2023.08.02.45
Files > Volume 8 > Vol 8 No 2 2023
Evaluation of humoral immunity by IgG measurement in broilers vaccinated with life and killed Newcastle vaccine
Douaa Y. Talib1,*, and Hazim T. Thwiny2
1 Department of Animal Production, College of Agriculture, University of Sumer, Thi Qar, Iraq
2 Department of Microbiology, College of Veterinary Medicine, University of Basrah, Basrah, Iraq
* Corresponding: [email protected]
Available from: http://dx.doi.org/10.21931/RB/2023.08.02.45
ABSTRACT
Current research objectives evaluated the humeral immunity induced by two different vaccination methods and estimated the efficiency of the commercially available Newcastle disease (ND) vaccine. A total number of 90-day-old unvaccinated chicks were divided equally into 3 groups; each group was vaccinated with different vaccination methods, while group 3 was the unvaccinated control group. Serum was collected from all groups, and five chickens from each group were slaughtered. ELISA measured the levels of IgG; there also were higher in the vaccinated groups (G1, G2) compared with the unvaccinated group. Group 2 also had the best IgG; The higher values of lymphoid organs (Bursa of Fabricius, thymus and spleen) indices were in. While the vaccinated groups were compared to non-vaccinated groups, between vaccinated groups, there was no significant difference (P < 0.05). The best-proven vaccine program in group 2(primary vaccine at 7th day of old by the intraocular vaccine (live vaccine), then boost potion dose at 21st day of old by drinking water vaccine(live vaccine).
Keywords: chicks, humeral immunity, vaccine.
INTRODUCTION
Newcastle Disease is a sensitive and highly fatal; contagious viral disease combined with neurological and respiratory signs that affect a widespread domestic and wild bird species throughout the five continents of the world1. Created by (NDV); an avian paramyxovirus type1 ND By pathotyping, NDV strains were classified into the highly pathogenic depending on the virulence in poultries: ketogenic, mesogenic and velogenice2,3.
The clinical form affecting the neurological, gastrointestinal, reproductive, and respiratory systems is often noted in naïve, unvaccinated, or poorly vaccinated birds. Clinical signs vary depending on the species of bird, the strain and challenge dose of the virus, and the immunity of the host4. The most common symptoms are respirational signs such as coughing, panting, sneezing and rales. Additional signs include falling wings, tedious legs, enlargement of tissues around the neck and eyes, twisted neck, rotating and interruption of egg creation.
Vaccination and appropriate hygiene measures are the most effective way to control and prevent ND. ND immunization plans include inactivated and attenuated vaccines to improve protection from infectious diseases5. Killed vaccines include viruses without pathogenic properties. It is inactivated through physical and chemical ways; it provides very high levels of antibodies against NDV and produces good protection against the virulent virus 6. It was applied by injection (S/C, IM) and has provided excellent antibody levels. In contrast, the live vaccine was applied by eye drops or drinking water technique to stimulate local defensive immunity managed by (Ig)A antibodies7.
N D V infection stimulates innate and humeral immunity; the innate immune response is an immediate reaction to control and inhibits the growth of viruses, spreading and aiding in developing pathogen-specific protection through the adaptive immune response 8, 9.
Humeral immunity eliminates extracellular pathogens (NDV) in body fluids and produces antibodies secreted by plasma cells after activation from B lymphocytes called IgG. The IgG is the most critical protective in avian immunity and can be measured in some cases in serum. The antibody titer levels still peak for 2-4 weeks from infection or vaccination 10.
In Iraq, numerous live vaccines with lentogenic strains of NDV, such as LaSota, are approved by several traders. However, these vaccines' efficiency in climatical state, spreading and transport are not inspected correctly and wisely by the trader or handler 11.
MATERIALS AND METHODS
Chicks
A total number of unvaccinated birds, 90 on 1st day of age, were taken from a local hatchery. Whole birds were adapted in their cages and provided with feeding and water during the experiment period; before the applied program, the birds were subdivided into 3 groups, each group included 30 birds, and a third group was considered the control group.
N D vaccines
This research used two types of vaccines; live vaccine Izovac NDV LASOTA and killed vaccine Nobilis ND BROILER
Experimental design
The experimentation persisted for 5 weeks, as The vaccination regimen (designed on the local recommended program, as shown in Table 1.
