2023.08.01.60
Files > Volume 8 > Vol 8 No 1 2023
Histological study and immunohistochemical expression of StAR protein in the suprarenal cortex of adult male rats associated with sleep disturbance
1 College of Medicine., Al-Nahrain University, Baghdad, Iraq
2 Computer Engineering Department, Bilad Alrafidain University College, 32001, Diyala, Iraq
*Corresponding author: [email protected] Phone No. +9647719539367
Available from: http://dx.doi.org/10.21931/RB/2023.08.01.60
ABSTRACT
The present study was designed to investigate the effects of sleep disturbance on histological features and evaluates the expression of StAR protein in the cortex of the adrenal gland of adult male rats. The suprarenal glands are endocrine organs that are directly affected by sleep deprivation. Sleep disturbance is a stress factor affecting steroidogenesis since it is regulated by the hypothalamic-pituitary axis (HPA). Its hormones are cholesterol-derived, and they use the Acut regulating protein of steroidogenesis StAR protein that plays an essential critical role in mediating cholesterol transfer to the inner mitochondrial membrane and the cholesterol side chain cleavage enzyme system. This research aims to investigate the effects of sleep disturbance (sleep disruption and deprivation) on the histological features and changes in StAR expression in the cortex of the adrenal glands of rats. Comparing experimental groups to controls, histological alterations such as cellular hypertrophy and vascular dilatation in the cortical zones of the adrenal cortex were found mainly in the Zona fasculata Zf. Immunohistochemistry was used to identify significant changes in the level of StAR, which showed a higher value in the sleep interruption group compared to the control and sleep deprivation groups at p-value ≤ 0.001. This indicates that sleep interruption has a more significant impact on steroidogenesis than sleep deprivation, which increases the level of StAR in the suprarenal gland.
Keywords: suprarenal gland; sleep disturbance; StAR protein; steroidogenesis; circadian rhythms.
INTRODUCTION
Modern societies are characterized by widespread problems with sleep and sleep loss, which interact with the hypothalamic-pituitary-adrenal axis (HPA) to cause undesirable changes in the neuroendocrine system. Sleep also has a bidirectional relationship with the central nervous system, which can result in metabolic disorders 1. The HPA axis, crucial for regulating alertness and rest, is supported by the sleep-wake cycle, also known as the circadian rhythm. The Circadian rhythms are responsible for the hormonal oscillation of steroid hormones within 24-h periods; it begins from an innate and genetically operated timekeeping system referred to as a "biological clock" 2,3. The anterior hypothalamus's suprachiasmatic nucleus (SCN) is the master circadian pacemaker 4. Sleep disturbance can change the activities of peripheral organs; substantial general evidence has already been introduced for the possible role of sleep in the modulation of adrenocortical and gonadal functions 5-12. In the case of adrenal steroids, the StAR protein is crucial in regulating steroid hormones necessary for life itself 13. The transport of cholesterol, the building block of all steroid hormones, from the outer to the inner mitochondrial membrane, which is the rate-limiting step in steroid biosynthesis, is primarily mediated by the steroidogenic acute regulatory protein (STAR). The first steroid, pregnenolone, is created at the inner membrane by the cytochrome P450 cholesterol side chain cleavage enzyme, cleaving the side chain of cholesterol. A series of enzymes then convert pregnenolone to numerous steroid hormones in different regions within the adrenal cortex s. The critical role of the STAR protein in regulating steroid production has been shown by scientific and clinical evidence 14. Adrenal steroid hormones (glucocorticoid GC) play an important role in the adaptive responses to different kinds of stress, and it's controlled by the hypothalamus–pituitary–adrenal gland (HPA) axis. Notably, disruption of the local adrenal clock influenced the daily steroid profiles 15,16, reflecting the importance of the adrenal peripheral clock of the steroid hormone in the circadian rhythm. This paper aims to investigate the effects of sleep disturbance on the histological features and changes in StAR expression in the cortex of the adrenal glands of rats.
