2023.08.03.95
Files > Volume 8 > Vol 8 No 3 2023
Prevalence and Risk Factors for Giardia Species in Livestock Animals of Iraq
1Iraq Natural History Research Center and Museum, University of Baghdad, Iraq
*Corresponding author: [email protected] , [email protected]
Available from: http://dx.doi.org/10.21931/RB/2023.08.03.95
ABSTRACT
Giardiasis is an infection caused by the protozoan flagellate parasite Giardia spp. in the intestine. G duodenalis, a species complex of diverse genotypes that tend to demonstrate host specificity, is responsible for most veterinary health-related infections; production animals, companion animals, and wildlife can all be infected. Abdominal pain and diarrhea, typically accompanied by steatorrhea, are the most common symptoms of Giardiasis. Cysts or antigens in feces are commonly used to diagnose the disease. Treatment regimens vary and are dependent on the indication. Control methods must include hygiene measures. Despite direct evidence of Giardia sp transmission to humans via polluted water supplies, our analysis will focus on some recent research and transition techniques for Giardia sp in goats. Both animals and people are at risk from this parasite.
Keywords: Giardia Species, Goats, Prevalence, Risk Factors, Giardiasis
INTRODUCTION
Giardia lamblia (Syn; Giardia duodenalis, Giardia intestinalis) is the causative microbe causing Giardiasis, a gastrointestinal infection that affects people and their pets, livestock, and wild animals. Giardia infection symptoms range from mild diarrhea to severe diarrhea, leading to chronic illness 1. It has a basic life cycle including rapid multiplications, trophozoites (non-invasive) on the intestinal mucosal surface, and environmentally resistant cysts excreted via the host's feces 2. Infected hosts' feces contain enormous quantities of infection cysts, contaminating drinking water, swimming pools, and food 3. Giardiasis is a zoonosis epidemic illness that affects humans and animals globally 4,5.
Most infected ruminants were asymptomatic; however, subclinical signs such as growth rate decline and feed modification efficiency impairment were identified, as well as sporadic prolonged diarrhea 6. Based on investigating the preserved genetic loci, the seven assemblages (A-G) have been documented for parasitic distinctive genes of Giardia. As a result, the A and B assemblages for humans and various mammalian hosts were identified 7. Because of the significant spread of zoonotic disease between humans and animals and the economic relevance, the A and B genotypes revealed the likely highest zoonotic risk to public health 8.
Scientific classification
Domain: Eukaryota
(unranked) : Excavata
Phylum: Metamonada
Order: Diplomonadida
Family: Hexamitidae
Subfamily: Giardiinae
Genus: Giardia 9.
Morphology
The extracellular protozoan parasite Giardia duodenalis (syn. G. lamblia, G. intestinalis) causes giardiasis and diarrheal disease in humans, livestock and companion animals worldwide. This protozoan parasite has two stages during its life cycle: a trophozoite (parasitic stage) and a trophozoite (parasitic stage)10. The trophozoites are pear-shaped and 15-20m in length, with four pairs of flagellae. They also feature a big anterior disc that adheres to the intestinal wall. Conversely, cysts are ten millimeters long, oval, and contain four nuclei but no flagella (Figure. 1).
