2019.04.03.9
Files > Volume 4 > Vol 4 No 3 2019
REPORTE DE CASOS / CASE REPORTS
Effect of Short-term Curcumin exposure and its Modulatory Role on Acute Cadmium Hepatotoxicity
Tammanna R. Sahrawat1, Ranbir C. Sobti2, Sukesh C. Sharma3, Uma N. Saikia4 and Madan L. Sharma5
Available from: http://dx.doi.org/10.21931/RB/2019.04.03.9
ABSTRACT
Curcumin, presents chemo-preventive, antioxidant and anti-inflammatory properties, whereas cadmium is a serious contaminant due to industrial and agricultural practices. Most of the work on curcumin deals with repeated exposures for longer durations. The present study was designed to investigate the effect of a single short-term (24 hour) curcumin treatment with FDA approved dose of 100 mg/kg body weight for humans, on Male Balb/c mice liver. Further, the modulatory role of its pre-treatment on acute cadmium hepatotoxicity was also studied. Animals treated with curcumin for 24 hours were divided into two groups, and one group was sacrificed. The other group was sacrificed after additional exposure to cadmium for 18 hours with corresponding positive and negative control groups. Oxidative stress was measured using a multi-parametric biochemical approach, and histopathological changes were studied using light and electron microscopes. Administration of curcumin for 24 hours resulted in an increase in oxidative stress in liver suggesting a pro-oxidant role, which might be due to the generation of reactive oxygen species, while post-treatment with Cd resulted in a synergistic effect on oxidative stress. Concurrent marked histological alterations were observed under the light microscope in the form of basophilic depositions, Kupffer cell hyperplasia, and lobular inflammation. Electron micrographs also revealed similar features along with pronounced damage to the endothelial cell fenestrations and bile canaliculi with crystalline deposits on hepatocytic surfaces. Therefore, it was concluded that after 24-hour exposure to curcumin, it acted as a pro-oxidant in mice liver and was not found to have an ameliorative effect on acute Cd-induced hepatotoxicity.
Keywords: Curcumin, Cadmium, acute exposure, liver, oxidative stress, pro-oxidant scanning electron microscopy, light microscopy.
INTRODUCTION
Antioxidants play an important role to nullify the deleterious effects of free radicals such as reactive oxygen species, thereby not only protecting the cells and tissues but also regulating various pathological and physiological processes. Curcumin, derived from the rhizome of the herb Curcuma longa (turmeric spice), is one such antioxidant which is a bioactive hydrophobic polyphenol compound. It is a safe nutritional dietary supplement that has been widely used in traditional medicine and as a spice/coloring agent since time immemorial1.
In India and Southeast Asia, the diverse biological functions of turmeric such as antioxidant activities, anti-inflammatory, and anti-mutagenic were realized since long, and it has been used in the treatment of inflammation, skin wounds, and tumors2. In recent years, there is growing evidence that curcumin is a potentially important chemopreventive agent against cancer and may play a crucial role in both the prevention and treatment of various neurodegenerative diseases such as Parkinson's and Alzheimer's disease3.
García-Niño and Pedraza-Chaverri (2014) in a review on the protective effects of curcumin against heavy metals-induced liver damage, gave an account of the various mechanisms by which curcumin confers protection. These mechanisms included suppression of oxidative stress, inflammation, and activation of stellate cells in the liver, with concomitant up-regulation of enzymes involved in detoxification and Keap1/ Nrf2/ARE pathway expression1. Various studies on guinea pigs, rats, monkeys, and pigs have reported that on the feeding of curcumin or turmeric there were no toxic effects4-6. On the other hand, Deshpande et al., (1998) reported that on feeding turmeric or ethanolic turmeric extract, in high doses for long-term exposure, to mice and rats, hepatoxicity was observed in the form of profuse focal necrosis along with decreased body weights7. Similarly, Kandarkar et al., (1998), while analyzing the effects of doses of whole spice turmeric or ethanolic turmeric extract reported being cancer protective, observed coagulative necrosis with areas of parenchymal regeneration8.
Cadmium (Cd) is a toxic heavy metal that has no physiological function in the body and causes deleterious effects on health following both acute and chronic exposures. Moreover, Cd accumulates in the body over time as in humans its biological half-life is 17-30 years9. Non-occupational exposure is generally via consumption of food and drinking water contaminated with Cd along with cigarette smoke. Occupational exposure mainly results from numerous modes of exposure, such as CdCl2 utilized in paint manufacture and Cd fume inhalation in battery industry10. Exposure to Cd has been reported to affect a myriad of organs in humans, but most commonly it affects kidney, lung, bone and skeletal, cardiovascular, and nervous systems11, 12.
The liver is a major organ that is involved in metabolism and detoxification reactions, through which all substances that are absorbed by the intestine pass. The liver is known to accumulate toxins, including heavy metals such as Cd following acute or chronic exposures that result in hepatotoxicity13-15.
Tarasub et al. in 2008 reported that following co-treatment with cadmium acetate and curcumin, curcumin was unable to ameliorate oxidative damage induced due to cadmium16. In another study, they reported that combined treatment of curcumin and vitamin C was more effective in protection against Cd-induced hepatic injury by scavenging free radicals17.
Hepatotoxic effects of Cadmium and beneficial effects of antioxidant compounds such as curcumin following long terms exposures have been studied in detail in previous studies. However, there is a lacuna in knowledge related to the effect of short-term treatment of curcumin on the liver and its modulatory role on subsequent treatment with cadmium chloride (CdCl2) in mice. Therefore, the present study was undertaken to investigate the effect of short-term exposure to curcumin in the liver of mice. Further, the ameliorative effects of short-term pre-treatment with curcumin in acute cadmium chloride induced hepatotoxicity would be evaluated by assessment of biochemical parameters of oxidative stress and ultrastructural alterations.
