2020.05.01.6
Files > Volume 5 > Vol 5 No 1 2020
INVESTIGATION / RESEARCH
Laboratory scale evaluation of Effective Microorganisms in the control of odor of organic waste from a market in the city of Riobamba, Ecuador
Cristina Calderón-Tapia1, Abigail Montero-Calderón2, María Núñez-Moreno1, Esteban Pazmiño-Arias2
Available from: http://dx.doi.org/10.21931/RB/2020.05.01.6
ABSTRACT
Inadequate waste management and poor storage conditions are a problem that still affects the population and environment. In the city of Riobamba (Chimborazo, Ecuador) some people feel affected by odor pollution generated by the accumulation of waste in landfills near to markets, corners, and public places. For minimizing this problem, the present work analyzed the potential odor reduction of organic waste from a market located in Riobamba, using Effective Microorganisms: Lactobacillus plantarum, Rhodopseudomonas palustris, Streptomyces albus, and Aspergillus oryzae. Four combinations of cocktails were formed subsequent to evaluating the antagonism of microbial strains. The odor sensory evaluation was carried out by 40 people using the American Society of Heating, Refrigeration and Air Conditioning Engineers odor scale, biochemical oxygen demand, chemical oxygen demand, temperature, pH, conductivity, turbidity, and color were measured in the treatment which reached imperceptible odor intensity. In this way, the cocktail formed by the four strains of Effective Microorganisms presents a reduction in the values of the physicochemical parameters of the leachate compared to the sample without the microorganisms, and furthermore, that cocktail controls bad smell produced by the decomposition of organic matter. Therefore, the application of Effective Microorganism opens up a possibility for the treatment of organic waste within local garbage collection stations.
Keywords. Odor, decomposition, control, Effective Microorganisms.
INTRODUCTION
Solid waste is the biggest problem of environmental impact worldwide; they affect the soil and air quality by the gases produced at their decomposition. Additionally, they transform water when they are deposited into it or dragged by rain. Organic waste resulting from animal, agricultural, and industrial production is the primary source of pollution in several countries1.
In 2014, approximately 11203.24 tons of solid waste were collected daily in Ecuador; of that, 62% was organic waste, 25% was a recyclable inorganic waste, and 13% was non-reusable hazardous waste2,3. In 2010, an average of 150 tons of solid waste per day was generated in the city of Riobamba (Chimborazo, Ecuador) by 225.74 habitants4. The biggest problem in Riobamba is odor pollution, due to the accumulation of waste in landfills located in markets, corners, and public places.
The decomposition of organic waste is a severe problem due to the large amount produced, also, air pollution due to bad odors has been increasing in recent years. In Spain for example, 25% of the population feels affected by this problem; in Ecuador, 26.76% of the population indicates having issues due to inadequate environmental odors5.
Some gases are generated as a result of waste rot: acetic acid, acetaldehyde, ammonia, amines, mercaptans, phenol, toluene, sulfuric acid, and other sulfur compounds6.
Additionally, the incorrect way of waste storage can generate the production of pathogens, which present a high risk to the health of the population, and mainly to people who handle the waste for final disposal7.
Effective Microorganisms (EM) are cultures of mixed organisms that degrade organic matter and allow its use for plants, improve soil characteristics and conditions for agriculture. The EM was formulated as a microbial cocktail using: photosynthetic bacteria, lactic acid bacteria, yeasts, fungi, and actinomycetes. In the process of rotting organic matter, the EM produces organic acids that are not usually in the soil, such as lactic acid, acetic acid, amino acids, malic acid, and vitamins that could be absorbed by plants8.
This research aimed to formulate a microbial cocktail that reduces odors caused by the decomposition of organic waste. Four strains of microorganisms were chosen to obtain an EM consortium: Lactobacillus plantarum, Rhodopseudomona palustris, Streptomyces albus, and Aspergillus oryzae to check the ability of the microbial consortium to reduce the substances that produce bad odors from the waste by removing pathogenic microorganisms through competitive exclusion.
