages of larval survival, although the results were still below those observed with Ivermectin. These findings are consistent with the results observed in the previous experiments. These results suggest that the aqueous extract from A. indica leaves has potential as a larvicidal agent against gastrointestinal nematodes in bovine species. However, further research is needed to evaluate the safety and efficacy of this extract in vivo.
Overall, the findings of this study demonstrate that the crude aqueous extract from A. indica leaves has a significant anthelmintic effect against gastrointestinal nematodes in ruminants. The extract could be a natural alternative to synthetic anthelmintics for veterinary clinical treatments in animal production systems. However, further research is needed to evaluate the safety and efficacy of the extract in vivo.
DISCUSSION
The study demonstrated that the aqueous extract of A. indica leaves has anthelmintic properties against sheep, goats, and cattle nematodes, suppressing larval development. Neem extract may interfere with the development and molting of parasitic nematodes. By disrupting the formation of new cuticles, Neem can disrupt the life cycle and decrease their ability to multiply. Jabbar et al.31 reported that A. indica leaves have been used in popular veterinary medicine as an anthelmintic for ruminants. However, Vieira et al.32 did not observe any anthelmintic effect of Neem at a dose of 30 g of dried leaves per goat/day for 5 days.
In another study by Rafique et al.15, the efficacy of aqueous, methanolic, and ethanolic extracts of dried leaves of medicinal plants Moringa oleifera and A. indica was tested for ovicidal and larvicidal activities in vitro against Haemonchus, Trichuris, Coccidia, and Trichostrongylus of wild sheep. The study evaluated six concentrations of these plant extracts (ranging from 1.56 to 50 mg/ml), which were different from those evaluated in the present experiment, and used the egg hatch assay (EHA) and larval development assay (LDA) in three replicates. The inhibitory effect on larval development was found in aqueous, methanolic, and ethanolic extracts of M. oleifera, with lethal concentration values (LC50) of 4.15 mg/ml, 2.75 mg/ml, and 1.9 mg/ml, respectively. Aqueous, methanolic, and ethanolic extracts of A. indica also showed inhibitory effects with LC50 values of 3.35 mg/ml, 1.89 mg/ml, and 2.85 mg/ml, respectively. The overall findings of this study demonstrate that the leaf extracts of M. oleifera and A. indica possess significant anthelmintic efficacy against sheep GIN, and could be a natural alternative to synthetic anthelmintics to treat worm infections in animals.
In a study by Wondimu & Bayu26, the in vitro and in vivo anthelmintic effects of crude methanolic extracts of leaves of A. indica, Vernonia amygdalina, Nicotiana tabacum, M. oleifera, Croton macrostachyus, and Hagenia abyssinica were investigated against gastrointestinal nematodes of the strongylus type in goats. The researchers used three graduated concentrations of crude extract (100 mg/ml, 50 mg/ml, and 25 mg/ml). They evaluated the in vitro anthelmintic effects using standard larval development assay, larval inhibition, and egg hatch tests. The results showed that crude extracts of N. tabacum, V. amygdalina, and C. macrostachyus were promising for the control of gastrointestinal nematodes, with a high (p<0.05) inhibition of egg hatch in vitro for C. macrostachyus compared to other plant species. The lowest inhibition was observed for M. oleifera and H. abyssinica, while N. tabacum caused 100% larvae (L3) mortality in three hours at 100 mg/ml and 50 mg/ml, unlike other plant extracts that did not show a substantial effect. The development of L1 to L3 larvae was stopped after exposure to extracts of N. tabacum, V. amygdalina, and C. macrostachyus. This study infers that crude methanolic extracts of A. indica leaves do not have biological activity against eggs and larvae of gastrointestinal nematodes of goats. However, in the present experiment, the crude aqueous extract of A. indica leaves showed in vitro biological activity with 100% larval mortality 72 h after applying the plant extract.
There are no published studies on the in vitro anthelmintic effect of A. indica on gastrointestinal nematode larvae of cattle. This medicinal plant is known to be rich in compounds that have antiparasitic, anti-inflammatory, and antimicrobial activities, such as azadirachtin, salannin, terpenoids, meliantriol, nimbin, saponins, alkaloids, tannins, acids, and steroids33,34. These agents target parasites and may have protective properties on organs in hosts infected by parasites35,36.
CONCLUSIONS
In conclusion, the current study demonstrated that the crude aqueous extract of A. indica leaves has in vitro anthelmintic activity against sheep, goats, and cattle gastrointestinal nematodes. The effective concentration of the extract was 0.06 mg/ml for sheep and goats but not for cattle. Concentrations of 0.07 mg/ml and 0.08 mg/ml were adequate for all three animal species, resulting in 100% larval mortality. These results suggest that A. indica leaf extract could be a natural alternative to synthetic anthelmintics for treating animal worm infections. However, more studies are needed to determine its efficacy and safety in live animals before widespread use.