MATERIALS & METHODS
Chemicals
All chemicals were of analytical grade specifications and obtained from HIMEDIA Ltd, India. Cadmium chloride (CdCl2) and curcumin were obtained from Sigma Chem. Co., St. Louis, MO, USA.
Animals and Treatments
Four to six-week-old Balb/c male mice weighing 20-25 grams were procured from the Central Animal House, Panjab University, Chandigarh. Mice were kept in cages, given food and water ad libitum and allowed to acclimatize for seven days, maintained at 12 hours light/dark regime before experimental use.
The animals were divided into five experimental groups, each containing seven mice and the doses was administered intraperitoneally. Curcumin dose of 100 mg/kg body weight (bw) was used, as it is the FDA approved dose for humans18. Curcumin was dissolved in DMSO (dimethyl sulfoxide), an amphiphilic compound that increases the permeability of the membranes, for its effective uptake by the cells19. A single i.p. Injection of 100 mg curcumin/kg bw dissolved in 100 ml DMSO was administered to the group T-II and animals were sacrificed after 24 hours of exposure, and therefore, a DMSO-treated group was also studied (group T-I). Cadmium chloride was dissolved in water and dose used in this study (0.8 mg CdCl2/kg bw) was two-thirds the experimentally determined LD50 value for intraperitoneal exposure in mice (calculated using Sun’s formula, 1963)20 to which group T-III were exposed for 18 hours.
The groups T-IV and T-V were injected 100 ml DMSO or 100 mg curcumin/kg bw dissolved in 100 ml DMSO, respectively and after 24 hours were given another treatment of 0.8 mg CdCl2/kg bw and finally sacrificed after 18 hours of the second treatment following various protocols and ethical procedures.
HEPATIC BIOCHEMICAL ESTIMATIONS
Preparation of Homogenate
10% homogenates of liver were prepared in 50mM Tris-HCl buffer (pH-7.4) using a Potter-Elvejhem homogenizer at 0-4°C. The homogenate was used for the spectrophotometric determination (using Jenway 6305 uv/vis spectrometer) of lipid peroxidation by measuring the tissue malondialdehyde (MDA) level21, superoxide anion-SA22, hydroperoxides-HP23, reduced glutathione-GSH24, and protein content25.
Preparation of Post Mitochondrial Supernatant -PMS
Liver homogenates were centrifuged at 9,200 rpm for 10 minutes at 4°C. The supernatant was stored at -20°C and used for the estimation of activities of Superoxide dismutase-SOD26, Catalase-CAT27, Glutathione peroxidase-GPx28, Glutathione reductase-GR29, and Glutathione-S-transferase-GST30, by continuous spectrophotometric rate determination (using Perkin Elmer Lambda 35 UV/vis spectrometer) and protein content25.
Histopathological studies
Tissue from the liver of mice was fixed in 10% buffered formalin and processed routinely. The blocks were embedded in paraffin wax. Sections of 5-6 μm thickness were cut by rotary microtome, stained with Haematoxylin-eosin (H&E) stain and examined under a light microscope (Leica DC 100, PC I Interface Digital Camera).
Scanning electron microscopy
The liver slices were washed with phosphate buffer and fixed in 4% glutaraldehyde in phosphate buffer. They were then dehydrated in ascending acetone grades and critical point dried through transitional fluid amyl acetate. The dried samples were fixed on metal stubs with double adhesive tape for gold sputtering. The stubs so prepared were examined using JEOL JSM 6100 Scanning Electron Microscope and captured at different magnifications to study the ultrastructural features.
Statistical analysis
Student’s t-test determined significance between pair of means for control and treated groups. The data were expressed as mean ± standard error of seven mice and the level of significance considered was P < 0.05.
RESULTS AND DISCUSSION
Effects of short-term curcumin treatment and pre-treatment for 24 hours, followed by acute Cd exposure for 18 hours were studied in male Balb/c mice. To investigate whether the exposure to curcumin alone or followed by Cd exposure causes hepatotoxicity resulting from oxidative stress, the levels of hepatic superoxide anion, hydroperoxides, GSH, total thiols, and MDA were measured in the liver homogenates of treated mice while activities of enzymes of the antioxidant system were measured in the PMS.
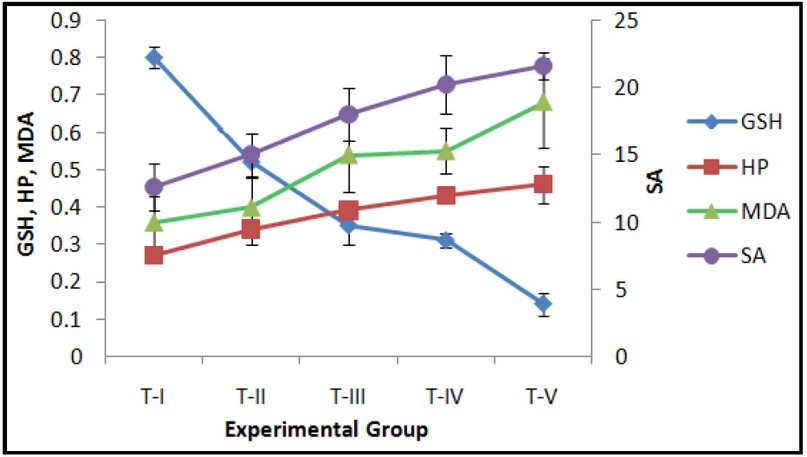
Fig. 1: Effect of exposure to Curcumin or Cd or their combinations on hepatic GSH (nmoles/mg protein), Superoxide anion (SA) (nmoles/mg protein), MDA (nmoles/mg protein) and Hydroperoxides (HP) (mmoles/mg protein) levels. Data expressed Mean ± SE (n=7). Level of significance P < 0.05.