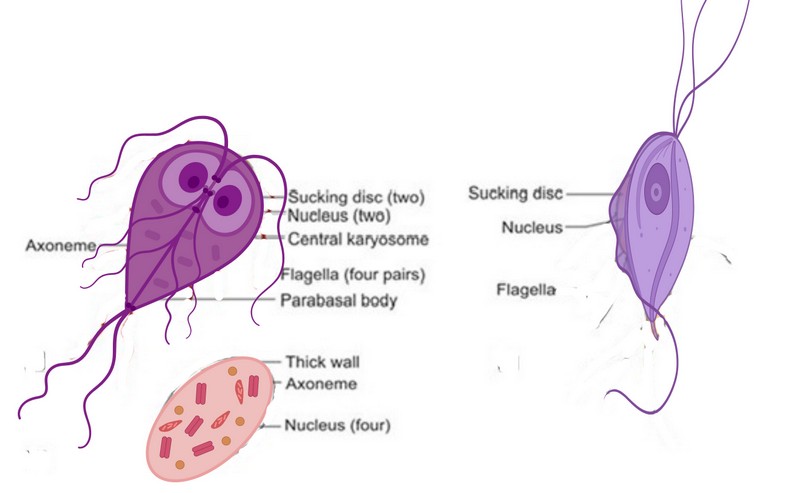
Figure 1. Morphology of Giardia
Life cycle
Giardia has a direct life cycle (no intermediate hosts) and spreads by feces-oral transfer. The infectious and resistant cysts are carried via the gastrointestinal tract and divided into two trophozoites per cyst to increase the odds of establishment after ingesting infected materials, food, or water. The pathogenic trophozoites will use their anterior disc to connect to the intestinal wall and feed on the contents. The trophozoites can replicate through binary fission within the small intestine lumen 12. It also suggested sexual reproduction; more recent research, 13, 14, supports this claim. However, the actual method of sexual reproduction and, as a result, its confirmation is unknown.
The trophozoites encyst a few days after infection and are discharged in the feces at irregular intervals 15. According to 16, the cysts are instantly infectious after excretion, and the life cycle can be completed in as little as 72 hours, despite the prepatent period typically being 4-10 days 17.

Figure 2. The life cycle of Giardia.
Review in the previous studies Distribution of Giardia species in Iraq and the world
Giardia. duodenalis has been found in some livestock animals in specific areas; epidemiological data on the parasite's incidence in sheep and goats is limited. Sheep and goats have been identified as common reservoirs of G. duodenalis. However, information on native breeds of these small ruminants in Iraq is scarce 19. A study on Giardia duodenalis infection in 50 small intestine and 50 gallbladder samples from cattle of various ages and sexes from an abattoir and a few butcher shops in Mosul City, Iraq, revealed that the percentages were 28% and 12%, respectively. Al-Qadissiya Province 20 discovered Giardiasis in a variety of animals. Twenty-one found Giardia duodenalis genotypes in goats in Iran at a rate of 15.9%. In an arid location in central Iran, 22 found that goats were infected with Giardia duodenalis at a rate of 5%.
In contrast, 23 found 2.9 % Giardia duodenalis in goats in Heilongjiang Province, China. In order to estimate the danger of zoonotic transmission and contribute to parasite epidemiology in Brazil, the first molecular genotyping of Giardia duodenalis from goats was documented. To determine the involvement of these animals in zoonotic transmission, more genetic investigations with samples from different geographical areas of the country, as well as other animal hosts and humans, are required 24.
16 out of the 375 specimens (4.3%) were positive for G. duodenalis, according to 25, with 13 of those sequenced successfully belonging to assemblage A and in Inner Mongolia, China, sheep, lambs had a much higher infection rate than ewes (8.6% vs 0.9%) respectively 26. 46.9% of the stool samples from 98 Kalahari Red goats in Nigeria showed signs of G. duodenalis infection. Twenty-seven wrote in Korean. They discovered that 44 samples (5.6%) of the calves were G. duodenalis-positive. Giardia infection was found in calves, lambs, and goat kids in Northern Ethiopia at rates of 39, 32, and 21%, respectively, according to 28. Giardia prevalence in cattle was 35.1% throughout Europe, according to 29, with neonatal animals having the most significant prevalence (39.6%), while the mean heard prevalence was 67.0%. Giardia assemblages A and B were more often discovered in younger animals, whereas mixed assemblages A and E infections were observed most frequently (55.6%).
Although G. duodenalis is found all over the globe, there are significant differences in its geographic and epidemiological distribution. Cysts are more resilient in chilly, damp environments. Therefore, Giardiasis is often seen as symptomatic waterborne outbreaks in temperate climes, typically low-prevalence locations 30. Giardiasis may be outbreak-related in the United States and is often linked to drinking or recreational water exposure. Outbreaks of food-borne illness do happen, although they are less often reported. Numerous variables, including direct and indirect, fecal contact and host characteristics, are risk factors for sporadic occurrences 31.