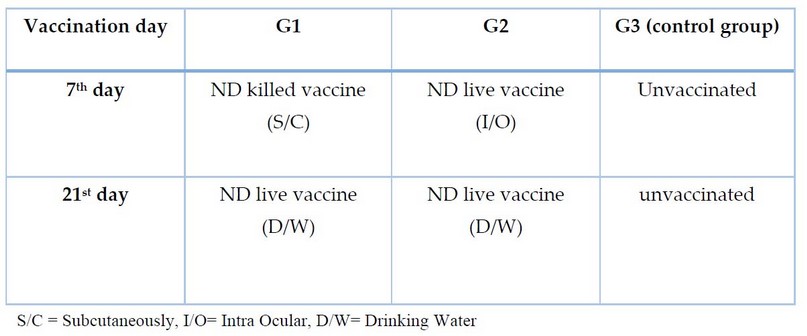
Table 1. The experimental design
Sample collection and sampling schedule
Two ml of blood was collected aseptically from each bird's wing or jugular vein and allowed to clot, and serum was separated using a bench centrifuge at 1500 rpm for 10 min. Then sera were separated and stored at -20 o C until the serological tests were carried out. Pre-vaccination sera were randomly collected from 25% of the birds used for the study on the seventh day following hatch to evaluate their maternal immune status. Six chickens were sampled randomly from each group.
Measurement of antibodies (IgG) against NDV by commercial ELISA kit
Used commercial ELISA kit (Newcastle Disease Virus Antibodies ELISA Kit, Catalog No: E-AD-E013). ELISA was performed as per the manufacturer's instructions; This ELISA kit applies to the in vitro quantitative determination antibodies against NDV
Microplate hemagglutination inhibition (H I) test
Microplate H I applied to limit the ab level of the obtained sera as of the bird in the three groups. The IgG level of the NDV in the serum was estimated by H I and cross HI tests in U-bottom microtiter plates using constant 8 H A units of LaSota strain as antigen with two-fold serum dilutions ( β method). HI, titers equal to or greater than 1/ 16 (24) were considered positive as the World Organization for Animal Health recommended.
Lymphoid organ indexes
In the last week of the experiment (35 days), each group was weighed individually; 5 birds of each group were sacrificed .than a thorough visual evaluation, the thymus, bursa of Fabricius and spleen were immediately removed, dry and individually weighed. Advanced significant lymphoid organ weight variation was assessed, and their directories were planned12.
Statistical analysis
The data collected in this research in different groups were statistically inspected via analysis methods of alteration (ANOVA). P<0.05 was measured as statistically significant.
RESULTS
Hemagglutination inhibition (HI) test
Hemagglutination was 1:128 at the last dilution of ag (antigen); the antigen titer( 8 HUA) was used. The antibody response of the chickens to vaccination schemes was assumed in Table (2); The mean pre-immunization HI antibody titer was found to be 4.6 at age 7 days, then declined to 2.6 and 0 at age 21 and 35 days, respectively.
Post-vaccination HI geometric mean titer (GMT) values were determined, and it was detected that on day 21 of age (14 days post-vaccination), all the birds in Group 1,2 seroconverted with a statistically significant increase (p < 0.05). Serum HI antibody titers that were protective. However, the birds in group 1, which had higher HI titers
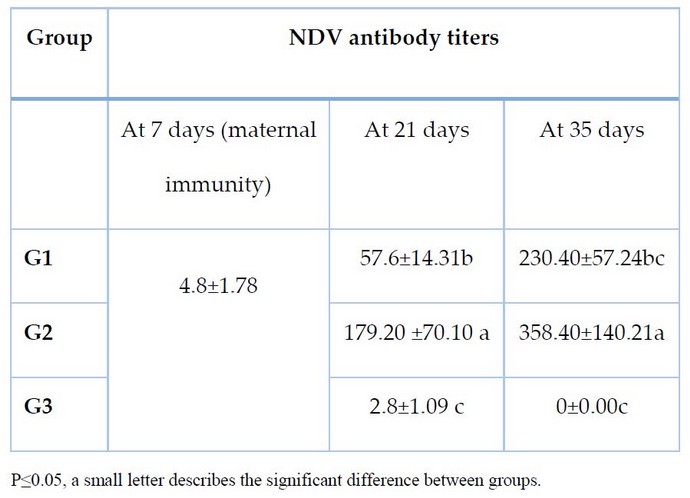
Table 2. The GMT of antibody titers against NDV in experimental groups measured by HI test
Titer of IgG concentration in serum
Serum antibody titers against NDV antigen at the first, third, and fifth week of age in all groups are obtainable in Table 3. The antibody titers against NDV was exhibited as a percentage of inhibition (IP), and the results show that maternal immunity dropped to a non-protective level at 7 days of age of chicks).
The immunized groups gave good IP comparison with the control or non-immunized groups at 14 days post-vaccination and post-booster doses at 21 days and 35 days of chicken age, respectively, as shown in Table 3.
The immunization program, which was practical in group 2, gave the highest IP, reaching 71 at 14 days post-vaccination (21 days of age) and 88 at 14 days post-booster vaccination dose (35 days of age). The result of the other group (group 1) was 58 and 73 at 21 days and 35 days of age, respectively.