DMSO treated control group T-I was compared with the treated groups T-II to T-V. The GSH and total thiol levels in the liver of group T-I were 0.80 ± 0.03 and 31.1 ± 2.1 nmoles/mg protein, respectively. Both GSH and total thiol levels decreased in all the treated groups T-II to T-V as compared to the controls (T-I). Exposure to curcumin for 24 hours (group T-II) or pre-treatment with curcumin followed by acute Cd exposure (group T-V) resulted in increased oxidative stress as seen by an increase in LPO, SA, HP and activities of antioxidant and detoxifying enzymes SOD, CAT, GPx, GR and GST, with a concomitant decrease in cellular antioxidant GSH for the groups T-II and T-V (Figs. 1-3). These observations indicate pro-oxidant effects following short-term exposure to curcumin in mice. The increase in activities of antioxidant/detoxifying enzymes SOD, CAT, GPx, GR and GST following treatments with curcumin, Cd or curcumin and Cd combinations is suggestive of the activation of the cellular protective mechanisms involving antioxidant and detoxifying enzymes. However, the protective mechanisms of the cells i.e. antioxidant and detoxifying enzymes were insufficient to neutralize the overwhelming effect of ROS generation, thereby resulting in the observed oxidative stress for the doses and duration of exposure in the present study.
The basal MDA level, indicative of lipid peroxidation, in control liver was 0.36 ± 0.07 nmoles/mg protein. MDA was significantly increased by 1.5, 1.6 and 1.8 fold, following Cd exposure in groups T-III to T-V respectively (Fig. 1). On the other hand, total thiols showed a significant decrease of 4.7, 5.4 and 8.1 fold for Cd-exposed groups, i.e. groups T-III to T-V, respectively, irrespective of the pre-treatment with curcumin in group T-V (Fig. 2). A corresponding decrease of 2.3, 2.5, and 5.7 fold in GSH levels in groups T-III to T-V, respectively, was observed (Fig. 2). Similar increase ranging between 1.1 to 2 fold was observed in the activities of the liver antioxidant enzymes SOD, GPx, CAT and GSH pathway enzymes GPx and GR for groups T-III to T-V (Figs. 2 and 3).
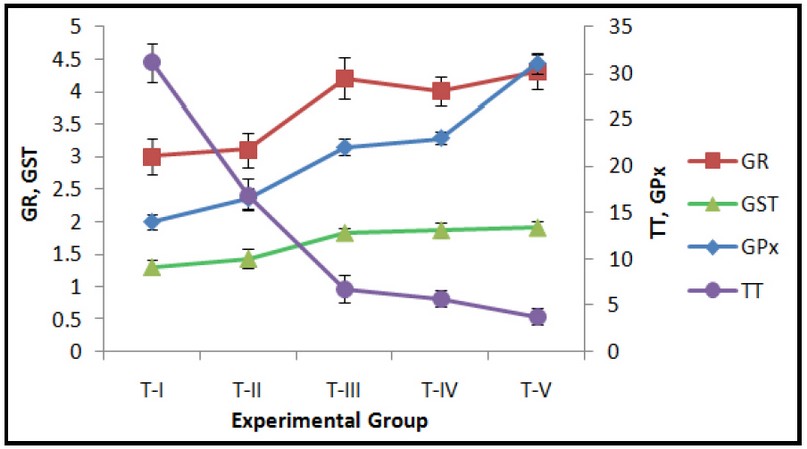
Fig. 2: Effect of exposure to Curcumin or Cd or their combinations on hepatic Glutathione peroxidase (GPx) (nmoles NADPH consumed/ min./mg protein), Glutathione reductase (GR) (nmoles NADPH oxidized/min./mg protein), Glutathione-S-transferase (GST) (GSH adduct formed/min./mg protein) and Total Thiols (TT) (nmoles/mg protein). Data expressed Mean ± SE (n=7). Level of significance P < 0.05.
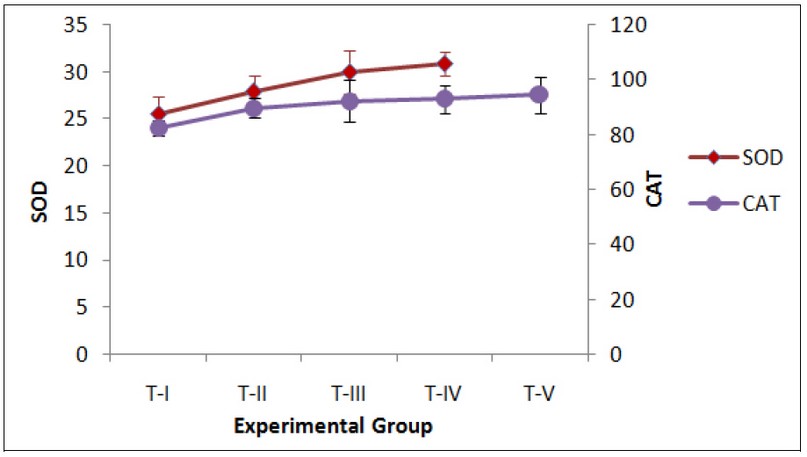
Fig. 3: Effect of exposure to Curcumin or Cd or their combinations on hepatic Superoxide dismutase (SOD)- Unit#/min/mg protein (Unit# - 1 unit enzyme activity is defined as the amount of enzyme-inhibiting 50% Nitroblue tetrazolium reduction), and Catalase (CAT)- mmoles H2O2 decomposed/min/mg protein and Glutathione peroxidase (GPx)- nmoles NADPH consumed/min/mg protein. Enzyme activities. Data expressed Mean ± SE (n=7). Level of significance P < 0.05.