MATERIAL AND METHODS
Thirty males (Rattus Norvagicus albinae) were selected for the present study with weights ranging from (200-250g). Rats are divided into three groups (10 animals per group).
Group A: animals in (GA) (sleep interruption group) were housed in conditions of 12 hours of light and 12 hours of darkness, with the creation of flashlights at three intervals of 2 hours each throughout their specified sleep period before the experiment.
Group B: In (GB) (Group for Sleep Reduction), animals were subjected to continuous flashlight stimulation for seven hours daily during the 14-day trial. The control group received no such treatment.
Group C: animals in (GC) were kept at a regular sleep rhythm of twelve hours in light and twelve hours in the dark.
All animals were treated following National Institute of Health (NIH) standards. The animal was euthanized with chloroform in a closed chamber, and the glands were dissected and removed from the abdominal cavity; then samples were fixed in 10% formaldehyde, and the tissue was prepared following the (Suvarna et al., 2018) 17, and examined under the light microscope for general histology. With StAR (polyclonal antibody) from abcam (ab203193), immunohistochemical evaluation for StAR was done by Aperio scope image analysis VII, total positivity was measured, and statistical analysis was done with SPSS software.
RESULTS
General Histological Features
In the control group, the three cortical zones are clearly defined, and each zone has the distinctive cellular pattern of the glands that make up the zona glomerulosa ZG, zona fasciculate ZF, and zona reticularis Zr, as shown in figure 1.
In group A, there is cortical vascular dilatation and engorgement with blood as well as an increase in the area of the spongeocyte's cytoplasm, which indicates an increase in its synthetic activity and cholesterol uptake, the zona fasciculate's relative thickness was found to have increased in comparison to other cortical layers, as shown in figure 2.
While in group B, there is a significant capsular hemorrhage, an increase in lipid material buildup in the cytoplasm of hypertrophied spongiocytes figure 3, and the presence of dilated blood vessels mostly in the Zf, as shown in figure 4.
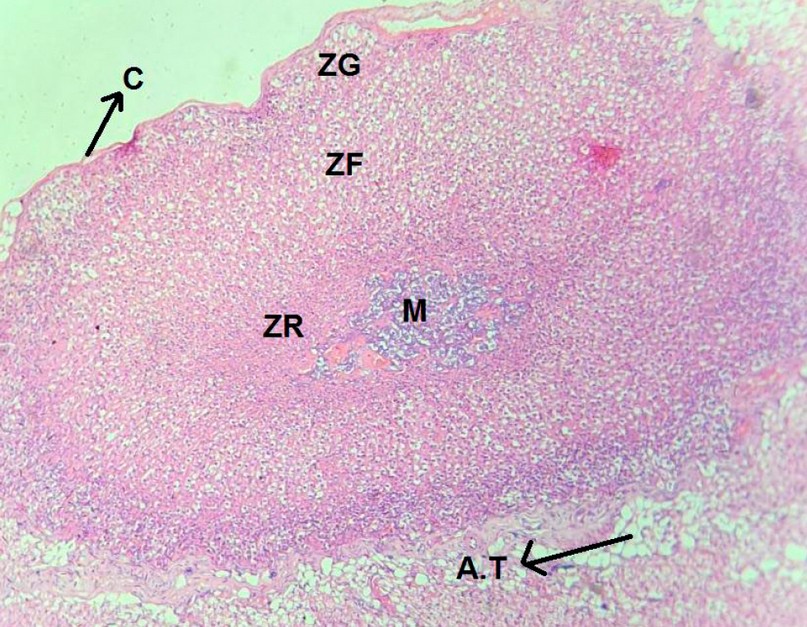
Figure 1. A cross-section in the suprarenal gland (control group) shows the capsule, adipose tissue (AT), zona glomerulusa (zg), zona sasiculata (zf), zona reticularis (zr) and medulla (M). (H&E, X4).
