2022.07.02.60
Files > Volume 7 > Vol 7 No 2 2022
1.2. University of Mosul/College of Environmental Science and Technology, Mosul, Iraq
* Correspondence: [email protected];
Available from: http://dx.doi.org/10.21931/RB/2022.07.02.60
ABSTRACT
It was using Neurospora crassa as a model organism to study the biological clock. Due to its use for more than 50 years in the circadian rhythm Study, this biological cycle has been studied by growing it on a Vogel's medium with a constant temperature and different light cycles (continuous light, light to dark, continuous darkness) with different concentrations of heavy metals (Lead Nitrate II, Zinc sulfate, Cadmium chloride, iron chloride III). It was also Measured protein CLOCK and hormone Melatonin By using Elisa Kit. Neurospora crassa was cultured in a thin layer of Vogel's medium using growth tubes (tube race) which grow in one direction only and are distributed by forming conida bands on the medium surface; each band represents one cycle circadian. However, examining Nerospora conidia on growth tubes is a simple but powerful tool for studying the circadian clock and detecting the effect of heavy metals and light on it. At the same time, the results indicated an apparent decrease of CLOCK protein at continuous light compared to light/dark for all treatments. It also recorded a rise in melatonin concentration in continuous darkness and decreased with continuous lighting. The results of the growth tubes also indicated a decrease in growth at continuous light compared to light/dark. Heavy metals also affected Neurospora crassa, as it had the highest inhibition when using lead nitrate II compared to iron chloride III. The study aimed to investigate the effect of heavy metals and differences in light and darkness on the biological clock of the fungus Neurospora crassa.Conclusion the effect on the biological clock disturbances of Neurospora crassa
Keywords. Neurospora crassa, Melatonin hormone, Clock protein, Heavy metals, light, dark.
INTRODUCTION
The circadian clock is a biological process at a specific frequency during 24 hours. This rhythm is regulated by the circadian clock that is widely observed in all living things genetics maps of clock gene . Although the circadian rhythm is an internal process, it is tuned and regulated with the surrounding environment by external signals, which include light, temperature and components of the surrounding environment. 1
Heavy metals are present in the ecosystem, some types of iron, copper, and cadmium and their danger lies in the fact that they are not degradable and persist in the environment for a long time. Although the minerals are necessary for metabolic activity at low concentrations, when they exceed specific concentrations, they produce harmful and toxic effects on living organisms, 2,3.
Although fungi are not capable of photosynthesis and are not able to observe neighboring objects, the majority of fungal species show some form of response to light at the wavelength 450 nm (blue) to 700 nm (red), affecting the growth of the fungus and its metabolic reprogramming to melatonin N-acetyl-5-methoxy tryptamine. It is an indole hormone that is synthesized by monocytes and multicellular organisms whose primary physiological function is to provide a time signal to regulate the circadian clock, which peaks at night and decreases during the day under normal environmental conditions; that is why it is called the dark hormone or the hand of the clock. It is activated by the light/dark cycle as light suppresses its secretion,4.
Neurospora crassa, a filamentous fungus, has been widely used as an experimental organism in different genetics. It consists of seven chromosomes and ten thousand proteins that code for the gene. The total length of the genetic map of the fungus is 1000 units and 10 % of the repetitive DNA. The base consists of 40 mica. And biochemical experiments. And the detection of circadian rhythms for several reasons is the speed of its growth, the ease of its spread on specific growth media, its moderate restriction, the flexibility of dealing with it in the laboratory, and the complete discovery of DNA genes of interest for researchers. As well as its use in biochemical tests. DNA repair isolation of mutation and gene silencing, this fungi is the mainstay in some new and important discoveries in genetics, 3.
MATERIALS AND METHODS
The standard strain of Neurospora crassa was obtained from the Fungal Genetics stock center (FGSC) in the USA, type A with the number 987#.
It was reactivated by culturing it on Vogel's medium, which is prepared by adding 4 ml Vogel's salts, 3 g agar, 1 ml Trace elements and 0.5 ml Biotin with 200 ml distilled water.
