2019.04.03.7
Files > Volume 4 > Vol 4 No 3 2019
INVESTIGATION / RESEARCH
Synthesis of new Carnitine Palmitoyltransferase I inhibitors derivatives of C75
Kamil Makowski1,2,4,*, Paula Mera2, Javier Ariza3,4, Dolors Serra2,3, Jordi Garcia3,4, Laura Herrero2,3, Marta López1, Alicia Venegas1
Available from: http://dx.doi.org/10.21931/RB/2019.04.03.7
ABSTRACT
Carnitine Palmitoyltransferase (CPT1) is an enzyme that catalyzes the transport of fatty acids from the cytosol into the mitochondria. CPT1 inhibition in the hypothalamus increases fatty acid levels, which produces an increased expression of anorexigenic neuropeptides, a sign of satiety. C75 acts as an antiobesity predrug. In vivo C75, is converted into C75-CoA adduct, which is a potent inhibitor of CPT1 and produces a loss of appetite and body weight. In this work, we present three new derivatives of C75, where the carboxylic group is replaced by a carnitine unit, malonic group, and a hydroxyl group with changes from trans to cis relative stereochemistry. Our results suggest that introducing a bigger group than carboxylic in β position or cis relative configuration of the lactone leads to a decrease of CPT1 inhibitory activity.
Keywords: C75, CPT1, Carnitine Palmitoyltransferase 1 inhibitor, α-Methylene-γ-butyrolactones
INTRODUCTION
Obesity is a global problem that is increasing at epidemic rates. According to the World Health Organization, in 2016, more than 1.9 billion adults were overweight, and more than 650 million are classified as truly obese1. Obesity is a risk factor for health as it is related to serious diseases such as type 2 diabetes mellitus, cardiovascular and Alzheimer's disease, and even some types of cancer2. Carnitine palmitoyltransferase 1 (CPT1), is an enzyme belonging to the family of carnitine acyltransferases whose function is to transport the long chain fatty acids coupled with coenzyme A (LCFA-CoA), from the cytosol to the mitochondria where LCFA are β-oxidized to satisfy the need for the energy required in the body3. Energy homeostasis is specifically regulated by the hypothalamic neurons, which can detect increased LCFA levels and regulate food intake by modulating appetite4. The inhibition of hypothalamic CPT1 increases cytosolic LCFA-CoA levels of hypothalamic neurons, which triggers the mRNA expression of anorexigenic neuropeptides and decreases the mRNA expression of orexigenic neuropeptides. This is a signal of satiety and nutrient abundance which produces a loss of appetite5. The activity of CPT1 can be modulated by its physiological inhibitor malonyl-CoA, which controls the balance between synthesis and oxidation of LCFAs6.
C75 is an α-methylene-γ-butyrolactone, which acts as an appetite suppressor when it is coupled with coenzyme A (C75-CoA) by inhibiting CPT1 enzyme. Studies in vivo showed that injection of free C75 is transformed into C75-CoA and causes suppression of appetite and loss of body weight in animals5. The majority of the studies were carried out with a racemic mixture of C75, however (+)-C75-CoA enantiomer is responsible for the strong inhibition of CPT1 and a decrease in food intake in animals7. Herein, we show the synthesis of some racemic C75 derivatives and the evaluation of their CPT1 inhibitory activity with the aim of better understanding the structural requirements for stronger CPT1 inhibition.
MATHERIALS AND METHODS
The organic synthesis was carried out by standard procedures. A thin layer, column chromatography, melting point, NMR, IR, and high-resolution mass spectrometry were used to characterize and identify the obtained products (data not shown).
The anorexigenic activity of the C75 derivatives was tested in vivo in Sprague-Dawley rats, with intracerebroventricular (i.c.v.) administrations5. To perform in vitro CPT1 inhibitory studies, all derivatives of C75 were previously converted into coenzyme A adducts. The determination of the CPT1 inhibitory activity was carried out using a radiometric method7
RESULTS
We started the design of new derivatives of C75 with changes in the β position of lactone. Since malonyl-CoA is a physiological inhibitor of CPT1 and carnitine is a substrate of that enzyme, we synthesized two derivatives which substitute carboxylic group by malonic and carnitine moiety. On the other hand, the importance of relative lactone stereochemistry is unknown, and cis-C75 is unstable due to the migration of exocyclic double bond into endocyclic. Thus, we synthesized an alcoholic derivative of cis-C75, which can be compared with the already reported trans compound (UB006)8.
The synthesis of the C75 derivatives is initiated by the reduction using BH3: SMe2 of the advanced acid mixture reported previously in the synthesis of C759 with a protected double bond as selenoether.
The mixture of alcoholic diastereomers could be separated with fewer difficulties than acids and were the straining point in the synthesis of designed derivatives. The final alcoholic product (±)-cis-UB006 where obtained straightforwardly by recovering the exocyclic double bond in oxidative conditions.