Other risk factors with lower odds ratios are still significant on a population level due to their high prevalence. These include daycare exposure, swimming in or drinking from natural water bodies, chronic gastrointestinal conditions, or antibiotics. Some risk factors, such as male-male sexual contact and international travel, have high odds ratios. Recent studies have not linked animal exposure to risk 32. However, there is still debate concerning animals' place in society. Animals have a modest role statistically, but there are human instances with relatively well-documented animal origins 33. Numerous studies of the relative importance of genotypes A (usually AII) and B have been reported, but the results of these studies need to identify a difference in epidemiology. In contrast, there is accumulating evidence that genotype AI is primarily a zoonotic infection 34.
Clinical signs of Giardiasis in Animals
Giardia infections appear to differ in appearance within animal groups. Giardia infection in cats or, more commonly, dogs can exist without causing symptoms and is only detected during routine fecal tests. However, in pups and kittens, it may be accompanied by chronic diarrhea or steatorrhea, which can be continuous or intermittent. It is also possible that you will lose weight. Feces in cats and dogs with clinical Giardiasis are frequently mushy, poorly shaped, pale, malodorous, include mucus, and appear greasy. Watery diarrhea is uncommon, and stools rarely contain blood. Vomiting is uncommon, but it can happen. Other forms of nutritional malassimilation must be distinguished from Giardiasis (e.g., exocrine pancreatic insufficiency and intestinal malabsorption). Clinical laboratory test results are commonly expected. Chinchillas, particularly kits, appear particularly vulnerable to Giardia infection in small animal pets. In contrast, clinical signs of disease appear reasonably expected; infection in otherwise healthy animals has been observed in surveys regularly.
Giardiasis can cause diarrhea in calves and, to a lesser extent, other producing animals that do not respond to antibiotic or coccidiostatic treatment. Giardiasis can be detected by pasty discharge to fluid stools with a mucoid appearance, especially in young animals (1–6 months old). Infection of goat kids, lambs, and calves in the lab resulted in lower feed efficiency and, as a result, lower weight gain. However, as with other species, asymptomatic excretion is typical; vast numbers of cysts can be found in the feces of cows and sheep with no apparent clinical indications 35.
Pathogenesis
A non-invasive minor intestinal pathogen called G. duodenalis causes a variety of clinical manifestations, such as persistent diarrhea and weight loss, postinfectious sequelae including irritable bowel syndrome and chronic exhaustion, growth retardation, and silent infections. It is incredibly challenging to understand the processes causing this wide variety of illnesses because of these various symptoms, which variations in the host, parasite, or microbiome may cause. Nevertheless, during the last 20 years, there has been a significant advancement in understanding some of the processes. There are also recent in-depth analyses of these advancements 36.
The likelihood of direct pathogenesis from the mechanical attachment has been postulated due to the trophozoites' strong adhesion to the small intestine mucosa. There is presently no proof to back up this hypothesis, however. Current research suggests that a combination of secreted proteases and other Giardia proteins, the host immune response, and the interaction of these factors with the intestinal microbiota 37,38 instead causes varied symptoms. During the immune response, individuals with biopsies for symptomatic Giardiasis only show flattening of the villi without any evident inflammatory alterations.
An inflammatory image may be visible, albeit it might not be related to where the trophozoites are. In a few instances, symptomatic patients' ileal biopsy specimens from ileocolonoscopy revealed trophozoites, whereas their duodenal biopsy specimens revealed inflammatory alterations 39. All the patients showed blunting or atrophy of the ileum; six of the eleven had neutrophilic infiltration, and one had symptoms compatible with celiac disease. The discovery of trophozoites in the ileum is in line with an animal model in which they sometimes appeared in the ileum or even the cecum but were primarily localized in the proximal small intestine 40 These discoveries bring up intriguing new issues about the pathophysiology of Giardiasis that are being pursued by ongoing studies.