Acute Cd exposure has been reported to be associated with an increase in LPO, SA and HP and disruption of the cellular antioxidant system with a subsequent decrease in GSH31, 32. Waisberg et al. (2003) had reported that Cd cannot directly generate free radicals and rather is involved in the indirect formation of ROS and RNS33. An imbalance between lipid peroxidation and antioxidant system results in oxidative stress. One of the main manifestations of oxidative damage is lipid peroxidation34. In the present study, the increase in MDA levels with a concurrent decrease in GSH and total thiols, indicates the depletion of reduced glutathione and total thiols, due to increased lipid peroxidation. The increase in the levels of hydroxyl radicals, superoxide anions, hydrogen peroxide along with the enzymes of the antioxidant system indicated that short-term exposure to curcumin and/or Cd results in oxidative stress.
Pro-oxidant effects of curcumin have also been previously reported, though mainly in terms of reactive oxygen species induced DNA damage35-37. Rukkumani et al. (2004) investigated the effect of curcumin on alcohol and poly-unsaturated fatty acid-induced oxidative stress and had also reported an increase in LPO and HP with an associated decrease in GSH following curcumin administration38. In a study following co-treatment with cadmium acetate and curcumin, it was reported that curcumin did not have a protective effect against Cd-induced oxidative stress16. Following long-term pre-exposure, to curcumin, there are reports that tissues are protected against LPO as curcumin increases intracellular glutathione concentration and acts as a powerful oxygen free radical scavenger39.
Masuda et al. (1999) explained the anti-oxidative role of curcumin, suggesting a two-stage mechanism involving a radical trapping stage and radical termination stage40. In a subsequent study, they elaborated that radical terminations were of two types; (i) dimer formation between two curcumin radicals and (ii) a coupling product between curcumin and lipid hydroperoxide with the latter being fundamental in the antioxidant mechanism of curcumin41. Thus, the increase in ROS generation observed in the groups exposed to curcumin viz. groups T-II and T-V may be explained based on the formation of free radicals, before the radical tapping stage during the 24 hours of exposure in the present study resulting in pro-oxidant effects.
Kawanishi et al. (2005) reported that like many antioxidants, curcumin could be a "double-edged sword," having carcinogenic and pro-oxidant effects on one hand and anticancer and antioxidant effects on the other42. The antioxidant, protective and ameliorative effects of curcumin have been reported after long term, sustained exposures of curcumin by exerting effects of a potent scavenger of different ROS including superoxide anion radicals (O2-.) and hydroxyl radicals (.OH)43.
In our previous study on dose-response effects following acute Cd exposures in mice, we reported that with an increase in CdCl2 dose there were significant changes in the biochemical parameters indicative of oxidative stress, while a certain amount of Cd load was required for histopathological changes to take place in hepatic tissue15. The hepatic tissues of the mice administered different treatments were further investigated to identify the correlation between oxidative stress and histopathological alterations.
Liver from the control group T-I and curcumin-treated group T-II did not exhibit any macroscopic alterations in the morphology of hepatic tissue of mice. On the other hand, exposure to Cd (groups T-III and T-IV) or curcumin pre-treatment followed by Cd exposure (group T-V) resulted in marked alterations in the histology of the liver as observed under the light and scanning electron microscopes.
Light micrograph examination of sections of the groups T-II, T-III/T-IV, and T-V revealed signs of cell injury as compared to control mice (group T-I) that had normal morphology and occasional foci of lobular inflammation (Fig. 4A). Multiple foci of lobular inflammation by mononuclear cells and Kupffer cell hyperplasia were observed in all the other groups T-II to T-V (Figs. 4 B-D), though cell injury was more pronounced in group T-V with prominent sinusoidal dilation and giant cell transformation along with depositions of basophilic material (Fig. 4D). The morphological changes were diffused and not localized to any specific area, suggesting that Cd and curcumin acted as general hepatotoxins following short-term exposures.
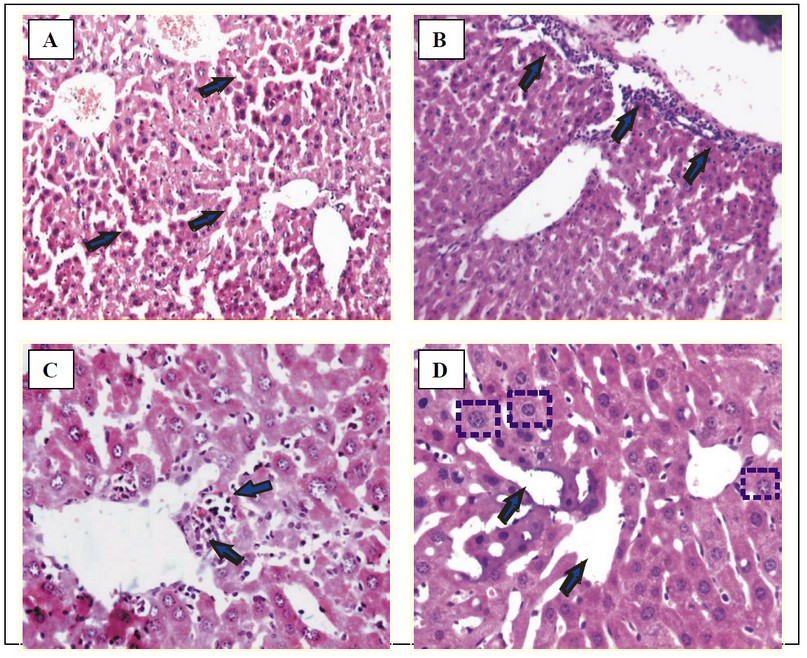
Fig. 4: Micrographs of liver of treated mice.
(A) Normal morphology with occasional foci of lobular inflammation in control group T-I. H & E × 550.