Preparation of Solutions
Vogel's salts: In 750 ml of distilled water, 150 g of aqueous sodium citrate, 250 g of potassium dihydrogen phosphate, 100 g ammonium nitrate, 10 g magnesium sulfate hydrated and 5 g Calcium chloride, 5. Solution trace elements: In 95 ml of distilled water dissolve 5 g of citric acid, 5 g of zinc sulfate, 1 g of ammoniac iron sulfate and 0.25 g copper sulfate, 0.05 g of boric, 0.05 of sodium molybdate. Biotin solution: In 50 ml of distilled water is dissolved 5 g of biotin, 6.
ELISA
A ready-made kit was used to measure the clock protein from the manufacturer sun long Biotech with the number SL3124Hu and the ready-made kit to measure me from the manufacturer sun long Biotech that carries the number SL1169Hu and using the device Elisa.
Using the ELISA technique to measure the hormone melatonin and the clock protein in Neurospora crassa
Vogel's medium was used to grow Neurospora crassa; a spore suspension of fungus was made and added to 15 glass flasks containing 200 ml of Vogel's medium and then divided into three groups: each group consisted of 5 glass flasks with different metals with specific concentrations were added, cadmium 0.0492g, zinc 0.1614g, iron 0.087g, lead 0,1212g and each group exposure for 12/12 hours dark/light, 24 hours light, 24 hours dark, And for each group one flask without metals for control and all flasks were incubated in the vibrating incubator of 75 rpm at 28 co for 7 days.
After the incubation, The fungal solution was filtered by filter paper and then finely ground in a ceramic motor, and 2 ml of normal saline was added. The material was carefully collected in tubes and centrifuged for 20 minutes at 2000-3000 rpm and the material was carefully separated and placed in a tube, and the measurement process was carried out as follows:
Prepared a standard according to the kit method for each measurement and put in the first 5 of the well for the ELISA Microelisa, leaving the 6 well as blanks control, 25 ml of buffer solution to dilute, and 25 ml of fungus sample into the well. It was left to incubate for 30 minutes at 37 co. The washing process was carried out 5 times, then 50 microliters of the reagent HRR-conjugate was added to each well except for the control well and left to incubate for 30 minutes at 37 co, and the washing process was 5 times after incubation 50 ml of solution chromogen solution A and 50 ml of solution B were added to each well, mixed with gentle stirring and incubated at 37 co for 15 min. Finally, 50 ml of stop solution was added to each molecule to finish the reaction. We note the color change from blue to yellow and then read the absorbance OD at 450 nm using a microtiter plate reader.
Growth tubes (race tubes)
The growth of Neurospora crassa and the effect of heavy metals and light were tracked in hollow glass tubes of about 35-40 cm in length and 16 mm in diameter and bent at both ends at an angle of 45 (to keep the Vogel's) in 15 tubes; it was divided into three groups:
Each group consisted of 5 tubes of different metals with specific concentrations of cadmium 0.0492g, zinc 0.1614g, iron 0.087g, lead 0,1212g and each group exposure for 12/12 hours dark/light, 24 hours light, 24 hours dark, And for each group one tube without metals for control all tubes were inoculated with standard isolate Neurospora crassa and incubate at 28 co for 6 days. The fungus growth was monitored, and the growth was determined every 24 hours by placing an arrow with a pen marker.
RESULTS AND DISCUSSION
The current study results indicate the extent of the extent to measure of the CLOCK protein of the fungus Neurospora crass in control 12light/12dark 1650.5pg/ml, as shown in the figure (1) to which the fungus is affected by heavy metals, light and darkness. It may be due to the interaction of heavy metals and their compounds with fungus in different ways depending on the type of metal, the organism and the environment, 7. We noticed the CLOCK protein's effect strongly on lead metal at continuous lighting, which amounted to 153.1 pg/ml compared to the control, which reached 360.1 pg/ml. While iron chloride showed a kind of resistance in continuous lighting, which reached 255 pg/ml.