MATERIALS AND METHODS
This study was carried out in the Molecular Biology-Genetics and Microbiology laboratory at Science Faculty, Escuela Superior Politécnica de Chimborazo (ESPOCH), Ecuador. The culture collection is belonging to Plantsphere Laboratories, Quito, Ecuador (Table 1).
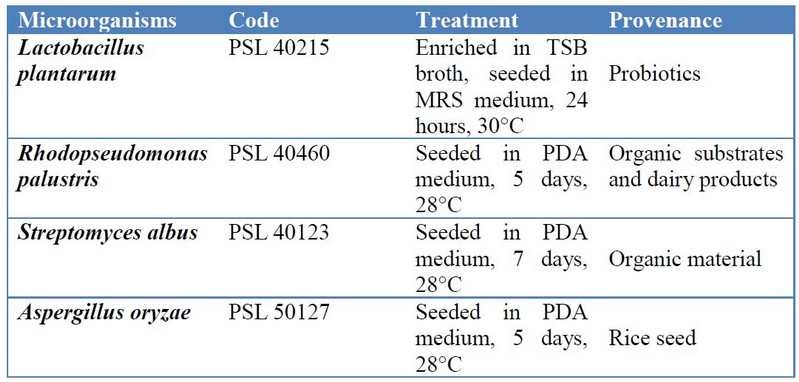
Table 1. Summary of the methods for microbial strains activation
Antagonism test
A confrontation was made between the four microbial strains in Petri dishes with PDA medium at 28°C for seven days. The microbial suspensions were prepared in concentrations of 1x104 CFU mL-1, in saline solution for L. plantarum and R. palustris, and tween 80 0.1% for S. albus. Then 5mm diameter discs of A. oryzae were taken, using sterile punches9. Three essays of the antagonistic effect and three controls were performed with the fungus.
The inhibition rate was determined by I = [(C-T) /C] x 100. Where, C is the radius of the mycelium of the control, T is the radius of the mycelium.
Treatment design
Four treatments (T1 to T4) and control treatment (T5) was performed with three repetitions each, with concentrations described in Table 210.
To determine a concentration of microorganism to work, the spore counts of A. oryzae and S. albus strains was performed in triplicate using a Neubauer chamber. Once the required concentration was found, the inoculums were stored at 5°C until the preparation of the EM cocktails.
Additionally, the inoculum of L. plantarum and R. palustris was suspended into 10 mL of saline solution, homogenized in a vortex for 5 minutes, and successive dilutions were made until 10-5. To define a concentration, each dilution was counted by triplicate in a cell counting chamber11.
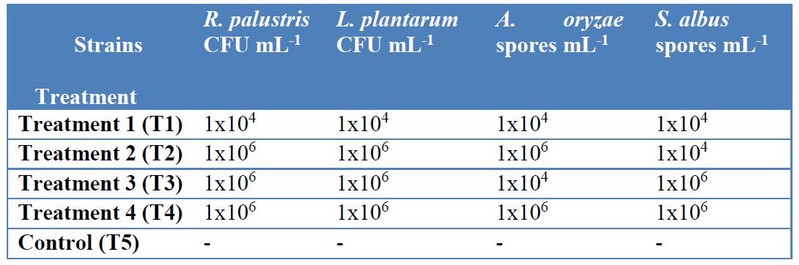
Table 2. Treatment design
Treatment Formulation
The cocktails were prepared with: 90% distilled water, 5% sterile molasses, and 5% of microbial inoculums (1.25% of each EM inoculum); they were incubated at 28°C for 24 hours. For each treatment, the ratio of cocktail to organic waste was 1:1000. The biomass used as a substrate was obtained from the organic waste of markets from Riobamba. The substrate was chopped for obtaining a homogeneous mixture of 200 Kg m-3.