(B) Portal tract inflammation and Mononuclear cell (MNC) infiltration in group T-III/T-IV. H & E × 550.
(C) MNC infiltration around the central vein, KC (Kupffer cell) hyperplasia and locular inflammation in group T-II. H & E × 280
(D) Hepatocytic damage with giant cell transformation, sinusoidal dilation and KC hyperplasia with basophilic deposits within heptocytes and sinusoids in group T-V. H & E × 550.
Investigation of the morphological features of the livers under the scanning electron microscope revealed that the mesothelial layer of the liver was apparently normal in the control group T-I, groups T-II and T-V (Figs. 5 A, B and D), whereas hyperplasia with necrotic damage was observed in the liver of mice in the groups T-III/T-IV (Fig. 5C). Kupffer cells were seen lying over the endothelial cells lining the sinusoids in groups T-I and T-II (Figs. 6 A & B). Similar to the observations of KC hyperplasia seen in the light micrographs of groups T-II and T-V (Figs. 4 C & D), KC lying over necrotic hepatocytes were also observed on the electron micrographs (Fig. 6 C) with crystalline deposits on the hepatocytic surface in the group T-V (Fig. 6 D).
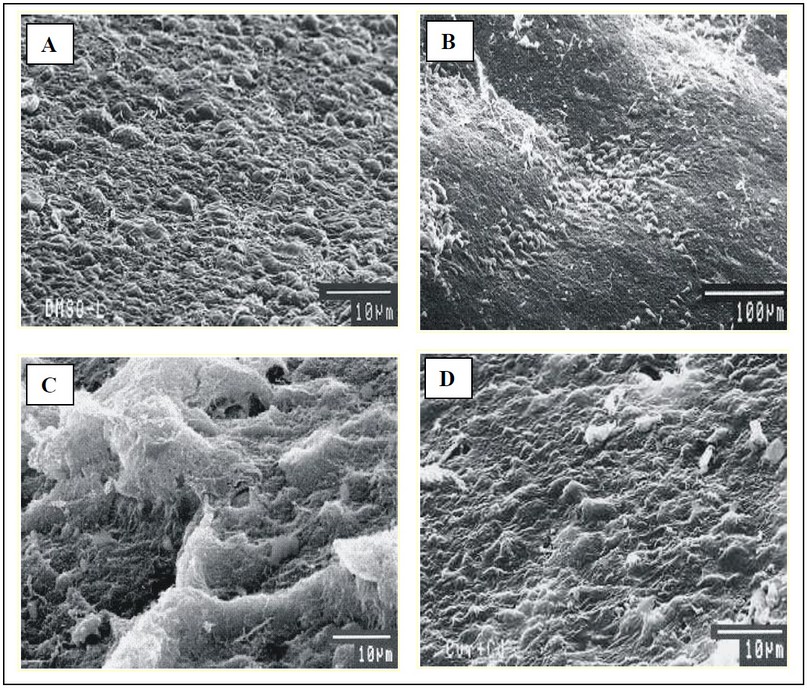
Fig. 5: Scanning electron micrographs of mesothelial layer of liver of mice.
(A) Group T-I (SEM 10 µm), (B) Group T-II (SEM 100 µm) and (D) Group T-V (SEM 10 µm) - Normal mesothelial layer.
(C) Group T-III/IV show hyperplasia and necrotic damage of mesothelial layer (SEM 10 µm).
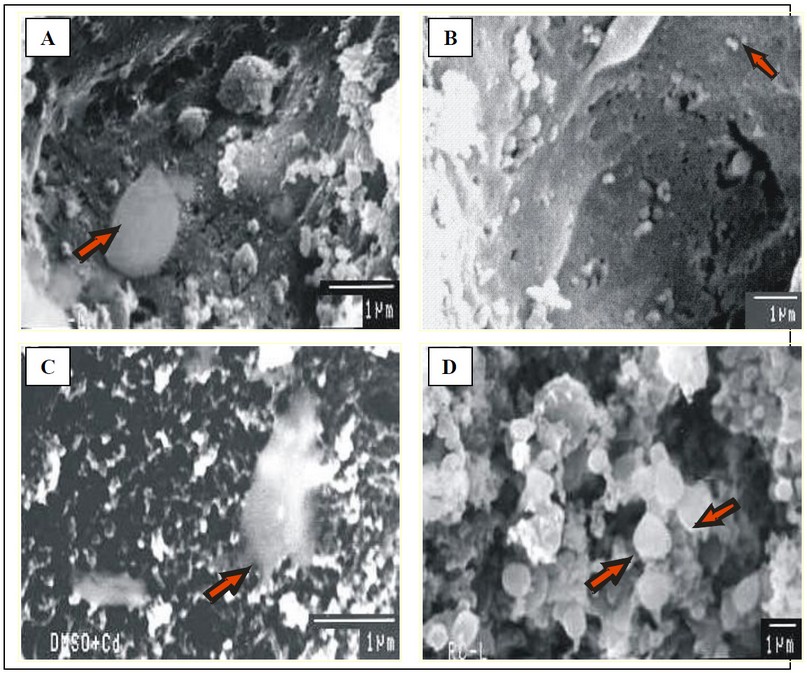
Fig. 6: Micrographs of liver showing
(A) Group T-I (SEM 1µm) and (B) Group T-II (SEM 1µm) Kupffer cell lying on the surface of normal endothelial cells lining the sinusoids
(C) Group T-III/IV has Kupffer cells lying over necrotic hepatocytes (SEM 1µm).
(D) Crystalline deposits observed on the hepatocyte surface group T-V (SEM 1µm).