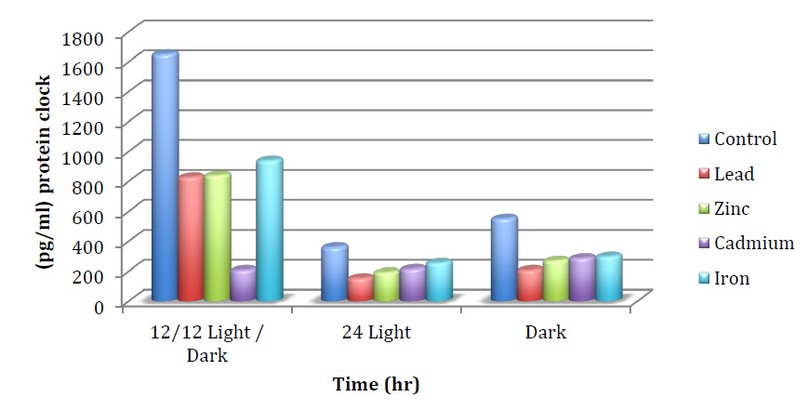
Figure 1. The concentration of protein CLOCK when treating N.crassa with minerals and different lights
This decrease may be because proteins are essential to heavy metal targets as metals interfere with the biological activity of natively folded proteins through a variety of patterns. It either binds to release thiols or other functional proteins or groups displaces basic metal ions in metallic proteins catalyzes side-chain oxidation, 8,9. Or perhaps the reason for this apparent decrease When the fungus was treated with heavy metals compared to the control is due to the metal reaction targeting the non folded proteins, as it has been proven that heavy metals prevent chemically forming denatured proteins from entering the body protein.
Folding in vivo causes the nascent accumulation of proteins in living cells by interfering with the folding of nascent or non-native proteins, and heavy metals also have a profound effect on protein homeostasis and cell vitality. They may enhance the cumulative tendency associated with proteinuria disease, 10. We also note a decrease in protein CLOCK in all treatments with continuous light compared to light /dark lighting. For example, Cadmium Chloride, where the concentration of clock protein in light/darkness was 908.1 pg/ml Compared to continuous light, whose concentration was 215.1 pg/ml. This is in agreement with Kallies et al.,11 studies, which observed a decrease in protein at constant light, and this was explained by the effect of light on the rates of protein synthesis within Neurospora crassa cells, which indicated the general effect of light on the metabolism membrane processes and shape of the fungus. Also, this apparent decrease of the clock protein. When light is disturbed is probably due to the effect of light on the proteins through either extracellular polypeptides that may play a role in sending signals between cells and then synchronizing the circadian clocks inside the mycelium or in protein secretion can be seen in light induction of differentiation and subsequent changes in aerial hyphae. The molecular clock consists of transcription and translation regulatory loops influenced by external conditions,12.
Whereas, Fonken et al. 13 indicated that exposure to dim light at night disrupted molecular circadian rhythms (at the gene and protein level) by causing changes in essential clock transcription factors within both the central clock and peripheral tissues.
Figure 2 also shows the effect of heavy metals light and dark on the hormone melatonin, a hormone that is secreted at night and acts as a time signal for the biological clock. The study results indicate a decrease in the hormone melatonin when treated with heavy metals compared to the control, and the reason for this is due to the role of heavy metals in lowering hormone levels and contributing to hormonal imbalance. Where the concentration of melatonin When using an iron at constant light reached 5.01 pg/ml compared to a control of 12.02 pg/ml, and this decrease may be due to the accumulation of heavy metals in the glands that make hormones or interfere with the signals of the review feeding cycle in the body or interfere or block hormone be receptor sites, as well as interference may be interference in the toxicity of enzymes that create hormones,14. The synthesis and release of melatonin are directly related to exposure to light, and natural circadian rhythms depend on exposure to light /dark patterns, 15. This matches our current study, where the concentration of melatonin When using Zinc sulfate in the dark reached 47.66 pg/ml compared to continuous light, which reached a concentration of 5.01 pg/ml for the same mineral. This is consistent with the Grivas and Savvidou, 16, study which indicated that short pulses of light of sufficient intensity and duration abruptly suppress melatonin production.
As well as Ovid et al 17 Which showed that exposure to light during the middle of the dark phase significantly reduces the production of melatonin. The hormone melatonin is the body's temporal pacemaker. Farhadi et al., 18 showed the effect of light and dark on melatonin concentration and noted that the highest concentration of melatonin in the continuous darkness compared to light /dark because of the long nights led to the prolongation of the period of melatonin secretion.