The malonic derivative (±)-UB001 was synthesized in three steps, condensation of starting trans alcohol with a monoprotected malonic acid using carbodiimide and 4-DMAP as a catalyst. Next, the benzyl group was removed with TFA. Finally, under oxidizing conditions, the double bond of the lactone was recovered obtaining the derivative UB001.
The last derivatives with carnitine moiety were obtained in two steps. The first hydroxyl group was replaced by bromine using Appel reaction conditions, and then L-carnitine was coupled. The reaction required relatively high temperature and in the same step double bond was formed indicating that selenoether is unstable during the heating. The product called UB079 is a mixture of diastereomers due using enantiopure carnitine, and this mixture was used for preliminary CPT1 activity studies.

Figure 1. Synthesis of three new derivatives of C75
To test the inhibitory power in vitro of the synthesized derivatives, these must be previously converted into their coenzyme A adducts. The synthesis of the coenzyme A adduct is carried out by mixing the derivative and the HSCoA in D2O at a pH ~ 8 in an NMR tube and reaction was finished before 4h in all cases.

Figure 2. Preparation of the adducts of derivatives with coenzyme A
Once coenzyme A adduct of the derivatives was prepared, the inhibitory activity of these compounds was tested in vitro. The malonic derivative, UB001-CoA showed a decrease in the inhibitory activity concerning C75. Whereas, the carnitine derivative, UB079-CoA, was inactive in the inhibition of CPT1 at concentrations IC50 > 50 mM. On the other hand, it is observed that the alcoholic analogous, compound (±)-cis-UB006-CoA, present much worst inhibitory capacity than C75-CoA and trans UB006-CoA.

Figure 3. Effect of C75 derivatives on CPT1 activity was assayed in the presence of increasing concentrations of UB001-CoA, UB079-CoA (A), UB006-CoA, cis-UB006-CoA (B). C75-CoA was used as a positive control. The results are represented as the mean ± S.E.M.
Of the three derivatives presented, the compound UB001-CoA was selected for in vivo study. For the analysis, the free C75 (positive control) and UB001 were administered by i.c.v. Injection as well as a vehicle without any drug as a control. Then, body weight and food intake were measured. The UB001, which present about 10-fold decrease of inhibitory in vitro activity, compared to C75, present complete loss of appetite suppressing activity (Figure 4B) when administered at the same concentration as C75 i.c.v. and did not cause a loss of body weight (Figure 4A).
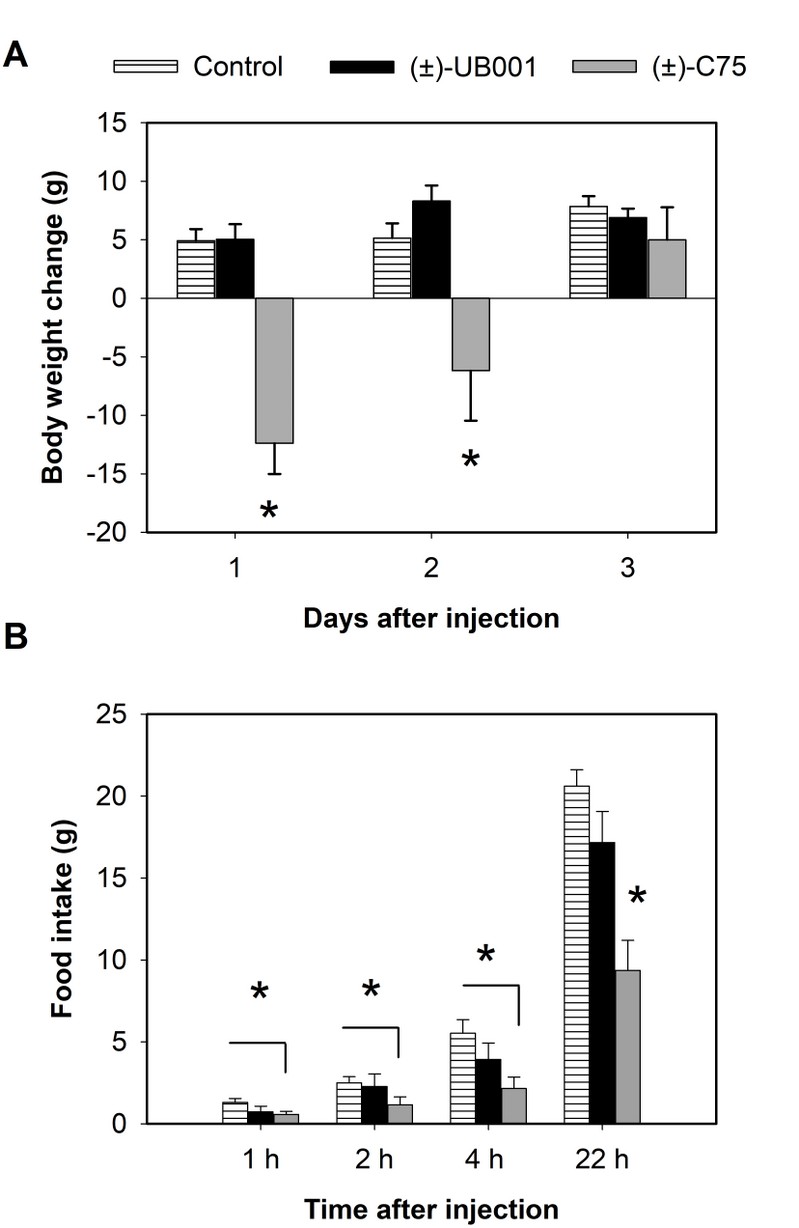
Figure 4. Effect of UB001 on body weight and food intake. (A). Accumulated food intake was measured after 1, 2, and 22 h after the injection (B). The results are expressed as the average ± S.E.M.