Endothelial cells lining the sinusoidal wall of the liver have characteristic fenestrations with well-defined porosity of bile canaliculi, as observed in the liver of groups T-I and T-II (Figs. 7 and 8: A and B). However, loss of endothelial cell fenestrations and blockage of bile canaliculi due to blebbing of the surface epithelia due to necrotic damage were seen in the liver of mice in the groups exposed to Cd, i.e. T-III/T-IV and T-V (Figs. 7 and 8: C and D).
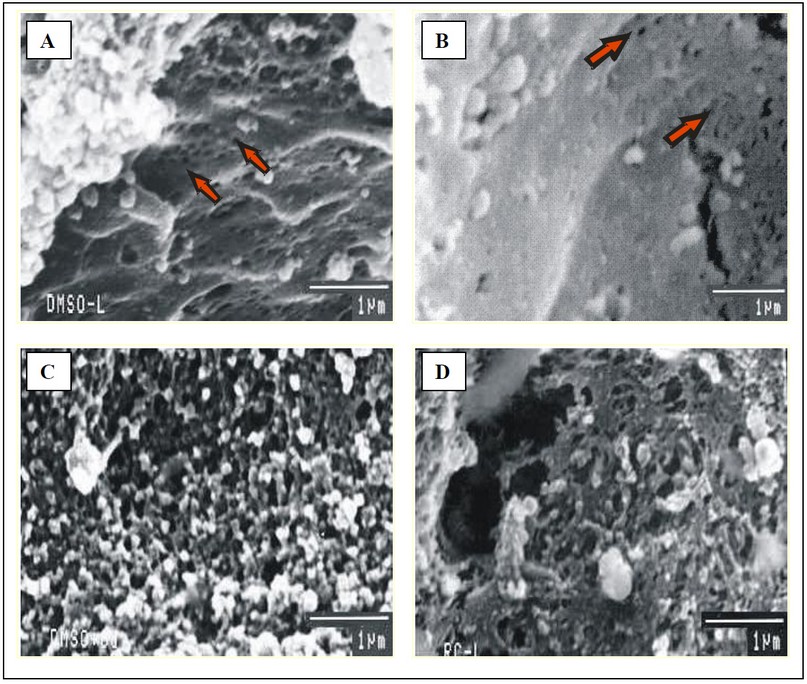
Fig. 7: Scanning electron micrographs of liver of mice with (A & B) Clear sieve plate fenestrations of endothelial cells lining the sinusoids in groups T-I and T-II (C & D) Sinusoidal damage observed as necrosis and loss of fenestrations in Cd exposed groups T-III/T-IV and T-V. (SEM 1 µm).
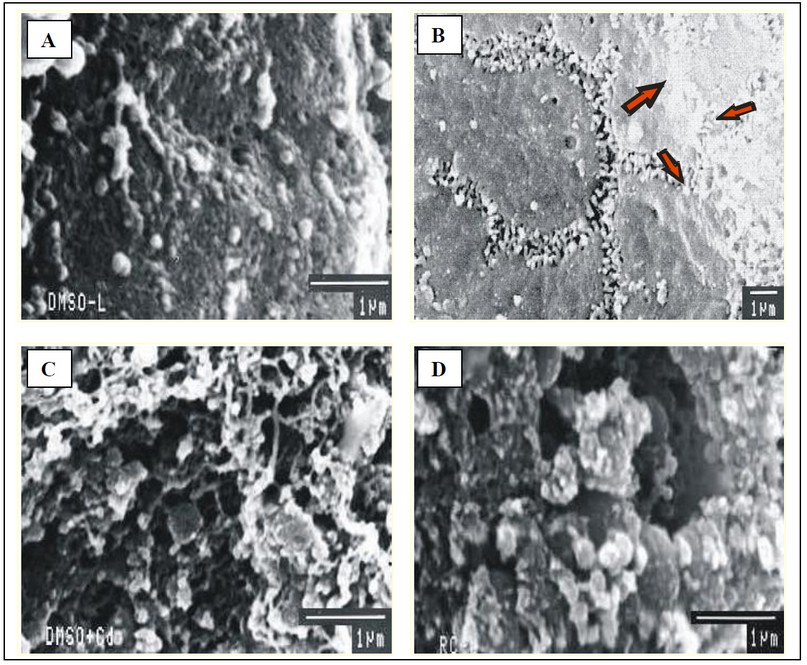
Fig. 8: Scanning electron micrographs of liver of mice showing well defined porosity of hepatocytes of bile canalculi in groups T-I and T-II (A & B) and necrotic damage resulting in loss of bile bile canalculi in Cd exposed groups T-III/T-IV and T-V (C & D). (SEM 1 µm).
A plausible mechanism of curcumin and Cd-induced hepatotoxicity may be that the damage results cumulatively due to release of endogenous inflammatory mediators (ROS) and concurrent Kupffer cell and mononuclear cell activation as seen on light and electron micrographs. Blockage of the bile canaliculi and loss of endothelial cell fenestrations may further result in ischemia44 with subsequent loss of function of hepatocytes.
CONCLUSION
In summary, the present results suggested that curcumin exposure for 24 hours or curcumin pre-treatment followed by Cd exposure for 18 hours, in mice resulted in increased oxidative stress that was measured in terms of increased levels of LPO, SA, HP and activities of enzymes of antioxidant and detoxifying systems with concomitant decrease in the GSH and total thiols. Corresponding histopathological alterations were observed in the liver sections in the light and electron micrographs of the treated mice, suggesting that on short-term exposure to curcumin, it acts as a pro-oxidant and has a synergistic effect on acute Cd hepatotoxicity.
Conflict of Interests
The authors declare that there is no conflict of interests.
ACKNOWLEDGEMENT
The author wishes to acknowledge financial assistance received from the Council for Scientific and Industrial Research, New Delhi, India and Indian Council of Medical Research, New Delhi, India during her doctoral research work at the Department of Biotechnology, Panjab University, Chandigarh, India.