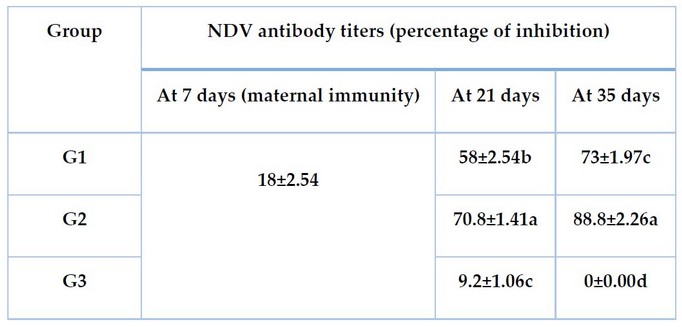
Table 3. The mean value of antibody titers (percentage of inhibition) against NDV in experimental groups measured by ELISA test
Lymphoid organs indices
The higher values seem in the immunized group compared with non-immunized groups at day 35 (4th week). In contrast, between immunized groups, there was no significant difference (P < 0.05) in lymphoid organ indices.
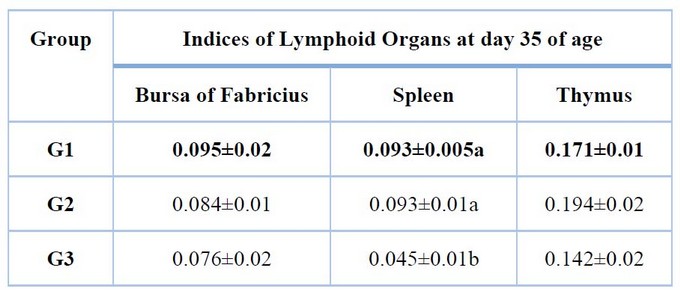
Table 4. Lymphoid organs indices of experimental groups
DISCUSSION
In the current research, the primary vaccination was approved on time as soon as the parental antibody titer was (GMT 4.8). The results showed that the maternal antibody titer on the 7th day of old was 4.8; the decline in antibody titer was recorded at the 3rd week of age and reached to untraceable at the 5th week of age measured by using hemagglutination inhibition test investigative test for N D . Early protection was necessary with traditional ND vaccines managed at an early period of age to avoid any possibility of infection.
We assessed whether the presently used vaccines under different vaccination schedules could induce effective immunity in chickens. The comparative efficacy of commercially available vaccines has been a challenge for scientists.
The results of the research showed that the antibodies titer was significantly different between treated groups, and the best-increased antibody levels were in group 2which used a live vaccine by eye drop at the 7th day of age and by drinking water at the 21st of age. Also, on days 21st and 35th of age, there were significant differences between the vaccinated and control groups (p ≤ 0.05).
In the control group, in which any N D vaccine was not used, antibody titers were decreased, and the unvaccinated chickens were susceptible to disease.
Researchers indicated that although immunization mainly provides good protection against disease and mortality, it may not provide sufficient protection against virus transmission to prevent or halt epidemics of Newcastle Disease.
Generally, analyses indicate that a high fraction of birds (>85%) needs to have a high antibody titer after vaccination to ensure that no epidemic spread is possible in vaccinated populations 13,14.
This result is also informed in research that intraocular administration produces higher protection for all vaccines than drinking water vaccine 15.
Parry 16 also found that live ND vaccines administered by eye drops or orally induce protective mucosal immunity mediated by IgA antibodies. It is speculated that the results obtained in our study indicate that the high antibody titer in group 2 on day 35 is due to the booster vaccination (day 21) with the La Sota virus, which replicated quickly in the mucosal membrane, inducing local immunity.
CONCLUSION
Current studies show that the vaccine given by the eye drop method is better than the drinking water method, and the live vaccine is better than the killed vaccine. The immunity detected in immunized birds indicated that the vaccines were effective. The antibody titer built-up in the tested birds was significant. The two-fold increase in humoral responses of the birds following the 'primer dose' and 'booster dose' was commeasurable with the efficacy of the imported vaccines. This is in tandem with the work of Abbas and Alexander 17, 18, who, in their various report, documented on NDV dose-response relationship 63 among the virus content, serological response and clinical protection.
REFERENCES
1. Zhu, W., Dong, J., Xie, Z., Liu, Q. and Khan, M.I. Phylogenetic and pathogenic analysis of Newcastle disease virus isolated from house. 2010;11456-062016-Reg. 689–698.
2. Alexander, D. J. Newcastle disease and avian paramyxovirus infections, p. 541–569. In B. W. Calnek, H. J. Barnes, C. W. Beard, and L. R. McDougald (ed.), Diseases of poultry, 10th ed. Iowa State University Press, Ames, Iowa. 1997.