CONCLUSIONS
We conclude that the groups tested in the β position of the derivatives, the carnitine unit in UB079 and the malonic group in UB001 cause the loss of CPT1 inhibitory activity and seems that active center of that enzyme does not support bulkier group than carboxylic on the β position of the lactone. On the other hand, the change of trans to cis relative stereochemistry produces a loss of CPT1 inhibitory activity.
These new findings suggest that the length of the spacer between coenzyme A and the carboxylic group should be less than seven bonds, and it will be considered in future studies of CPT1 inhibitors derivatives of C75.
Acknowledgments
The Ministry of Spain supported this study – MINECO (SAF2014-52223-C2-1-R to JG and DS, co-funded by the Fondos Europeos de Desarrollo Regional de la Unión Europea [FEDER] and SAF2013-45887-R to LH), the Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN) (Grant CB06/03/0001 to DS), the Generalitat de Catalunya (2014SGR465 to DS), Fundació Marató TV3 (201627-30-31 to DS) and the European Foundation for the Study of Diabetes (EFSD)/Janssen-Rising Star and L’Oréal-UNESCO “For Women in Science” research fellowships to LH. KM is grateful to IBUB (Universitat de Barcelona) for a fellowship.
REFERENCES
1 Obesity and overweight. World Heal. Organ. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed 24 Mar2019).
2 Tseng Y-H, Cypess AM, Kahn CR. Cellular bioenergetics as a target for obesity therapy. Nat Rev Drug Discov 2010; 9: 465–482. doi:10.1038/nrd3138.
3 Thupari JN, Landree LE, Ronnett G V, Kuhajda FP. C75 increases peripheral energy utilization and fatty acid oxidation in diet-induced obesity. 2002; 99: 9498–9502.
4 Wolfgang MJ, Lane MD. The Role of Hypothalamic Malonyl-CoA in Energy. 2006; 281: 37265–37269. doi:10.1074/jbc.R600016200.
5 Mera P, Bentebibel A, López-Viñas E, Cordente AG, Gurunathan C, Sebastián D et al. C75 is converted to C75-CoA in the hypothalamus, where it inhibits carnitine palmitoyltransferase 1 and decreases food intake and body weight. Biochem Pharmacol 2009; 77: 1084–1095. doi:10.1016/j.bcp.2008.11.020.
6 López M, Lelliott CJ, Vidal-Puig A. Hypothalamic fatty acid metabolism: a housekeeping pathway that regulates food intake. Bioessays 2007; 29: 248–61. doi:10.1002/bies.20539.
7 Makowski K, Mera P, Paredes D, Herrero L, Ariza X, Asins G et al. Differential pharmacologic properties of the two C75 enantiomers: (+)-C75 is a strong anorectic drug; (-)-C75 has antitumor activity. Chirality 2013; 25: 281–7. doi:10.1002/chir.22139.
8 Makowski K, Mir JF, Mera P, Ariza X, Asins G, Hegardt FG et al. (−)-UB006: A new fatty acid synthase inhibitor and cytotoxic agent without anorexic side effects. Eur J Med Chem 2017; 131: 207–221. doi:10.1016/j.ejmech.2017.03.012.
9 Sanchez C, Makowski K, Mera P, Farras J, Nicolas E, Herrero L et al. Convenient synthesis of C75, an inhibitor of FAS and CPT1. RSC Adv 2013; 3: 6564–6571. doi:10.1039/C3RA40913A.
Received: 24 March 2019
Acepted: 8 july 2019
Kamil Makowski1,2,4,*, Paula Mera2, Javier Ariza3,4, Dolors Serra2,3, Jordi Garcia3,4, Laura Herrero2,3, Marta López1, Alicia Venegas1
1School of Chemical Sciences and Engineering, Yachay Tech University. Ecuador. 2Department of Biochemistry and Physiology, School of Pharmacy and Food Sciences, Institut de Biomedicina de la Universitat de Barcelona (IBUB), Universitat de Barcelona, Barcelona, Spain. 3Centro de Investigación Biomédica en Red de Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Instituto de Salud Carlos III, Madrid, Spain. 4Department of Inorganic and Organic Chemistry, Section of Organic Chemistry, Facultat de Química, Universitat of Barcelona, Spain.
*corresponding autor e-email:[email protected]