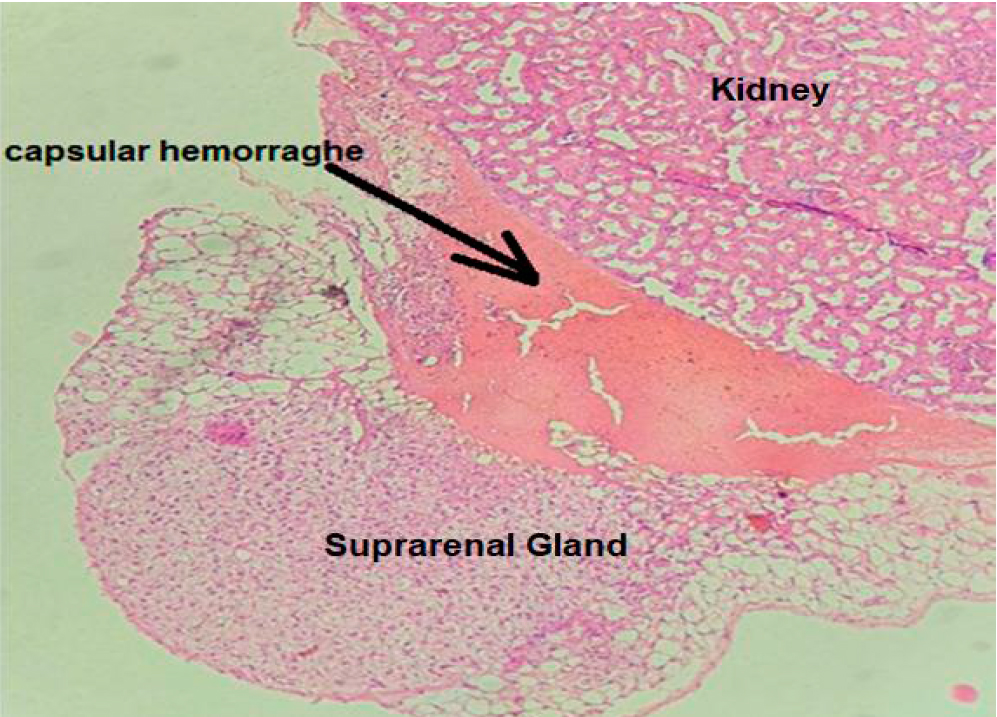
Figure 2. Cross section in suprarenal cortical group A showed a relative increase in the relative thickness of Zona fasiculata compared to other zones (H&E, X 10).
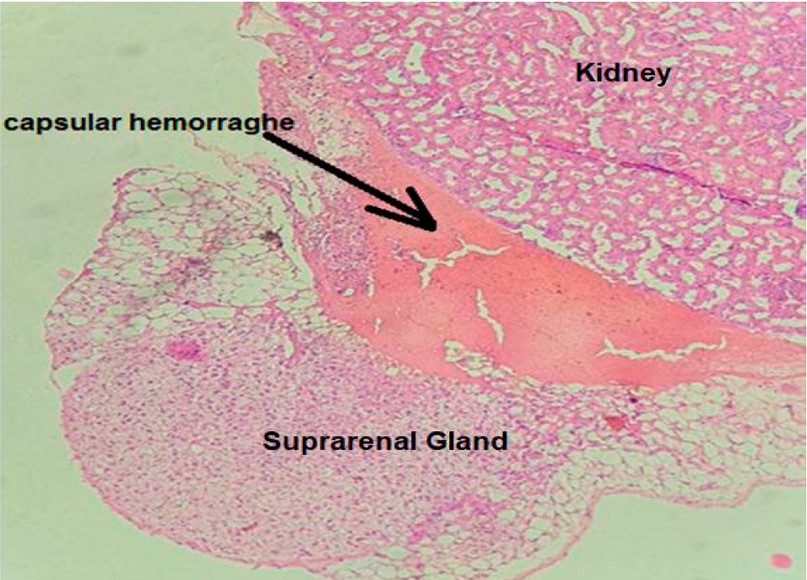
Figure 3. Cross section in suprarenal gland cortex of group B show capsular hemorrhage (H&E, X40).