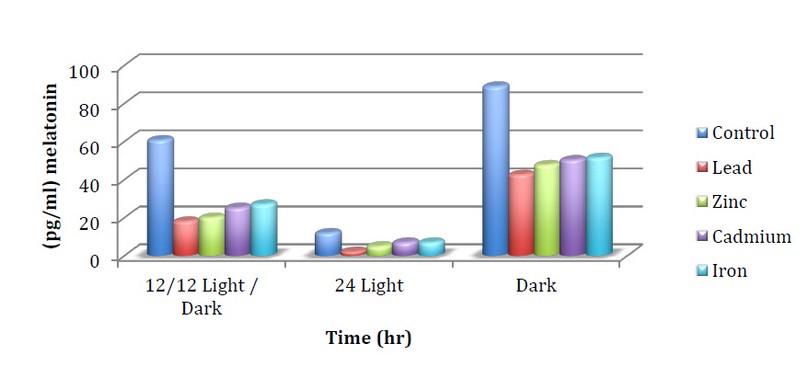
Figure 2. The concentration of melatonin when treating mushrooms with minerals and different lights
Growth tubes (race tubes)
Figures 3,4,5 indicate the growth of the fungus Neurospora crassa on the medium of the Vogels in the presence of heavy metals and exposure to different light periods. The results showed a decrease in the growth of the fungus in all treatments with metals compared to the control.
The fungus growth when using zinc metal on the first day reached 1.5cm compared to the control, which reached 3.5 cm under continuous light. It may be due to the toxic effect of heavy metals on the growth of fungus at a specific concentration, 7. While the growth on the third day when using Lead metal reached 2cm at continuous light compared to the control, which reached 6.4 cm in growth, We note that the effect of fungus on lead was the highest inhibition compared to iron, whose growth reached 4.1cm. This is in agreement with the study of Qasim et al., 2 which indicated a decrease in the growth of fungi when treated with lead metal compared with iron, which showed more resistance
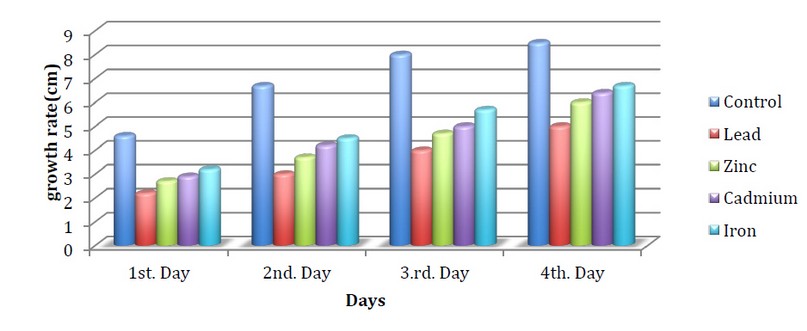
Figure 3. Speed of N.crassa growth when treated with minerals with 12/12 hours of light/dark
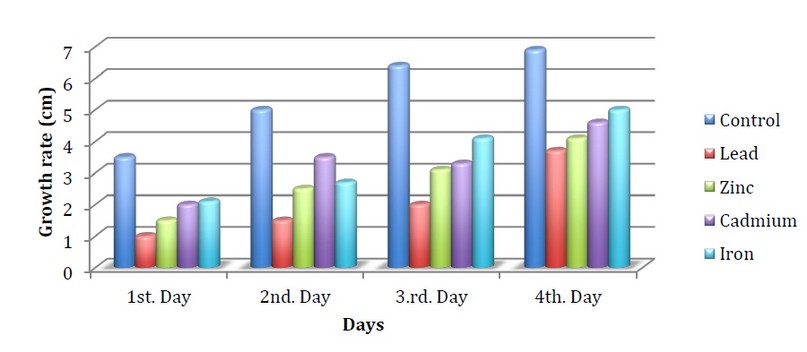
Figure 4. Speed of N.crassa growth when treated with minerals with 24 hours of light
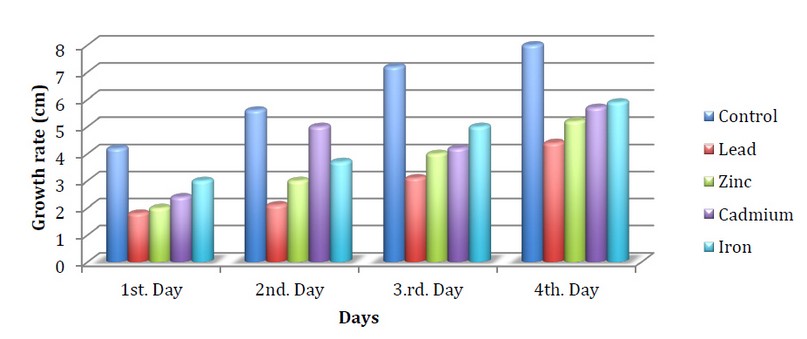
Figure 5. Speed of N.crassa growth when treated with minerals with 24 hours of dark
This decrease is due to the heavy metals having a high density, five times more than the density of water and their inability to decompose over time and their accumulation inside the bodies of living organisms due to their long half-life.