REFERENCES
1. García-Niño, W. R., & Pedraza-Chaverri, J. (2014). Protective effect of curcumin against heavy metals-induced liver damage. Food and Chemical Toxicology, 69, 182-201.
2. Iqbal, M., Sharma, S. D., Okazaki, Y., Fujisawa, M., & Okada, S. (2003). Dietary supplementation of curcumin enhances antioxidant and phase II metabolizing enzymes in ddY male mice: possible role in protection against chemical carcinogenesis and toxicity. Pharmacology & toxicology, 92(1), 33-38.
3. Ambegaokar, S. S., Wu, L., Alamshahi, K., Lau, J., Jazayeri, L., Chan, S., ... & Timiras, P. S. (2003). Curcumin inhibits dose-dependently and time-dependently neuroglial cell proliferation and growth. Neuroendocrinology Letters, 24(6), 469-469.
4. Wahlstrom, B., & Blennow, G. (1978). A study on the fate of curcumin in the rat. Acta Pharmcol. Toxicol., 43: 86-92.
5. Bhavanishankar, T.N., Shanta, N.V., Ramesh, H.P., Indira, Murthy A.S., & Sreenivasmurthy, V. (1980). Toxicity studies on turmeric (Curcuma longa): Acute toxicity studies in rats, guinea pigs and monkeys. Ind. J. Exp. Biol.,18, 73-75.
6. Bille, N., Larsen, J.C., Hansen, E.V. & Wurtzen, G. (1985). Subchronic oral toxicity of turmeric oleoresin in pigs. Food Chem. Toxicol., 23, 967-973.
7. Deshpande, S.S., Lalitha, V.S., Ingle, A.D., Raste, A.S., Garde, S.G., & Maru, G.B. (1998). Subchronic oral toxicity of turmeric and ethanolic turmeric extract in female mice and rats. Toxicol. Letters, 95,183-193.
8. Kandarkar, S.V., Sawant, S.S., Ingle, A.D., Deshpande, S.S., & Maru, G.B. (1998). Subchronic oral hepatotoxicity of turmeric in mice - Histopathological and ultrastructural studies. Ind. J. Exp. Biol., 36, 675-679.
9. Kjellström, T., & Nordberg, G. F. (1978). A kinetic model of cadmium metabolism in the human being. Environmental research, 16(1-3), 248-269.
10. Agency for Toxic Substances and Disease Registry (ATSDR). 2003 & 2012. Toxicological profile for Cadmium. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service.
11. Ognjanović, B. I., Marković, S. D., Ðorđević, N. Z., Trbojević, I. S., Štajn, A. Š., & Saičić, Z. S. (2010). Cadmium-induced lipid peroxidation and changes in antioxidant defense system in the rat testes: Protective role of coenzyme Q10 and Vitamin E. Reproductive Toxicology, 29(2), 191-197.
12. Jaishankar, M., Tseten, T., Anbalagan, N., Mathew, B. B., & Beeregowda, K. N. (2014). Toxicity, mechanism and health effects of some heavy metals. Interdisciplinary toxicology, 7(2), 60-72.
13. Souza, V., Bucio, L., Jay, D., Chavez, E., & Gutierrez-Ruiz, M. C. (1996). Effect of cadmium on calcium transport in a human fetal hepatic cell line (WRL-68 cells). Toxicology, 112(2), 97-104.
14. Saïdi, S. A., Azaza, M. S., Windmolders, P., van Pelt, J., & El-Feki, A. (2013). Cytotoxicity evaluation and antioxidant enzyme expression related to heavy metals found in tuna by-products meal: an in vitro study in human and rat liver cell lines. Experimental and toxicologic pathology, 65(7-8), 1025-1033.
15. Sahrawat, T.R., Sobti, R.C., Sharma, S.C., Saikia, U.N., & Sharma, M.L. (2019). Hepatotoxicity on acute exposure to cadmium chloride in mice. Journal of Emerging Technologies and Innovative Research (JETIR), 6(5), 384-390.
16. Tarasub, N., Narula, K., & Ayutthaya, W.D.N (2008). Effects of curcumin on cadmium-induced hepatotoxicity in rats. Thai. J. Toxicol. 23, 100–107.
17. Tarasub, N., Junseecha, T., Tarasub, C., & Ayutthaya, W.D.N. (2012). Protective effects of curcumin, vitamin C, or their combination on cadmium-induced hepatotoxicity. Journal of basic and clinical pharmacy, 3(2), 273.
18. Chainani-Wu, N. (2003). Safety and anti-inflammatory activity of curcumin: a component of tumeric (Curcuma longa). The Journal of Alternative & Complementary Medicine, 9(1), 161-168.
19. Perkins, S., Verschoyle, R. D., Hill, K., Parveen, I., Threadgill, M. D., Sharma, R. A., ... & Gescher, A. J. (2002). Chemopreventive efficacy and pharmacokinetics of curcumin in the min/+ mouse, a model of familial adenomatous polyposis. Cancer Epidemiology and Prevention Biomarkers, 11(6), 535-540.
20. Sun, R. (1963). A forthright and practical method for comprehensive calculation of LD50. Learned J. Pharm, 10, 65.
21. Beuge, J.A., & Augst, S.D. (1978). Microsomal lipid peroxidation. Method Enzymol., 52:302-310.
22. Beutler, E., Duron, O., & Kelley, B.M. (1963). Improved method for detection of blood glutathione. J. Lab. Clin. Med., 61,882-888.
23. Elferink, J.G. (1984). Measurement of the metabolic burst in human neutrophils: a comparison between cytochrome c and NBT reduction. Research communications in chemical pathology and pharmacology, 43(2), 339-342.
24. Jiang, Z.Y., Hunt, J.Y., & Wolf. S.P. (1992). Detection of lipid hydroperoxides using the ‘fox method’. Anal. Biochem., 202, 384-389.