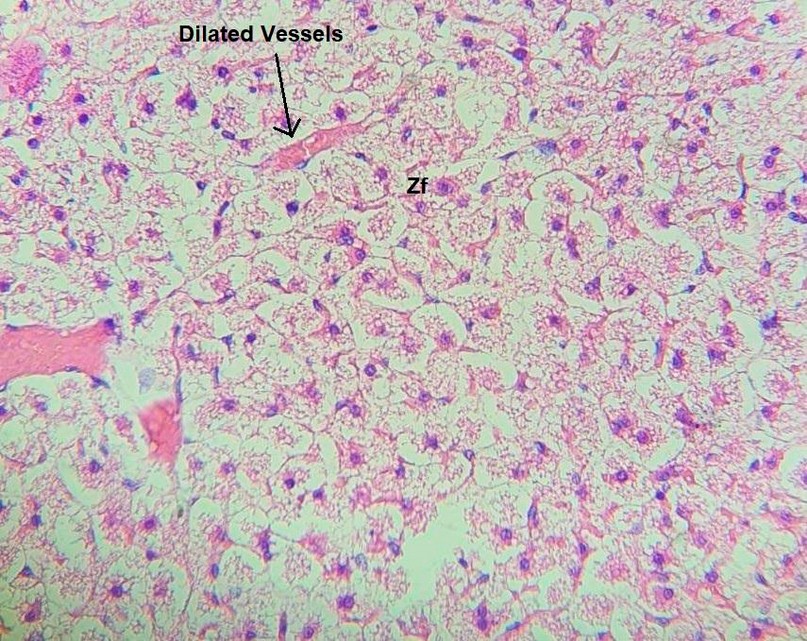
Figure 4. Cross section in suprarenal gland cortex of group B show hypertophy in Zf cells with vessels dilation (H&E X10).
Immunohistochemical Evaluation of StAR Protein in Suprarenal Gland Cortex in Control and Test Groups.
Weak patching distribution of the StAR protein in the three cortical zones of the suprarenal gland (Figure 5) with total positivity 0.160±0.008 pixel / micrometer² (Table 1), with different significance between the control group and group A in (p≤0.001) (Table 2).
Immunohistochemical expression of StAR protein in group A show wide strong distribution in all three zones of the suprarenal gland (Figure 5).
The total positivity was (0.715±0.018) pixel / micrometer² (Table 1), and the differences were found to be significant at (p≤0.001) between (GA vs. Control) and (GA vs. GB) (Table 2).
Immunohistochemical expression of StAR protein in group B shows strong wide distribution in all three zones of the suprarenal gland (Figure 5).
The total positivity was (0.598±0.026) pixel / micrometer² (Table 1), the differences was found to be significant (p≤0.001) between (GB vs Control) and (GA vs GB) (Table 2).
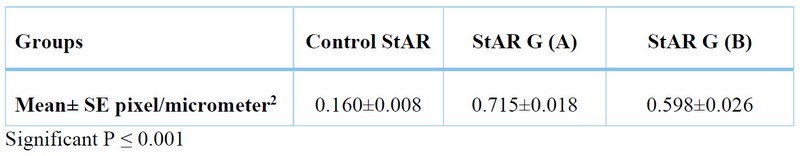
Table 1. Total positivity comparison of StAR Antibody in suprarenal cortex by Aperio scope image among groups by ANOVA.
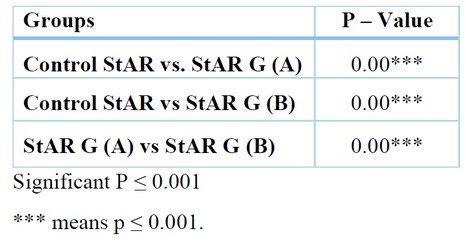
Table 2. The relationship between StAR Antibody in suprarenal cortex among groups using unpaired T-Test.