Diagnosis
Light microscopy, combined with zinc sulfate centrifugation for cyst concentration in stool specimens, is the most practicable method for diagnosing Giardia in a clinical environment 41. Due to the sporadic nature of cyst excretion, many stool samples should be investigated over 4–5 days. Coproantigens can be detected using a variety of enzyme-linked immunosorbent assay (ELISA)-based technologies, which function well but are somewhat expensive. Due to their high cost, indirect immunofluorescence and PCR are typically used in epidemiological studies and as research tools. Copro-ELISAs are available but costly, and immunofluorescence and PCR, like Giardia, are not feasible therapeutically.
The advantage of microscopy for Giardia and Cryptosporidium is that it is non-specific; thus, additional parasites can be detected, which could help determine the source of non-specific symptoms like diarrhea. It is also worth remembering that Giardia and Cryptosporidium can be present in household animals without causing any symptoms. 42.
Diagnostic molecular tool of Giardia sp
Giardia has been better-understood thanks to the development of powerful new tools in molecular biology, which have also revolutionized our understanding of the taxonomy, population genetics, and epidemiology of Giardiasis in humans and domestic animals. The most modern molecular methods are utilized to differentiate Giardia at the species/assemblage and genotype levels, despite relatively straightforward PCR tests being employed to identify Giardia in clinical and environmental samples. These instruments are often used to determine the genotypes of G. duodenalis in clinical specimens 43.
The genome (genotype AI) was the first to be disclosed, and it was followed by those (genotype B), then (genotype AII). In addition, the dog isolates with genotypes C and D and the livestock genotype (E) from a pig 44 have all been sequenced. In order to sequence the genotypes C and D, DNA from individual cysts was amplified. Genotypes C and D belong to the same group phylogenetically, although they differ from one another roughly as much as genotypes A and B do. Mammals harbor all eight genotypes of G. duodenalis. A and B are two often seen in people and sporadically in other animals.
Based on variations in sequencing and biology, two groups (AI and AII) of genotype A isolates have been identified. Sequence variations across isolates are uncommon in the relatively homogenous Genotype AI group, according to 45 research. The initial Giardia genome was released in 2007 and has since been improved using optical mapping 46, longer reads with optical mapping 47, and other methods. However, it has been argued that for organisms like Giardia, a clonal or near-clade approach should be utilized and that naming distinct species is premature 48. The need for molecular typing, which is not presently accessible, would make it difficult to detect them at a clinical level. The term "G. duodenalis" is used for all of these genotypes for this review, even though there is still no agreement on the preferable nomenclature.
The target gene (such as the SSU rRNA, gdh, tpi, ef1, bg, and variant surface protein [vsp] genes), the number of loci analyzed, the assay's specificity (Giardia specific, G. duodenalis specific, or assemblage specific), and the subsequent steps (RFLP analysis or DNA sequencing of PCR products) all affect the usefulness of molecular diagnostic tools. Recently, 43 examined the use of these loci for genotyping and subtyping of G. duodenalis and their sequencing features. There are a few frequently used primers for identifying the species, genotype, and subtype of Giardia isolates in animal and human specimens. The SSU rRNA, gdh, tpi, and bg genes are among those that are commonly targeted.
Treatment and Vaccination
Although the need to treat infections in ruminants is debatable, no licensed medicine is currently available to treat Giardiasis in ruminants. Several medications have been demonstrated to be effective against Giardiasis in calves see 49, for a list of these drugs and their dosages. Treatment alone is insufficient to control Giardia infection in ruminants since re-infection occurs quickly, and daily drug administration would be required due to the high degree of environmental contamination 50. In all species, good husbandry, including quick removal of feces from the environment, is likely to reduce the odds of re-infection and transmission of Giardia.