Acknowledgments: This result is part of the Territorial and International Project: FSPI Agrecocaribe: Use of Beneficial Autochthonous Microorganisms and Natural Products in Agricultural Systems. We want to thank all the individuals who contributed to this result, especially Dr. Josmel Sajas Romero, professor at the University of Camaguey "Ignacio Agramonte y Loinaz", Dr. Carlos Armando Mazorra Calero and Dr. Jorge Martínez Melo, professors at the University of Ciego de Ávila "Máximo Gómez Báez", and Naisy Fole Companioni, ATD corresponding to the same university.
Conflicts of interest: The authors declare no conflict of interest.
REFERENCES
1. Sierra M, Flórez P, Morales E, Vásquez M, Calle M, Sierra R. Determinación de la carga parasitaria gastrointestinal en Bovinos de la zona rural de Río de Oro y el Municipio de Aguachica, Cesar, por la técnica de McMaster. Rev Fac Cienc Salud UDES. 2016;3(1.S1):20.
2. Scott J. Influence of an internal Parasite Control on Cattle Grazing Behavior and Production. Theses, Dissertations and Student Research in Agronomy and Horticulture. 2017;133.
3. Alzahrani F, Al-Shaebi EM, Dkhil MA, Al-Quraishy S. In vivo anti-Eimeria and in vitro anthelmintic activity of Ziziphus spina-christi leaf extracts fares. Pakistan Journal of Zoology. 2016;48(2):409-413.
4. López OS, Chaparro GJJ, Gómez OLM. Overview of Poultry, Eimeria, life cycle and host-parasite interactions. Frontiers in Veterinary Science. 2020;7:384.
5. Gari JA. Biodiversity and indigenous agroecology in Amazonia. The Indigenous peoples of Pastaza. Economic Geography Research Group. School of Geography and the Environment. Etnoecologica. 2001;7:21-37.
6. Salgado MS, Carrillo DF, Escalera VF, Delgado CC. Tests to identify resistant sheep to gastrointestinal parasites in San Pedro Lagunillas Nayarit. Revistas abanico. 2017;7(3):63-71.
7. Torres FRA, Higuera PRI. In vivo anthelmintic activity of terpenes and essential oils in small ruminant. Journal MVZ Cordoba. 2021;26(3):e2317.
8. Azam F, Munier S, Batool M, Ahmad B, Abbas G. A review on advancements in ethnomedicine and phytochemistry of Tribulus terrestris; a plant with multiple health benefits. International Journal of Biosciences. 2019;14:21-37. DOI: 10.12692/ijb/14.1.21-37
9. Majeed Y, Shaukat MB, Abbasi KY, Ahmad MA. Indigenous plants of Pakistan for the treatment of diabetes: A review. Agrobiological Records. 2021;4:44-63.
10. Hassan NMF, Sedky D, Abd El-Aziz TH, Shalaby HA, Abou-Zeina HAA. Anthelmintic potency and curative effect of pomegranate peels ethanolic extract against H. contortus infection in goats. International Journal of Veterinary Science. 2020;9:210-216.
11. Adoho ACC, Konmy BBS, Olounladé PA, Azando EVB, Hounzangbé-Adoté MS. Phytochemistry and larval toxicity of Ipomea asarifolia, Commelina diffusa, Acalypha ciliata and Eleusine indica against Artemia salina. International Journal of Veterinary Science. 2022;11:121-128.
12. Abbas RZ, Zaman MA, Sindhu ZUD, Sharif M. Anthelmintic effects and toxicity analysis of herbal dewormer against the infection of Haemonchus contortus and Fasciola hepatica in goat. Pakistan Veterinary Journal. 2020;40:455-460.
13. Eguale T, Tadesse D, Giday M. In vitro anthelmintic activity of crude extracts of five medicinal plants against egg-hatching and larval development of Haemonchus contortus. Journal of Ethnopharmacology. 2011;137:108-113.
14. Sobhy H, AboElnaga TR, Behour TS, Razin EA. In vitro trypanocidal activity of essential oils of some plants against Trypanosoma evansi. International Journal of Veterinary Science. 2021;10:191-195.
15. Rafique A, Mahmood MS, Abbas RZ, Ali S, Mian A, Rizwan A. Anthelmintic activity of Moringa oleifera and Azadirachta indica against gastrointestinal nematodes of wild sheep. Journal of the Hellenic Veterinary Medical Society. 2022;73(2):3989-3996. DOI: 10.12681/jhvms.25876
16. Bandeira M, Garcia S, Almeida DG, Oliveira A. Efeito do extrato aquoso das folhas de nim indiano (Azadirachta indica) sobre o crescimento inicial de plantas daninhas. Revista Gaia Scientia. 2015;9(1):1-6.
17. Souza AM, Pereira CE, de Paula EM, de Freiras RM, Kikuti AL. Bioatividade de extratos vegetais de nim, jambu e pimenta de macaco sobre sementes de alface. Revista Científica Eletrônica de Agronomía. 2016;30:43-50.
18. Rahman AH, Almatroudi A, Alrumaihi F, Khan A. Pharmacological and Therapeutic Potential of Neem (Azadirachta indica). Pharmacognosy Reviews. 2018;12(24).