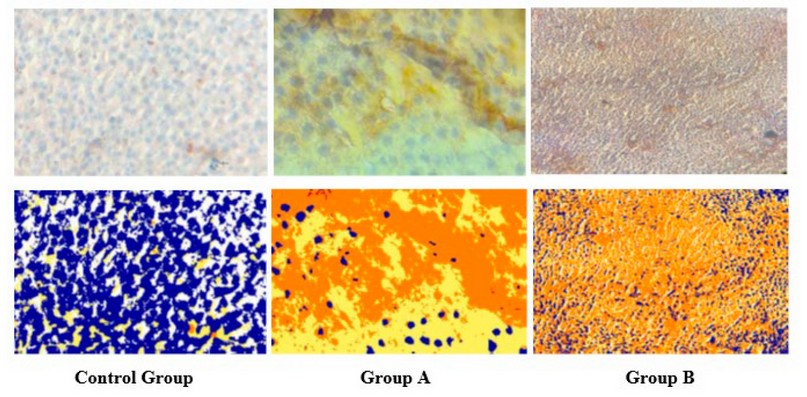
Figure 5. Immunohistochemical reaction of StAR of adrenal cortex in control and test group with their markup images by Aperio scope image analysis software. IHC.StAR.X10.
Immunohistochemical Mean ± SE evaluation of cortical StAR antibody with their significance between control and test groups that repressed in Figure 6.
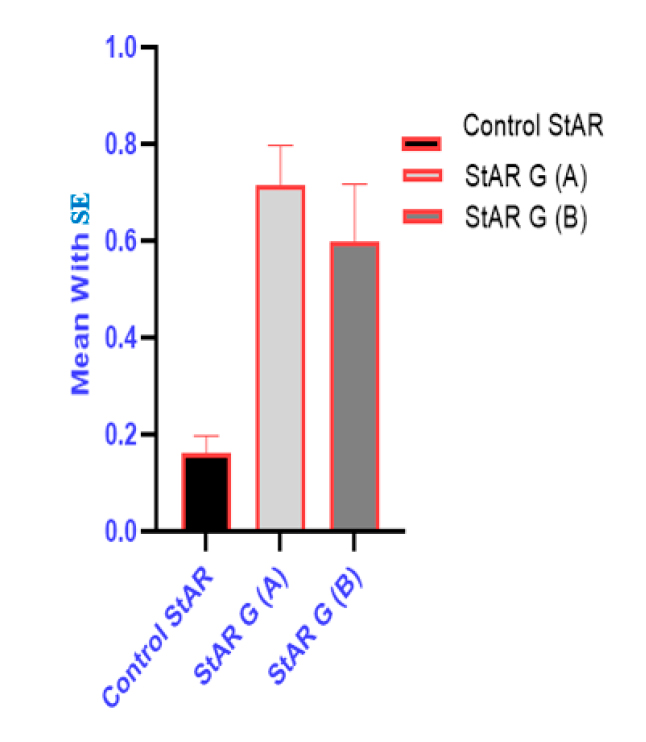
Figure 6. Comparison of StAR antibody groups with Mean±SE in suprarenal cortex.