A decrease in growth was also observed when using Cadmium metal, where the growth on the first day reached 2.4 cm. When it was dark compared to the control, which reached 4.2, this corresponds to what was reached by Baldrian and Gabriel, 19, who observed the inhibition of the growth of the fungus when using cadmium metal accompanied by a total change in the microscopic morphology. He also saw a change in the color of the mycelium with high intensity and without the formation of aerial hyphae. We also note the effect of growth at different illuminations, where Continuous light had an apparent inhibitory effect compared to light /darkness in all treatments, and this is consistent with what was reached by Sargent. 20 When studying the effect of light on the fungus Neurospora crassa, it was noticed that deformation was observed when the fungus was exposed to continuous light by inhibiting the circadian rhythm of the fungus.
Studies have also indicated that the fungus Neurospora crassa is exposed to light in the last part of the night; this means that dawn has arrived, and therefore the light signal must work to shift the phase of the clock forward (morning) While when exposing the fungus in the first part of the night to light, it means that the sun Not yet set resulting in circadian disturbances.
CONCLUSIONS
The results indicated an apparent decrease of CLOCK protein at continuous light compared to light/dark for all treatments. It also recorded a rise in melatonin concentration in continuous darkness and decreased with continuous lighting. The results of the growth tubes also indicated a decrease in growth at continuous light compared to light/dark. Heavy metals also affected Neurospora crassa, as it had the highest inhibition when using lead nitrate II compared to iron chloride III.
Acknowledgments: The authors want to thank the University of Mosul for the provision of equipment, which improved the quality of this work.
Conflicts of Interest: The authors declare no conflict of interest
REFERENCES
1. Reddy S, Reddy V, Sharma S. Physiology, Circadian Rhythm [Internet]. StatPearls Publishing, Treasure Island (FL); 2021. Available from: http://europepmc.org/books/NBK519507
2. Alkazaz SR, Khalil MI, Fadhel MN. Isolation and molecular identification of microorganisms isolated from soils contaminated with heavy metals in Mosul city. Bionatura [Internet]. 2021 Nov 15;6(4):2196–201. Available from: https://www.revistabionatura.com/2021.06.04.10.html
3. Khalil MI. In silico detection of DNA methylation in fungi Neurospora crassa genes, rid-1 and dim-2. In: AIP Conference Proceedings [Internet]. 2020. p. 020072. Available from: http://aip.scitation.org/doi/abs/10.1063/5.0000105
4. Liu L, Labani N, Cecon E, Jockers R. Melatonin Target Proteins: Too Many or Not Enough? Front Endocrinol (Lausanne) [Internet]. 2019 Nov 15;10(November):1–13. Available from: https://www.frontiersin.org/article/10.3389/fendo.2019.00791/full
5. Vogel HJ. Distribution of Lysine Pathways Among Fungi: Evolutionary Implications. Am Nat [Internet]. 1964 Nov;98(903):435–46. Available from: https://www.journals.uchicago.edu/doi/10.1086/282338
6. Gonçalves AP, Videira A. Programmed Cell Death in Neurospora crassa. New J Sci [Internet]. 2014 Mar 2;2014:1–7. Available from: https://www.hindawi.com/journals/njos/2014/479015/
7. Anahid S, Yaghmaei S, Ghobadinejad Z. Heavy metal tolerance of fungi. Sci Iran [Internet]. 2011 Jun;18(3):502–8. Available from: http://dx.doi.org/10.1016/j.scient.2011.05.015