The presence of the microbial strains was checked, before and after the assays, by observation under the microscope: fungal staining using lactophenol blue dye12 and the Gram staining technique was used for bacteria13.
Odoriferous evaluation of cocktails.
A group of 40 heterogeneous people from 19 to 23 years old was selected. An eight days assay was performed14. The microbial cocktails were scattered on biomass on days: one, three, and six; and the odor evaluation was performed on days: one, three, six and eight. On day one, the organic waste was placed into experimental devices; the panel checked in each treatment if the smell was homogeneous or if it was in advanced decomposition. The scale used for the analysis of the tests is shown in Table 3 and is proposed by the American Society of Heating Refrigerating and Air Conditioning Engineers (ASHRAE). Additionally, some parameters as temperature, pH and conductivity were measured for each treatment into experimental devices15.
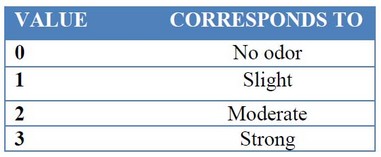
Table 3. Relative odor force scale
Leachate Analysis
The leachates from the treatment with the highest efficiency on odoriferous evaluation and the control treatment were collected for determining: biochemical oxygen demand (BOD5), chemical oxygen demand (COD), turbidity, color, temperature, pH, and conductivity (Table 4). These analyses were performed in the Water Quality laboratory at Science Faculty, ESPOCH, Ecuador.
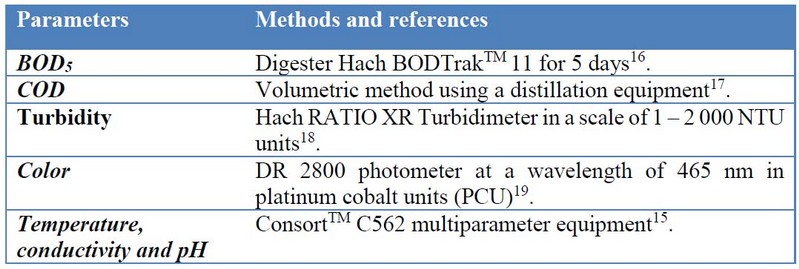
Table 4. Methods for monitoring leachate
RESULTS AND DISCUSSION
Antagonistic activity
The inhibition percentage associated with: L. plantarum, R. palustris, S. albus, and A. oryzae, ranged from 4% to 7.8%. The low antagonistic degree of A. oryzae, equal to 1, suggests that the fungus can invade ¼ of the surface of other microorganisms without damaging it. Additionally, the results show no formation of inhibition halos among the four microbial cultures, so its use as a single consortium of microorganisms is recommended due to the symbiotic effect presented. Therefore fungi, actinomycetes, and bacteria can co-exist in a mixed culture8; and they can be included in a biological treatment system for odor abatement20.
Evaluation of the growth of the culture in the microbial consortium
The four microbial cultures, which formed the initially mixed consortium, were remained after the treatments (Figure 1). Despite the notorious presence of A. oryzae, the growth of R. palustris, L. plantarum, and S. albus, was not inhibited.
Figure 1. The consortium of microorganism: a) S. albus; b) L. plantarum; c) R. palustris; and d) A. oryzae.
The efficiency of a biological treatment system for odour reduction depends on its heterotrophic microbial consortium20. Lactobacillus plantarum, Streptomyces albus, and Aspergillus oryzae are heterotrophic microorganisms while Rhodopseudomonas palustris has a versatile metabolism; for that reason, when they were placed into organic waste and molasses as substrate, they had nutrients necessary for gain energy21 and growing after the treatments.
Odor analysis
The application of EM consortium had a significant effect (p<0.05) on the differentiation of odor levels from organic waste, in fact, the panel perceived odor variations during the period of treatment until day eight. The use of pure cultures in biological treatment system for odor reduction in air, like they used in the mixed consortium for T1 to T4, ensures the early action against the potential pathogens that cause possible emissions of odour22; likewise, Fan, et al.23, determined the reduction of time for elimination of pungent odor coming from the decomposition of organic matter on home scale organic waste composting, so the unpleasant smells of compost with EM varied to earthy smell on week five compared to control treatment (without EM) which generated earthy smell on week seven.