DISCUSSION
Circadian rhythm plays important role in supporting sleep –wake cycle, the HPA axis play important role in alertness and modulating sleep. Dysfunction in this axis level, meditate by the SCN which regulates the daily rhythm of the (HPA) axis. Cortisol, as well as a glucocorticoid, serves as a supplementary messenger between the central clock and peripheral tissues. since the sleep and the stress response share the same pathway on (the HPA axis) so when something disburses (like the light, as seen in this present study) the function of this axis, this will result in sleep disturbance and stress response 18,19. Light remarkably influences the adrenal gland; exposure to light causes corticosterone to be secreted via the SCN stimulation route 20. The hypothalamic link between the retina and descending automatic circuits to peripheral organs, such as the adrenal gland is assumed to be the SNC clock cell network 21. The primary association between stress and slow wave of sleep SWS has been discovered in recent studies as they reset the responses of HPA and its effect on peripheral organs. This explains the findings of this study, which indicated that sleep disruption could directly impact the adrenal cortical layers. The cross-histological features of the adrenal gland showed progressive changes, including a marked increase in cellular size with an increase in the thickness of the entire cortical layers, especially the ZF layer of the adrenal cortex, indicating the stimulatory effect of light on the adrenal gland combined with the increase in StAR protein expression in sleep interruption group, confirmed by the findings by Vgontzas et al.;(2001) who discovered the presence of intrinsic circadian oscillators in the adrenal gland served as clock genes to cause an oscillation in glucocorticoid secretion 22. We can therefore conclude that StAR protein is one of the proteins that undergoes diurnal oscillation in response to sleep disturbance as tested in GA while the sleep deprivation as in GB show a Significant reduction in the immunohistochemical expression of StAR protein compared to group A; this reduction is caused by the effect of sleep deprivation on HPA, which initially activates steroid synthesis and increases cholesterol uptake by spongeocytes, but eventually causes desensitizing of HPA to stress due to loss of SWS pattern responsible for resting of the HPA which results in a loss of steroidogenic activity as expressed by a significant reduction in StAR protein in GB this due to lack of SWS which has an inhibitory role on HPA 23, that associated with an increase in intracellular cholesterol deposition leads to mitochondrial injury and further reduction in StAR dependent steroidogenesis 24.
CONCLUSION
A stress factor that affects the secretory activity of the adrenal glands through the hypothalamic-pituitary axis is light-induced sleep disruption. As the secretion of HPA-dependent steroid hormones increases, as well as cortical vascular supply, vascular dilatation, and capsular hemorrhage. Interruptions in sleep have a stimulatory effect on HPA, which is transported to the adrenal cortex and increases synthetic steroid activity and spongiocyte hypertrophy. Interruption occurs at varying intervals and further stimulates the production of steroid hormones in the adrenal cortex. At the same time, sleeplessness due to prolonged exposure to light had a desensitizing effect on the HPA, which decreased the production of adrenal cortical steroids due to a defect in StAR protein and intracellular cholesterol deposition, which increased the size of spongiocytes and severely damaged their organelles. We can conclude that StAR protein in group B showed that significant reduction in immunohistochemical expression compared with group A.
REFERENCES
1. Seetho IW, Wilding JP. Sleep-disordered breathing, type 2 diabetes and the metabolic syndrome. Chronic respiratory disease. 2014 Nov;11(4):257-75.
2. Reppert SM. A clockwork explosion!. Neuron. 1998 Jul 1;21(1):1-4.
3. Dunlap JC. Molecular bases for circadian clocks. Cell. 1999 Jan 22;96(2):271-90.
4. Thomas GK, Cheng KC, Wang JZ. SHIRAZ: an automated histology image annotation system for zebrafish phenomics. Multimedia tools and applications. 2011 Jan;51(2):401-40.
5. Åkerstedt T, Palmblad J, de la Torre B, Marana R, Gillberg M. Adrenocortical and gonadal steroids during sleep deprivation. Sleep. 1980 Mar 1;3(1):23-30.
6. Gonzalez-Santos MR, Gajá-Rodíguez OV, Alonso-Uriarte R, Sojo-Aranda I, Cortes-Gallegos V. Sleep deprivation and adaptive hormonal responses of healthy men. Archives of andrology. 1989 Jan 1;22(3):203-7.
7. Opstad PK. Circadian rhythm of hormones is extinguished during prolonged physical stress, sleep and energy deficiency in young men. European journal of endocrinology. 1994 Jul 1;131(1):56-66.
8. Gonzalez-Ortiz M, Martinez-Abundis E, Balcazar-Munoz BR, Pascoe-Gonzalez S. Effect of sleep deprivation on insulin sensitivity and cortisol concentration in healthy subjects. Diabetes, nutrition & metabolism. 2000 Apr 1;13(2):80-3.