19. Dublín DR, Roque LE, Estrada OJ. Efficiency of the Neem Azadirachta indica A. Juss leaf extract in the control of gastrointestinal nematodes in Pelibuey sheep. Rev. electrón. Vet. 2012;13(7):1-16.
20. Barrabí PM, Arece GJ. In vitro antihelmintic activity of an accuos extract of Neem (Azadirachta indica A. Juss) leaves and seeds. I. Inhibition of eggs hatching and larval. Revista de Salud Animal. 2013;35(2):103-8.
21. Cruz CA, Hortúa LLC, Moreno FG, González PAC. Evaluación del Efecto de Azadirachta indica y Thymus vulgaris sobre el recuento de huevos de helmintos y coccidias en corderos. REDVET. 2017;18(9):1-13.
22. Moya MA, Escudero VG. Las plantas medicinales en el control de nemátodos gastrointestinales en cabras: potencial de las plantas que crecen en la región de Coquimbo, Chile. Revista Brasileira de Plantas Medicinais. 2015;17:480–494.
23. Radhakrishnan L, Gomathinayagam S, Balakrishnan V. Evaluation of anthelmintic effect of Neem (Azadirachta indica) leaves on Haemonchus contortus in Goats. Research Journal of Parasitology. 2007;2:57-62.
24. Rahman W, Lee R, Sulaiman SF. In vitro anthelmintic activity of Neem plant (Azadirachta indica) extract against third-stage Haemonchus contortus larvae form goats. Global Veterinaria. 2011;7:22-26.
25. Sakti AA, Kustantinah K, Nurcahyo RW. In vitro and in vivo anthelmintic activities of aqueous leaf infusion of Azadirachta indica against Haemonchus contortus. Tropical Animal Science Journal. 2018;41(3):185-190.
26. Wondimu A, Bayu Y. In Vitro and In Vivo Anthelmintic Effects of Medicinal Plants Against Gastrointestinal Nematodes of Goats at Ha-ramaya University Farm, Eastern Ethiopia. East African Journal of Veterinary and Animal Sciences. 2019;3(1):55-62.
27. Jamra N, Das G, Singh P, Haque M. Anthelmintic efficacy of crude neem (Azadirachta indica) leaf powder against bovine strongylosis. Journal of Parasitic Diseases. 2015;39(4):786-788.
28. Schmutterer H. The Neem Tree. Source of unique natural products for integrated pest management, medicine, industry and other purposes. Weinheim. Edition VCH. 1995;pp 36.
29. Toledo M, Ovies D, López J, Crombet H, Lugo R. Plantas Medicinales y otras técnicas de uso práctico para la medicina veterinaria cubana. Instituto de Medicina Veterinaria. La Habana. 1990;p. 3-4.
30. Gurr S, McPherson J, Bowles D. Lignin and associated phenolic acids in cell walls. Molecular Plant Pathology and Practical Approach. 1992;3:62.
31. Jabbar A, Iqbal Z, Khan MN. In vitro anthelmintic activity of Trachyspermum ammi seeds. Pharmacognosy Magazine. 2006;2:126-129.
32. Vieira RFC, Barbosa TS, Morinishi CK, Ciarlini PC, Bomfim SRM. Volume globular de cães determinado por impedância e método de microhematócrito (strumia). Acta Scientiae Veterinariae Porto Alegre. 2007;35:433-435.
33. Singh B, Pandya D, Mankad A. A Review on different pharmacological and biological activities of Azadirachta indica A. Jussm. and Melia azedarach L. Journal of Plant Science and Research. 2020;36(1-2):53-59.
34. Malar TRJ, Antonyswamy J, Vijayaraghavan P, Ock Kim Y, Al-Ghamdi AA, Elshikh MS, Hatamleh AA, Al-Dosary MA, Na SW, Kim H. In-vitro phytochemical and pharmacological bio-efficacy studies on Azadirachta indica A. Juss and Melia azedarach Linn for anticancer activity. Saudi Journal of Biological Science. 2020;27(2):682-688.
35. Masood S, Abbas RZ, Iqbal Z, Mansoor MK, Sindhu ZD, Zia MA, Khan J. Role of natural antioxidants for the control of coccidiosis in poultry. Pakistan Veterinary Journal. 2013;33(4):401-407.
36. Wunderlich F, Al-Quraishy S, Steinbrenner H, Sies H, Dkhil MA. Towards identifying novel anti-Eimeria agents: trace elements, vitamins, and plant based natural products. Parasitology Research. 2014;113(10):3547-3556. DOI: 10.1007/s00436-014-4101-8.
Received: 20 June 2023/ Accepted: 25 August 2023 / Published:15 September 2023
Citation: Correa Herrera N, Martínez Montero M E, Cepero Rodríguez O, Jumbo Romero M J, Linares Rivero C, Fontes Marrero D, Cuello Portal S, Quiñones-Galvez J, Rodríguez Benalcázar V M, Borja Ramos A T. Effect of aqueous extract of Azadirachta indica leaves on gastrointestinal nematodes in ruminants: an in vitro study. Revis Bionatura 2023;8 (3) 45. http://dx.doi.org/10.21931/RB/2023.08.03.45