Figure 2. Odor levels by treatments with capable microorganisms (T1 to T4) and control without EM (T5).
According to the sensory procedure performed14, T4 can be considered as an effective way to odor control of organic waste. The parameter “Strong odor” was: 25%, T1; 17.5%, T2; 2.5%, T3; and 0%, T4; as shown in Figure 2. The threshold level of olfactory identification for some malodorous compounds is: 42 ppm, acetone; 17 ppm, ammonia, and 0.00041 ppm, hydrogen sulphide21. In this way, T4 could have eliminated the perception for unpleasant smell compared to control T5 which kept the parameter “Strong odor” on 80% of the panel.
By comparison, the T4 contained the highest concentration of EM: ~106 CFU mL-1 of L. plantarum and R. palustris, and ~106 spores mL-1 of S. albus and A. oryzae. Namasivayam and Kirithiga24 verified that native microorganism increased when EM consortium also increased, they used high concentrations of compost with EM (12.1x106 CFU g-1 of bacteria, 21.3x105 CFU g-1 of actinomycetes, and 15.1x104 CFU g-1 of yeast and mold) for improving the soil nitrogen, phosphorus, and potassium levels.
Effect of temperature and pH
The temperature had a gradual increase in all treatments (Figure 3), but the treatment without EM presented the lowest temperature (23°C) at the end of the assay.
Changes in the temperature of the composting of organic wastes are closely related to microbial activity25; in this way, each increase of 10°C in the medium, is directly related to the microbial metabolic rate.
Figure 3. Temperature variations (°C) for treatments with EM (T1 to T4) and control without EM (T5).
The highest temperature (33°C) was reached at T4 on day 6. This behavior is similar to the presented by Song, et al. .25, for the decomposition of organic waste, where a higher temperature was observed in the treatment with a microbial consortium instead of those without microbial inoculation.
On the other hand, the tests with EM had a pH equal to 6 on day 8, while the maximum pH reached by the control treatment was balanced to 5 at the same time (Figure 4). The pH range suggested26 to carry out an appropriate degradation of organic matter with EM consortium is between 6 to 8.5; considering that in the initial phase of decomposition, the pH decreases during the first days; then it has a gradual increase until reaching values of 8.16 (at day 15) and 7.90 (at day 30).
Figure 4. pH variations for treatments with EM (T1 to T4) and control without EM (T5).
The variation in pH can be related to the production of odors since acidification, neutralization, and alkalization of pH in composting processes are closely related to microbial activity through the release of ammonia and the conversion of organic acids into CO225. Likewise, Miller, Macauley and Harper27, identified that a pH between 8 to 9 leads to the loss of nitrogen through the volatilization of ammonia, which is a compound identified as causing the bad smell in compost.
Leachate analysis
The values of BOD5, COD, turbidity, and conductivity obtained for T4 against T5, were as shown in Table 5. In leachates generated from vegetable waste in composting processes in laboratory28, COD concentration varies between ranges from 18 to 68 g L-1, and for BOD5 between 10 and 46 g L-1; in this way, the COD values obtained for the leachates of T4 and T5 are within the typical range, while the BOD5 is below the lower limit.
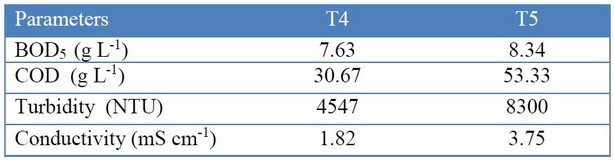
Table 5. Analysis of organic waste leached from T4 (with EM) and T5 (control).
