2022.07.04.7
Files > Volume 7 > Vol 7 No 4 2022
Chemotherapy's impact on a few blood parameters
1 University of Knfa, Iraq .
2; University of Knfa;Iraq .
Available from: http://dx.doi.org/10.21931/RB/2022.07.04.7
ABSTRACT
Cyclophosphamide (CP), Ifosfamide (IFO), Paclitaxel (PTX) and Doxorubicin (DOX) are commonly used cytostatic drugs. The present study investigates the ecotoxicity and genotoxicity of CP, DOX, and PTX, their human metabolites/transformation products (TPs) cyclophosphamide (NCP) as individual compounds and as a mixture. The three-parent compounds ( Further ecotoxicity studies of metabolites. The measured toxicity of the cross was lower than the toxicity predicted by the concentration addition model indicating potentiating effects of the CPCOOH toxicity. Revealed genotoxic activity of CP and the mixture in the presence The degradation study with UV irradiation of samples containing (CP, cyclo, and DOX ) showed efficient degradation of compounds and remained toxic. They are suggesting that no stable with adverse effects was formed. This is the first study describing the ecotoxicity and genotoxicity of the commonly used cytostatics CP, cyclo, and DOX, their known metabolites, and their mixture. The results indicate the importance of toxicological evaluation and monitoring drug metabolites as they may be more hazardous to humans than parent compounds.
Keywords. Paclitaxel. Doxorubicin. Cyclophosphamide. toxicity
INTRODUCTION
The most common reason cancer patients experience low blood counts is as a side effect of chemotherapy. Chemotherapy involves the use of drugs to destroy cancer cells. Chemotherapy works by destroying cells that proliferate, a characteristic of cancer cells. Unfortunately, chemotherapy also affects normal cells that overgrow, such as cells in the bone marrow that produce red blood cells, white blood cells, and platelets.Cancer is the second leading cause of death worldwide, with approximately 9.6 million cancer-related deaths in 20181. Cancer is a generic term for a large group of diseases that can affect any body part. Other terms used are malignant tumors and neoplasms. One defining feature of cancer is the rapid creation of abnormal cells that grow beyond their usual boundaries, which can invade adjoining body parts and spread to other organs; the latter process is referred to as metastasis. Metastases are the primary cause of death from cancer2. In compliance with this trend of increasing cancer prevalence, new ANP drugs are also being designed, tested, and manufactured at an increasing rate3. More than over the past few years, 70 new ANPs (antineoplastic drugs) drugs have been released to treat 20 variants of tumors (cancerous growths), and the number of antineoplastic drugs (ANP) drugs has expanded by more than 60%. More than 500 companies are currently pursuing ANP drug development, with 300 companies having cancer drugs under clinical development stages4, so the chemotherapy or antineoplastic agents are designed to interact directly or indirectly with DNA by damaging its structure, inhibiting, altering and disrupting mechanisms of its transcription, replication, and synthesis. Derivatives of nitrogen mustards were developed, including the DNA alkylating agents cyclophosphamide, chlorambucil, and melphalan, widely used in clinical therapeutics6,7, and chemotherapy may be administered to cancer patients through usual routes of intravenous, intramuscular or subcutaneous injection. In general, the fate of chemical biotransformation includes the generation of metabolites, which may be desired for the therapeutic purpose or not, due to toxicity. Briefly, drug pharmacokinetics occurs in two phases of biotransformation, comprised of the functionalization (Phase I) and conjugation (Phase II) of the compound load, to increase its polarity and facilitate its elimination through excretion8. Phase I relies on an enzymatic system of defense against most of the xenobiotic compounds, composed of members of the cytochrome 450 gene family (CYP 450), flavin-containing monooxygenases (FMO), monoamine oxidases (MAOs), and xanthine oxidase/aldehyde oxidase. Those enzymes are differentially expressed in tissues but broadly distributed in the liver, kidney, and intestine, employing introducing, modifying, or unmasking reactive functional groups at the parent drug. and the