In North America, a G. duodenalis vaccine made from trophozoites obtained from sheep is available for dogs and cats 51. Puppies and kittens who were subcutaneously vaccinated with the vaccine and then challenged with illness showed no clinical indications of Giardiasis. They showed that intestinal trophozoites and fecal cyst excretion were reduced or eliminated and that vaccinated mice gained more weight than non-vaccinated animals 52. However, several additional trials have failed to show that the vaccine has a meaningful effect on diseased animals 53, 54, Anderson et al., 2004). There is currently no Giardia vaccination available for both humans and animals.
.
CONCLUSIONS
G. lamblia is the most common protozoal infection and is crucial for human and animal health. Despite its wide range of effectiveness on the validity of human hosts, G. lamblia remains a reckless parasite that infects many people. The prevalence and insistence related to control planning for eradication of the parasite, mainly cleaning the source of drinking water, are still limited, so more future research on the mechanism of infection resistance and strategy for suitable control measures should be taken to eradicate the giardiasis infection is required.
Conflicts of Interest
The authors declare no conflict of interest.
REFERENCES
1- Feng, Y; Xiao, L. Zoonotic potential and molecular epidemiology of Giardia species and Giardiasis. Clin Microbiol Rev. (2011);24(1):110–40.
2- Adam, R.D. Biology of Giardia lamblia. Clin Microbiol Rev. (2001) 1;14(3):447–75.
3- Leber, A.L; Novak-Weekley S. Intestinal and urogenital amebae, flagellates, and ciliates. In: Manual of Clinical Microbiology, 10th Edition. American Society of Microbiology; (2011). p. 2149– 71.
4- Chatterjee, K.D. Parasitology: Protozoology and Helminthology. Thirteen Edition. Calcutta, New Delhi: Thomson Press; (2009).
5- Roberts, L.S; Janovy, J.; Gerald, D. Schmidt; Larry, S. Roberts’ Foundations of Parasitology. (2009).
6- Lalle, M; Pozio, E; Capelli, G; Bruschi, F; Crotti, D.; Caccio, S.M. Genetic heterogeneity at the β-giardin locus among human and animal isolates of Giardia duodenalis and identification of potentially zoonotic subgenotypes. Int J Parasitol. (2005) ;35(2):207–13.
7- Monis, PT; Andrews, R.H; Mayrhofer, G; Ey, P.L. Genetic diversity within the morphological species Giardia intestinalis and its relationship to host origin. Infect Genet Evol. (2003) ;3(1):29–38.
8- Xiao, L; Fayer, R. Molecular characterization of species and genotypes of Cryptosporidium and Giardia and assessment of zoonotic transmission. Int J Parasitol. (2008) ;38(11):1239–55.
9- Künstler, J. "Sur cinq protozoaires parasites nouveaux". C. R. Acad. Sci. Paris. (1882); 95: 347–349.
10-Halliez, M.C; Motta, J.P; Feener, T.D;Guerin, G; LeGoff, L; Francois, A; Colasse, E; Favennec, L; Gargala, G; Lapointe, TK . Giardia duodenalis induces paracellular bacterial translocation and causes postinfectious visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol (2016); 310(8):G574-585; PMID:26744469.
11-Sankar, A. S ; Sandhya, B. K. Chapter-04 Flagellates (Intestinal and Genital) Essentials of Medical parasitology ,(2014); 14 p.
12- Meloni, p.B; , AJ; Thompson, R.C. Characterization of Giardia isolates using a non-radiolabeled DNA probe, and correlation with the results of isoenzyme analysis. Am J Trop Med Hyg. (1989) ;40(6):629-37.doi: 10.4269/ajtmh.1989.40.629.
13- Logsdon, S. Encyclopedia of Soil Science.(2008). Soil Science Society of America Journal. 72, Issue 3. https://doi.org/10.2136/sssaj2008.0003br.
14-Birky, CW Giardia sex? Yes, but how and how much? Trends Parasitol., 26 (2010), pp. 70-74. https://doi.org/10.1016/j.cub.2004.12.055.