In order to assess the level of contamination caused by organic matter, it is necessary to calculate the ratio of BOD/COD to elucidate the biodegradability of the leachate28. In this sense, the leachate generated by the treatment with EM is moderately biodegradable (ratio T4 BOD/COD = 0.25) compared to the treatment without inoculated microbial consortium, which has leaching with low biodegradability (ratio T5 BOD/COD = 0.16).
For the conductivity, the values of T4 and T5 (from 1.42 to 82.6 mS cm-1) are adjusted to the typical range for leachates of degradation processes from vegetable waste obtained in the laboratory28. Besides, T4 showed a 30% reduction in color units (232.34 PCU) compared to the treatment without the application of EM.
CONCLUSIONS
The application of the microbial consortium formed by: Lactobacillus plantarum, Rhodopseudomonas palustris, Streptomyces albus, and Aspergillus oryzae had reduced odors produced by the decomposition of residues from a market of Riobamba, Ecuador. The present study was conducted only on organic wastes (legumes peel, vegetables and fruit leaves); for that reason, it should be considered as a preliminary study for the control of odors from another kind of waste. The ME concentrations used (~ 106 CFU mL-1 and ~ 106 spores mL-1) achieved a reduction in the perception of strong odor according to the panel. The species of ME used in the microbial consortium were observed at the beginning and at the end of the treatments, which proves their symbiotic action within the biological deodorization treatment system. During the treatments, the measurement of pH and temperature was necessary for its use as operating parameters that guarantee the biological activity of the microorganisms.
Finally, the parameters: BOD5, COD, turbidity, color, and conductivity of the treatment inoculated with L. plantarum, R. palustris, S. albus, and A. oryzae were lower compared to the values of the treatment without EM inoculated; that demonstrates the microbiological action in the purification of leachates.
REFERENCES
- Cruz N. Aprovechamiento y manejo de desechos organicos de cocina utilizando Microorganismos Eficientes de Montana MEM aislados de dos bosques secundarios de Costa Rica. Cartago: Instituto Tecnologico de Costa Rica; 2010.
- Instituto Nacional de Estadistica y Censos. Estadistica de información ambiental economica en gobiernos autonomos descentralizados municipales 2014. Quito: INEC; 2014.
- Agencia Pública de Noticias del Ecuador y Sudamérica. Cerca del 50% de residuos sólidos que se produce en Ecuador proviene de Quito y Guayaquil [audio]. Quito: ANDES; 2015.
- Cadena N. Plan de desarrollo y ordenamiento territorial 2015-2019. Riobamba: Consejo Cantonal; 2015.
- Fernandez A. Contaminacion por malos olores: un problema en aumento. Consumer [Internet]. Bizkaia: Fundacion Eroski; [updated 2014 Jan 02; cited 2019 Dec 22]. Available from: https://www.consumer.es/medio-ambiente.
- Enciclopedia Ambiental Ambientum. Tratamiento de olores procedentes de la fermentacion. Ambientum [Internet]. Madrid; [updated 2015; cited 2019]. Available from: https://www.ambientum.com/enciclopedia_medioambiental/suelos/
- Bernache G. Cuando la basura nos alcance: el impacto de la degradación ambiental. Mexico: CIESAS; 2006.
- Higa T, Parr J. Beneficial and effective microorganisms for a sustainable agriculture and environment. Atami: International Nature Farming Research Center; 1994.
- Suarez F, Vargas M, Lopez M, Capel C, Moreno J. Antagonistic activity of bacteria and fungi from horticultural compost against Fusarium oxysporum f. sp. melonis. Crop Protection. 2007. 26: 46-53.
- Huang R, Zong F. Screening of several efficient microbial combinations for deodorization. Hubei Agricultural Sciences. 2011.14.
- Sanz S. Practicas de microbiologia. 2nd ed. Logroño: Universidad de La Rioja; 2011.