8. Lemire JA, Harrison JJ, Turner RJ. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat Rev Microbiol [Internet]. 2013 Jun 13;11(6):371–84. Available from: http://dx.doi.org/10.1038/nrmicro3028
9. Sharma SK, Goloubinoff P, Christen P. Cellular Effects of Heavy Metals [Internet]. Banfalvi G, editor. Cellular Effects of Heavy Metals. Dordrecht: Springer Netherlands; 2011. Available from: http://link.springer.com/10.1007/978-94-007-0428-2
10. Ramadan D, Rancy PC, Nagarkar RP, Schneider JP, Thorpe C. Arsenic(III) Species Inhibit Oxidative Protein Folding in Vitro. Biochemistry [Internet]. 2009 Jan 20;48(2):424–32. Available from: https://pubs.acs.org/doi/10.1021/bi801988x
11. Kallies A, Mohsenzadeh S, Rensing L. Effects of light on protein secretion in Neurospora crassa. Arch Microbiol [Internet]. 1992 Jan 1;157(2):104–6. Available from: http://link.springer.com/10.1007/BF00245276
12. Hurley JM, Loros JJ, Dunlap JC. Circadian Oscillators: Around the Transcription–Translation Feedback Loop and on to Output. Trends Biochem Sci [Internet]. 2016 Oct;41(10):834–46. Available from: http://dx.doi.org/10.1016/j.tibs.2016.07.009
13. Fonken LK, Aubrecht TG, Meléndez-Fernández OH, Weil ZM, Nelson RJ. Dim Light at Night Disrupts Molecular Circadian Rhythms and Increases Body Weight. J Biol Rhythms [Internet]. 2013 Aug 8;28(4):262–71. Available from: http://journals.sagepub.com/doi/10.1177/0748730413493862
14. Azeh Engwa G, Udoka Ferdinand P, Nweke Nwalo F, N. Unachukwu M. Mechanism and Health Effects of Heavy Metal Toxicity in Humans. In: Poisoning in the Modern World - New Tricks for an Old Dog? [Internet]. IntechOpen; 2019. p. 23. Available from: https://www.intechopen.com/books/poisoning-in-the-modern-world-new-tricks-for-an-old-dog-/mechanism-and-health-effects-of-heavy-metal-toxicity-in-humans
15. Ostrin LA. Ocular and systemic melatonin and the influence of light exposure. Clin Exp Optom [Internet]. 2019 Mar 1;102(2):99–108. Available from: https://www.tandfonline.com/doi/full/10.1111/cxo.12824
16. Grivas TB, Savvidou OD. Melatonin the "light of night" in human biology and adolescent idiopathic scoliosis. Scoliosis [Internet]. 2007 Dec 1;2(1):6. Available from: https://scoliosisjournal.biomedcentral.com/articles/10.1186/1748-7161-2-6
17. Ovid D, Hayes TB, Bentley GE. Melatonin Administration Methods for Research in Mammals and Birds. J Biol Rhythms [Internet]. 2018 Dec 24;33(6):567–88. Available from: http://journals.sagepub.com/doi/10.1177/0748730418795802
18. Farhadi N, Gharghani M, Farhadi Z. Effects of long-term light, darkness and oral administration of melatonin on serum levels of melatonin. Biomed J [Internet]. 2016 Feb;39(1):81–4. Available from: http://dx.doi.org/10.1016/j.bj.2015.09.003
19. Baldrian P, Gabriel J. Effect of heavy metals on the growth of selected wood-rotting basidiomycetes. Folia Microbiol (Praha) [Internet]. 1997 Oct;42(5):521–3. Available from: http://link.springer.com/10.1007/BF02826566
20. Sargent ML, Briggs WR, Woodward DO. Circadian Nature of a Rhythm Expressed by an Invertaseless Strain of Neurospora crassa. Plant Physiol [Internet]. 1966 Oct 1;41(8):1343–9. Available from: https://academic.oup.com/plphys/article/41/8/1343-1349/6090912
Received: 10 February 2022 / Accepted: 20 April 2022 / Published:15 May 2022
Citation. Al-Sardar N, Ibrahim Khalil M. Study of the effect of heavy metals and differences of light / dark cycle in the melatonin hormone protein Clock and growth of Neurospora crassa fungi. Revis Bionatura 2022;7(2) 60. http://dx.doi.org/10.21931/RB/2022.07.02.60