25. Lowry, O.H., Rosebrough, N.J., Farr, A.L., & Randall, R.J. (1951). Protein measurement with the Folin phenol reagent. Journal of biological chemistry, 193, 265-275.
26. Kono, Y. (1978). Generation of superoxide radicals during auto-oxidation of hydroxyl-amine hydrochloride an assay for SOD. Arch Biochem Biophys, 186, 189-195.
27. Luck H. (1963). Catalase. In: Methods of enzymatic analysis Ed. H.U. Bergmeyer, Academic Press, New York, pp. 885-889.
28. Paglia, D.E., & Valentine, W.N. (1967). Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. The Journal of laboratory and clinical medicine, 70(1), 158-169.
29. Horn H.D. (1971). Glutathione reductase. In: Methods of enzymes analysis. Ed. Bergmeger, H.U:Academic Press, New York, pp. 875-881.
30. Habig W.H., Pabst M.J., & Kakoby W.B. (1974) Glutathione-S-transferase. J. Biol. Chem., 249:7130-7139.
31. Sarkar, S., Yadav, P., Trivedi, R., Bansal, A. K., & Bhatnagar, D. (1995). Cadmium-induced lipid peroxidation and the status of the antioxidant system in rat tissues. Journal of Trace Elements in Medicine and Biology, 9(3), 144-149.
32. El‐Maraghy, S. A., Gad, M. Z., Fahim, A. T., & Hamdy, M. A. (2001). Effect of cadmium and aluminum intake on the antioxidant status and lipid peroxidation in rat tissues. Journal of Biochemical and Molecular Toxicology, 15(4), 207-214.
33. Waisberg, M., Joseph, P., Hale, B., & Beyersmann, D. (2003). Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology, 192(2-3), 95-117.
34. Stohs, S. J., & Bagchi, D. (1995). Oxidative mechanisms in the toxicity of metal ions. Free radical biology and medicine, 18(2), 321-336.
35. Ahsan, H., Parveen, N., Khan, N. U., & Hadi, S. M. (1999). Pro-oxidant, anti-oxidant and cleavage activities on DNA of curcumin and its derivatives demethoxycurcumin and bisdemethoxycurcumin. Chemico-biological interactions, 121(2), 161-175.
36. Kelly, M.R., Xu, J., Alexander, K.E., & Loo, G. (2001). Disparate effects of similar phenolic phytochemicals as inhibitors of oxidative damage to cellular DNA. Mutation Research/DNA Repair, 485(4), 309-318.
37. Urbina-Cano, P., Bobadilla-Morales, L., Ramírez-Herrera, M.A., Corona-Rivera, J.R., Mendoza-Magaña, M.L., Troyo-Sanromán, R., & Corona-Rivera, A. (2006). DNA damage in mouse lymphocytes exposed to curcumin and copper. Journal of applied genetics, 47(4), 377-382.
38. Rukkumani, R., Aruna, K., Varma, P.S., Rajasekaran, K.N., & Menon, V.P. (2004). Comparative effects of curcumin and an analog of curcumin on alcohol and PUFA induced oxidative stress. J Pharm Pharm Sci, 7(2), 274-283.
39. Ciftci, O., Ozdemir, I., Tanyildizi, S., Yildiz, S., & Oguzturk, H. (2011). Antioxidative effects of curcumin, β-myrcene and 1, 8-cineole against 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin-induced oxidative stress in rats liver. Toxicology and Industrial Health, 27(5), 447-453.
40. Masuda, T., Hidaka, K., Shinohara, A., Maekawa, T., Takeda, Y., & Yamaguchi, H. (1999). Chemical studies on antioxidant mechanism of curcuminoid: analysis of radical reaction products from curcumin. Journal of agricultural and food chemistry, 47(1), 71-77.
41. Masuda, T., Toi, Y., Bando, H., Maekawa, T., Takeda, Y., & Yamaguchi, H. (2002). Structural identification of new curcumin dimers and their contribution to the antioxidant mechanism of curcumin. Journal of agricultural and food chemistry, 50(9), 2524-2530.
42. Kawanishi, S., Oikawa, S., & Murata, M. (2005). Evaluation for safety of antioxidant chemopreventive agents. Antioxidants & redox signaling, 7(11-12), 1728-1739.
43. Nanji, A. A., Jokelainen, K., Tipoe, G. L., Rahemtulla, A., Thomas, P., & Dannenberg, A. J. (2003). Curcumin prevents alcohol-induced liver disease in rats by inhibiting the expression of NF-κB-dependent genes. American Journal of Physiology-Gastrointestinal and Liver Physiology, 284(2), G321-G327.
44. Liu, J., Kershaw, W. C., Liu, Y. P., & Klaassen, C. D. (1992). Cadmium-induced hepatic endothelial cell injury in inbred strains of mice. Toxicology, 75(1), 51-62.
Received: 3 July 2019
Accepted: 30 July 2019
Tammanna R. Sahrawat1, Ranbir C. Sobti2, Sukesh C. Sharma3, Uma N. Saikia4 and Madan L. Sharma5
1Assistant Professor, Centre for Systems Biology and Bioinformatics, Panjab University, Chandigarh, India.
2Professor Emeritus, Department of Biotechnology, Panjab University, Chandigarh, India.
3 Professor, Department of Biochemistry, Panjab University, Chandigarh, India.
4 Professor Department of Histopathology, P.G.I.M.E.R., Chandigarh, India.
5CIL/SAIF, Panjab University, Chandigarh, India.
Corresponding Author: Dr. Tammanna R. Sahrawat* Ph.D.
Centre for Systems Biology Bioinformatics, Panjab University, Chandigarh, India.
Email: [email protected]