- Lopez L, Hernandez M, Colin C, Ortega S, Ceron G, Franco R. Las tinciones basicas en el laboratorio de microbiologia. Mexico: Medigraphic; 2014. p. 10-18.
- Madigan HT, Martinko JM, Dunalp PV, Clark DP. Biologia de los microorganismos. 12th ed. Madrid: Pearson Education; 2009.
- Ministerio de Ambiente y Desarrollo Sostenible Republica de Colombia. Protocolo para el monitoreo, control y vigilancia de olores ofensivos. Bogota: MinAmbiente; 2014.
- Alvarez RJ. Instructivo de uso del multiparametrico WTW, modelo MULTI 340i y medición de muestras. Huaraz: Unasam; 2014.
- Ingelab. Manual de instrucciones DBO logic. Buenos Aires: Ingelab; [date unknown].
- Garay J, Betancourt J, Ramirez G, Marin B, Cadavid B, Panizzo L, Lesmes L, Sanchez J, Lozano H, Franco A. Manual de tecnicas analiticas para la determinacion de parametros fisicoquimicos y contaminantes marinos: aguas, sedimentos y organismos. Serie de documentos generales 13. Santa Marta: Invemar; 2003.
- Carpio T. Turbiedad por nefelometría: método B. Bogota: Instituto de Hidrologia, Meteorologia y Estudios Ambientales; 2007.
- Aguilar M. Water analisis - Determination of color platinum cobalt in natural, wastewaters and wastewaters treated: test method NMX-AA-045-SCFI-2001. Mexico city: Diario oficial de la Federacion; 2001.
- Nanda S, Sarangi PK, Abraham J. Microbial biofiltration technology for odour abatement: an introductory review. Journal of Soil Science and Environmental Management. 2012. 3(2): 28-35.
- Wysocka I, Gebicki J, Namiesnik J. Technologies for deodorization of malodorous gases. Environmental Science and Pollution Research. 2019. 26(10): 9409-34.
- Rybarczyk P, Szulczynski B, Gebicki J, Hupka J. Treatment of malodorous air in biotrickling filters: a review. Biochemical Engineering Journal. 2019. 141: 146-162.
- Fan YV, Lee CT, Klemes JJ, Chua LS, Sarmidi MR, Leow CW. Evaluation of Effective Microorganisms on home scale organic waste composting. Journal of Environmental Management. 2018. 216: 41-48.
- Namasivayam KR, Kirithiga R. Effect of formulation of Effective Microorganism EM on post treatment persistence, microbial density and soil macronutrients. Recent Research in Science and Technology. 2010. 2(5): 102-6.
- Song C, Zhang Y, Xia X, Qi H, Li M, Pan H, Xi B. Effect of inoculation with a microbial consortium that degrades organic acids on the composting efficiency of food waste. Microbial Biotechnology. 2018. 11(6): 1124-36.
- Jusoh ML, Manaf LA, Latiff PA. Composting of rice straw with Effective Microorganisms EM and its influence on compost quality. Iranian Journal of Environmental Health Science & Engineering. 2013. 10: 17.
- Miller FC, Macauley BJ, Harper ER. Investigation of various gases, pH and redox potential in mushroom composting phase I stacks. Australian Journal of Experimental Agriculture. 1991. 31(3): 415-23.
- Roy D, Azais A, Benkaraache S, Drogui P, Tyagi RD. Composting leachate: characterization, treatment, and future perspectives. Reviews in Environmental Science and Bio/Technology. 2018. 17(2): 323-49.
Received: 13 January 2020
Accepted: 30 January 2020
Cristina Calderón-Tapia1, Abigail Montero-Calderón2, María Núñez-Moreno1, Esteban Pazmiño-Arias2
1Faculty of Science professor. Escuela Superior Politécnica de Chimborazo (ESPOCH)
2Researcher Yachay Tech University
Corresponding author:
Cristina Gabriela Calderón
ORCID: https://orcid.org/0000-0002-8574-103X