3. Farhan, S. M., Abdulateef, S. M. Al-Enzy, A. F. M, Mohammed, Th. T., Saeid, Z. J. M., Al-Khalani, F. M. H. & Abdulateef, F. M. Effect of heat stress on blood alkalinity of broiler chicks and its reflection in improving the productive performance. Indian Journal of Ecology, 2020; 47: 107-109.
4. Miller, P.J, Koch, G.Newcastle disease. In: Swayne, D. E., Glisson, J.R, McDouglad , L.R, Nolan , L.K, Suarez , D.L, Nair, V(E ds.)Disease of Poultry .Wiley –Blackwell, Hoboken , New Jersey , 2013; pp.89-138. http://refhub.elsevier.com/S0378-1135(16)30804-5/sbref0290
5. Marangon, S. and Busani L. The use of vaccination in poultry production. Rev. Sci. Tech., Off. Int. Epiz., 2006; 26 (1): 265-274
6. Furuya, Y., Regner, M., Lobigs, M., Koskinen, A., Müllbacher, A., & Alsharifi, M. Effect of inactivation method on the cross-protective immunity induced by whole 'killed'influenza A viruses and commercial vaccine preparations. Journal of General Virology, 2010; 91(6), 1450-1460.
7. Jayawardane, G. W. L., Spradbrow P. B. Mucosal immunity in chickens vaccinated with the V4 strain of Newcastle disease virus. Vet. Microbiol., 1995; 46: 69-77.
8. Sick, C., Schneider, K., Staeheli, P., Weining, K.C. Novel chicken CXC and CC chemokines. Cytokine. 2000; 12, 181–186.
9. ALobaidy, B. .; Al-Falahi, M. N. .; Almarie, A. A. . Effect Of 6-Bap Growth Regulator On Seed Priming Of Several Bread Wheat Varieties Under Water Irrigation Salinity Stress. JLSAR 2021, 2, 21-26.
10. Russell, P.H. Immunity to respiratory disease.Poult Immunology. 1998; 24: 235-242.
11. Al-Shammari, A. M., Al-Nassrawei H. A., Murtadha A. M. H. Molecular diagnosis of Newcastle disease Iraqi Virulent strain virus HN gene by specific primers design. Kufa journal for Veterinary Medical Sciences. 2014;5 (2): 196-203.
12. Al-Bazy, F. I. .; Abdulateef, S. M. .; Sulimn, B. F. . Impact Of Feeds Containing Optifeed®, Vêo® Premium, And Oleobiotec® On The Lipid Peroxidation Of Male Broilers Under Heat Stress. JLSAR 2022, 3, 25-31..
13. Van Boven M, Bouma A, Fabri THF, Katsma E, Hartog L, et al., Herd immunity to Newcastle disease virus in poultry by vaccination. Avian Pathol, 2008; 37(1): 1–5. Experimental Pathogenesis for Chickens, Turkeys and Pigeons of exotic Newcastle disease from an utbreak in California during 2002-2003. Veterinary Pathology,43
14. Al-Bayar, M. A., Abdulateef, S. M., Farhan, S. M., Shawkat, S. S. & Mohammed, Th. T. Role of Nitroglycerine injection in Japanese Quail (Coturnix japonica) testes tissues parameters. Indian Journal of Ecology, 2020; 47 (10): 251-255.
15. Rehmani S. F. Newcastle disease vaccination: A comparison of vaccines and routes of administration in Pakistan Prev Vet Med, 1996; 25, 241.
16. Parry, S .H., Aitken, I.D.: Immunoglobulin A in the respiratory tract of the chicken following exposure to Newcastle disease virus. Vet. Rec., 1973; 93: 258-260.
17. Abbas, T., Muneer, M.A., Ahmed, M.D., Khan, M.A., Younus, M. and Khan, I. Comparative Efficacy of Five Different Brands of Commercial Newcastle Disease LaSota Virus Vaccines In Broilers. Pakistan Vet. J., 2006; 26(2): 55-58
18. Alexander, D.J. Newcastle disease. In Y.M.Saif, Barnes, H.J.,Lisson, J.R., Fadly, A.M., McDougald, L.R., Swayne, D.E. (ed). Disease of Poultry, 2003; pp 64-87. Iowa State University Press
KO, Oluwa OK, Omomigbehin EO. (2006). Antimicrobial activity of crude xtracts of three medicinal plants used in South-West Nigerian folk medicine on some foodborne bacterial pathogens. Afr J Tradit
Received: 15 May 2023/ Accepted: June 10 2023 / Published:15 June 2023
Citation: Talib D Y, Thwiny H T. Evaluation of humoral immunity by IgG measurement in broilers vaccinated with life and killed Newcastle vaccine. Revis Bionatura 2023;8 (2) 45. http://dx.doi.org/10.21931/RB/2023.08.02.45