15-Taylor, G. D; Wenman, W. M; Tyrrell, D. L. J. Combined metronidazole and quinacrine hydrochloride therapy for chronic Giardiasis. Can Med Assoc J. 1987;136:1179–1180.
16-Thompson, R.C.A Reynoldson, J.A. Mendis, A.H.W. Giardia and Giardiasis. Advances in Parasitology. (1993). 32; 71-160.
17-Xiao,L; Herd, R. P. Infection pattern of Cryptosporidium and Giardia in calves. Vet Parasitol.1994 Nov;55(3):257-62. doi: 10.1016/0304-4017(93)00645-f.
18-Rajurkar, M.N; Lall,N; Basak,S ; Mallick, S.K. A Simple Method for Demonstrating the Giardia Lamblia Trophozoite. (2012). 6(9): 1492–1494. doi: 10.7860/JCDR/2012/4358.2541.
19-Alhayali, NS. Detection of Giardia duodenalis in Cattle in Mosul City, Iraq. Egyptian Journal of Veterinary Sciences .2020. 51(.3), pp. 381-390.
20- Jasim, J.A . Alkhanaq, M.N. Al_Ardi , M.H. Abd Ali Alrammah, H.S Use of Molecular Method to Detect Giardiasis in Different Animal in Al-Qadissiya Province – Iraq. Indian Journal of Forensic Medicine & Toxicology, (2021), Vol. 15, No. 3.
21-Hedieh, J; Mohammad, H.R; Masoud, SA; Mohammad, R.H . Determination of Giardia duodenalis genotypes in sheep and goat from Iran J Parasi Dis (2012). 38(1): 81–84.
22- Narges, K; Ali, f; Akram, A; Alireza, S; Alireza, Z; Zohre, F; Behnam, E; Arefeh, D. Una, R ; Fateme, A. , Molecular typing of Giardia duodenalis in cattle, sheep and goats in an arid area of central Iran Infection Genetics and Evolation, (2019), Volume 75.
23- Weizhe, Z; Xiaoli, Z; Rongjun, W; Aiqin, L; Yujuan, S; Hong, L; Jianping, C; Fengkun, Y; Xiaoyun, Z; Longxian, Z. Genetic characterizations of Giardia duodenalis in sheep and goats in Heilongjiang Province, China and possibility of zoonotic transmission. PLoS Negl Trop Dis; (2012) , 6(9):e1826.
24- Semenza, J.c; Sudre, B; Miniota,J; Rossi,M; Hu, W; Kossowsky,D; Jonathan E. Suk, Bortel,W; Khan, K. International Dispersal of Dengue through Air Travel: Importation Risk for Europe. PLoS Negl Trop Dis. 2014. 8(12): e3278. https://doi.org/10.1371/journal.pntd.0003278.
25- Ye, J; Xiao, L; Wang, Y; Guo, Y; Roellig, D.M ; Feng, Y. Dominance of Giardia duodenalis assemblage A and Enterocytozoon bieneusi genotype BEB6 in sheep in Inner Mongolia, China. Veterinary Parasitology 2015;210:235-239. DOI: http://dx.doi.org/10.1016/j.vetpar.2015.04.011.
26- Akinkuotu, O. A; Okwelum, N; Famakinde, S. A; Akinkuotu, A. C;Oseni, O. T. Giardia Infection in Recently Acclimatized Kalahari Red Goats in Nigeria . Nigerian Veterinary Journal. (2016). 37(1); 16-23pp.
27- Oh,S; Jung ,S; Lee , H; Choe ,C; Hur ,T; So, K. Multilocus Genotyping of Giardia duodenalis Occurring in Korean Native Calves. Veterinary Sciences. .(2021);8(7), 118; https://doi.org/10.3390/vetsci8070118.
28- Kifleyohannes,T; Nødtvedt,A; Debenham, J; Terefe,G; Robertson,L Cryptosporidium and Giardia in Livestock in Tigray, Northern Ethiopia and Associated Risk Factors for Infection: A Cross-Sectional Study. Frontiers in Veterinary Science, .(2022).8;(1-11)pp.