9. Heiser P, Dickhaus B, Schreiber W, Clement HW, Hasse C, Hennig J, Remschmidt H, Krieg JC, Wesemann W, Opper C. White blood cells and cortisol after sleep deprivation and recovery sleep in humans. European archives of psychiatry and clinical neuroscience. 2000 Feb;250(1):16-23.
10. Redwine L, Hauger RL, Gillin JC, Irwin M. Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. The Journal of Clinical Endocrinology & Metabolism. 2000 Oct 1;85(10):3597-603.
11. Chapotot F, Buguet A, Gronfier C, Brandenberger G. Hypothalamo-pituitary-adrenal axis activity is related to the level of central arousal: effect of sleep deprivation on the association of high-frequency waking electroencephalogram with cortisol release. Neuroendocrinology. 2001;73(5):312-21.
12. Charloux A, Gronfier C, Chapotot F, Ehrhart J, Piquard F, Brandenberger G. Sleep deprivation blunts the night time increase in aldosterone release in humans. Journal of sleep research. 2001 Mar 4;10(1):27-33.
13. Manna PR, Dyson MT, Stocco DM. Regulation of the steroidogenic acute regulatory protein gene expression: present and future perspectives. Molecular human reproduction. 2009 Jun 1;15(6):321-33.
14. Manna PR, Stetson CL, Slominski AT, Pruitt K. Role of the steroidogenic acute regulatory protein in health and disease. Endocrine. 2016 Jan;51(1):7-21.
15. Lin D, Sugawara T, Strauss III JF, Clark BJ, Stocco DM, Saenger P, Rogol A, Miller WL. Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science. 1995 Mar 24;267(5205):1828-31.
16. Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocrine reviews. 2011 Feb 1;32(1):81-151.
17. Suvarna KS, Layton C, Bancroft JD, editors. Bancroft's theory and practice of histological techniques E-Book. Elsevier health sciences; 2018 Feb 27.
18. Gotlieb N, Moeller J, Kriegsfeld LJ. Circadian control of neuroendocrine function: implications for health and disease. Current opinion in physiology. 2018 Oct 1;5:133-40.
19. Santiago GT, de Menezes Galvão AC, De Almeida RN, Mota-Rolim SA, Palhano-Fontes F, Maia-de-Oliveira JP, De Araújo DB, Lobão-Soares B, Galvão-Coelho NL. Changes in cortisol but not in brain-derived neurotrophic factor modulate the association between sleep disturbances and major depression. Frontiers in behavioral neuroscience. 2020 Apr 28;14:44.
20. Ishida A, Mutoh T, Ueyama T, Bando H, Masubuchi S, Nakahara D, Tsujimoto G, Okamura H. Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell metabolism. 2005 Nov 1;2(5):297-307.
21. Kiessling S, Sollars PJ, Pickard GE. Light stimulates the mouse adrenal through a retinohypothalamic pathway independent of an effect on the clock in the suprachiasmatic nucleus. PloS one. 2014 Mar 21;9(3):e92959.
22. Vgontzas AN, Bixler EO, Lin HM, Prolo P, Mastorakos G, Vela-Bueno A, Kales A, Chrousos GP. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. The Journal of Clinical Endocrinology & Metabolism. 2001 Aug 1;86(8):3787-94.
23. Bierwolf C, Struve K, Marshall L, Born J, Fehm HL. Slow Wave Sleep Drives Inhibition of Pituitary‐AdrenalSecretion in Humans. Journal of neuroendocrinology. 1997 Jun;9(6):479-84.
24. Bose HS, Sugawara T, Strauss JF, Miller WL. The pathophysiology and genetics of congenital lipoid adrenal hyperplasia. New England Journal of Medicine. 1996 Dec 19;335(25):1870-9.
Received: January 15, 2023 / Accepted: February 25, 2023 / Published:15 March 2023
Citation: Mohammed H A, Kamoona H R, Mahmood Khudhur A. Histological study and immunohistochemical expression of StAR protein in the suprarenal cortex of adult male rats associated with sleep disturbance. Revis Bionatura 2023;8 (1) 60. http://dx.doi.org/10.21931/RB/2023.08.01.60