29- Mateusa,M; Ozolin, Z ; Terentjeva,M ; Deksne,G. Giardia duodenalis Styles, 1902 Prevalence in Cattle (Bos taurus Linnaeus, 1758) in Europe: A Systematic Review. Microorganisms, .(2023). 11(2);309 https://doi.org/10.3390/microorganisms11020309
30- Coffey, C.M; Collier, S.A; Gleason, M.E; Yoder, J.S; Kirk, M.D; Richardson, A.M; Fullerton, K.E; Benedict, K.M. Evolving epidemiology of reported giardiasis cases in the United States, 1995–2016. Clin Infect Dis (2021). 72:764–770. doi: 10.1093/cid/ciaa128.
31- Benedict, K.M; Collier, S.A; Marder, E.P; Hlavsa, M.C; Fullerton, K.E; Yoder, J.S.. Case-case analyses of cryptosporidiosis and Giardiasis using routine national surveillance data in the United States—2005–2015. Epidemiol Infect (2019).147:e178. doi: 10.1017/S0950268819000645
32- Reses, HE; Gargano, J.W; Liang, J.L; Cronquist, A; Smith, K; Collier ,S.A; Roy, S.L; Vanden Eng, J; Bogard, A; Lee, B; Hlavsa, M.C; Rosenberg, E.S; Fullerton, K.E; Beach, MJ; Yoder, J.S. Risk factors for sporadic Giardia infection in the USA: a case-control study in Colorado and Minnesota. Epidemiol Infect (2018). 146:1071–1078. doi: 10.1017/S0950268818001073.
33- de Lucio, A.; Bailo, B.; Aguilera, M.;Cardona, G.A; Fernandez-Crespo, J.; Carmena, D. No molecular epidemiological evidence supporting household transmission of zoonotic Giardia duodenalis and Cryptosporidium spp. from pet dogs and cats in the province of Alava, Northern Spain. Acta Trop, (2017). 170:48–56. doi: 10.1016/j.actatropica.2017.02.024.
34-Ankarklev, J; Lebbad, M; Einarsson, E; Franzen, O; Ahola, H; Troell, K; Svard, S.G. A novel high-resolution multilocus sequence typing of Giardia intestinalis assemblage A isolates reveals zoonotic transmission, clonal outbreaks and recombination. Infect Genet Evol (2018).60:7–16. doi: 10.1016/j.meegid.2018.02.012.
35- Koudela, B; Vítovec, J. Experimental giardiasis in goat kids. Vet Parasitol
. (1998 ) 15;74(1):9-18. doi: 10.1016/s0304-4017(97)00146-5.
36- Certad, G; Viscogliosi, E; Chabe, M; Caccio, SM. Pathogenic mechanisms of Cryptosporidium and Giardia. Trends Parasitol , (2017.33:561–576.
37- Fink, M.Y; Singer, S.M. The intersection of immune responses, microbiota, and pathogenesis in Giardiasis. Trends Parasitol , 2017.33:901–913.
38- Allain, T; Fekete, E; Buret, AG Giardia cysteine proteases: the teeth behind the smile. Trends Parasitol 2019.35:636–648.
39- Oberhuber, G. Mesteri, I. Kopf, W; Muller, H. Demonstration of trophozoites of G. Lamblia in ileal mucosal biopsy specimens may reveal Giardiasis in patients with significantly inflamed parasite-free duodenal mucosa. Am J Surg Pathol 2016.40:1280–1285.
40-Barash, NR; Nosala, C; Pham, J.K; McInally, S.G; Gourguechon, S; McCarthy-Sinclair, B; Dawson, S.C. Giardia colonizes and encysts in high-density foci in the murine small intestine. mSphere 2017.2:e00343-16.
41- Zajac, A.M ; Johnson, J; King, S.E. Evaluation of the importance of centrifugation as a component of zinc sulfate fecal flotation examinations. Journal of the American Animal Hospital Association. (2002) ;38:221–224.
42- Elliot, A; Morgan, U.M; Thompson, R.C.A. Improved staining method for detecting Cryptosporidium oocysts in stools using Malachite Green. Journal of General and Applied Microbiology. (1999);45:139–142.
43- Wielinga, C. M., and R. C. Thompson. . Comparative evaluation of Giardia duodenalis sequence data. Parasitology (2007). 134 : 1795-1821.
44- Jerlstrom-Hultqvist, J; Franzen, O; Ankarklev, J; Xu, F; Nohynkova, E; Andersson, J.O; Svard, S.G; Andersson, B. Genome analysis and comparative genomics of a Giardia intestinalis assemblage E isolate. BMC Genomics (2010). 11:543. doi: 10.1186/1471-2164-11-543.
45- Tsui, C.K; Miller, R; Uyaguari-Diaz, M; Tang, P; Chauve, C; Hsiao, W; Isaac-Renton, J; Prystajecky, N. Beaver fever: whole-genome characterization of waterborne outbreak and sporadic isolates to study the zoonotic transmission of Giardiasis. (2018).mSphere 3:e00090-18. doi: 10.1128/mSphere.00090-18.
46- Perry, D.A; Morrison, H.G; Adam, R.D. Optical map of the genotype A1 WB C6 Giardia lamblia genome isolate. Mol Biochem Parasitol( 2011). 180:112–114. doi: 10.1016/j.molbiopara.2011.07.008.
47- Xu, F; Jex, A; Svard, S.G. A chromosome-scale reference genome for Giardia intestinalis WB. Sci Data,(2020). 7:38. doi: 10.1038/s41597-020-0377-y.
48- Tibayrenc M, Ayala ,F.J. Cryptosporidium, Giardia, Cryptococcus, Pneumocystis genetic variability: cryptic biological species or clonal near-clades? (2014).PLoS Pathog 10:e1003908. doi: 10.1371/journal.ppat.1003908
49- O’Handley, R.M; Olson, M.E. Giardiasis and cryptosporidiosis in ruminants. Veterinary Clinics of North America Food Animal Practice. (2006) ;22:623–643.
50- Geurden, T; Claerebout, E; Dursin, L; Deflandre, A; Bernay, F; Kaltsatos, V; Vercruysse, J. The efficacy of an oral treatment with paromomycin against an experimental infection with Giardia in calves. Veterinary Parasitology.(2006);135:241–247.
51- Olson, M.E; Ceri, H; Morck, D.W. Giardia vaccination. Parasitology Today. (2000);16:213–217.
52- Olson, M.E; Morck, D.W; Ceri, H. Preliminary data on the efficacy of a Giardia vaccine in puppies. Canadian Veterinary Journal. (1997);38:777–779.
53-Payne, PA;Ridley, R.K; Dryden, M.W; Bathgate, C; Milliken, G.A; Stewart, P.W. Efficacy of a combination febantel–praziquantel–pyrantel product, with or without vaccination with a commercial Giardia vaccine, for treatment of dogs with naturally occurring Giardiasis. Journal of the American Veterinary Medicine Association. (2002);220:330–333.
54- Stein, J.E; Radecki, S.V; Lappin, M.R. Efficacy of Giardia vaccination in the treatment of Giardiasis in cats. Journal of the American Veterinary Medical Association. (2003);222:1548–1551.
55- Anderson, K.A; Brooks, A.S; Morrison, A.L; Reid-Smith, R.J; Martin, S.W; Benn, D.M; Peregrine, AS Impact of Giardia vaccination on asymptomatic Giardia infections in dogs at a research facility. Canadian Veterinary Journal. (2004) ;45:924–930.
Received: 25 June 2023/ Accepted: 15 June 2023 / Published:15 September 2023
Citation: Makawi Z A and Jassim S Y. Prevalence and Risk Factors for Giardia Species in Livestock Animals of Iraq. Revis Bionatura 2023;8 (3) 95 http://dx.doi.org/10.21931/RB/2023.08.03.95