Phase II reactions occur with the introduction of acetyl, sulfate, glucuronide acid, glutathione, and amino acids functional groups, either in the parent molecule or in a phase I metabolite structurally changed, enabling binding sites for conjugation. These reactions are mostly catalyzed by the enzyme uridine 5'- diphosphate (UDP)-glucuronosyltransferase (UGTs), sulfotransferases (SULTs), glutathione S-transferase (GSTs), and N-acetyltransferases (NATs). Biotransformation mechanisms present low specificity to pharmaceuticals and, in general, enhance the polarity of compounds, thus favoring excretion. Nevertheless, this change is often incomplete, with parent compounds excreted together with the metabolites in a variable proportion14. So The pharmaceuticals elected for the toxicity assessment in the present thesis were: cisplatin, cyclophosphamide, and tamoxifen. They were chosen according to a combination of 50 criteria regarding their consumption and environmental occurrence. Despite the increase in the number and quality of innovative new drugs currently released in the pharmaceutical market5, the first drug(CP), The advent of chemotherapy fundamentals, emerged in the first four decades of the 20th century. Breakthrough advances undertaken in World War II after an accidental spill of sulfur mustards led to the marked depletion of bone marrow and lymph nodes in men exposed to those chemicals, following discoveries of potential anticancer therapeutic9. In this course, cyclophosphamide (CP) was developed as a mustard prodrug with cytotoxic and alkylating purposes10. The CP dosage commonly administered in humans varies widely depending on the clinical indication, and can be defined as low (1–3 mg kg-1 or 40–120 mg m-2 ), daily orally administered; intermediate (15–40 mg kg-1 or 600–1,500 mg m-2 ), via intravenous, every 3 to 4 weeks; and high (> 120 mg kg-1 or > 5,000 mg m-2 ), every two days. After consumption, CP undergoes subsequent activation and transformation by the CYP 450 enzymes to yield the major cytotoxic species activation, the phosphoramide mustard (PAM) and acrolein. These species form labile covalent DNA adducts and inter-strand crosslinks, accountable for blocking DNA replication and avoiding cell proliferation11. According to Bagley et al. 12, not more than 20% of injected CP is excreted intact in urine at any dose level. Chemotherapy-induced alterations and incomplete blood count may cause significant problems in clinical practice. Anemia, neutropenia, and thrombocytopenia induced by chemotherapy regimens may cause life-threatening complications such as severe infections and hemorrhagic complications. Moreover, these side effects may also necessitate dose reduction and delay in schedules of chemotherapy treatment13, and the reason for choosing this study is to know the toxicity of chemotherapy drugs and their effect on some blood parameters (WBC, HB, PLT) Table1
MATERIALS AND METHODS
In this study, samples were collected from the Middle Euphrates cancer center in Al-Najaf city, Iraq collected 40 samples were from patients 20 patients treated with different types of chemotherapy-treated withe(Cyclophosphamide, DOX rubicin, paclitaxel) take the sample in (Nov and Dec -2021) Serum hemoglobin Table 6 (HGB) levels, white blood cell Table4 (WBC), platelet Table5 (PLT) count, and 20 patients considered as control (Table 1,2). And work (UV) to know the wavelength of three drugs (figure 1, 2,3)
RESULTS
After working in UV spectrophotometer measure, the wavelength of each drug is taken urine and serum after and before taking drugs so camper between this process the rate before took drugs and this test show the percentage of drugs in the patient's body like this (figure 1) after and before taking the drugs and measure samples by (UV) to know the wavelength to three drugs
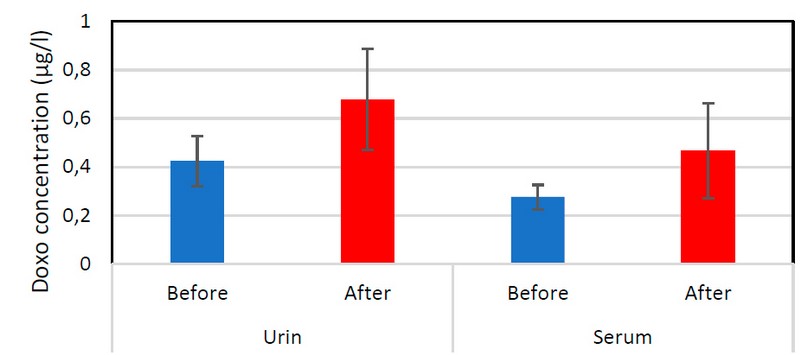
Figure 1. Shows the process of measuring serum and urine samples by (UV) spectrophotometer to know the wavelength of drugs and, after learning the wavelength according to published research, the toxicity percentage for each drug
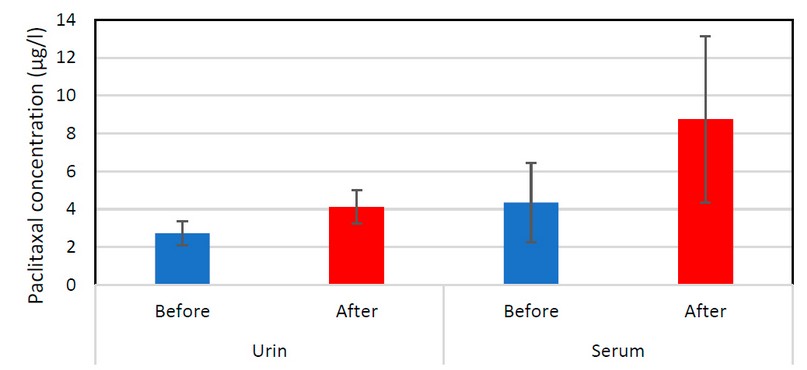
Figure 2. Paclitaxel drugs(urine, serum) after and before taking the drugs show an increased rate of drugs after taking the drug as a result of the difficulty of breaking down these compounds inside the patient's body
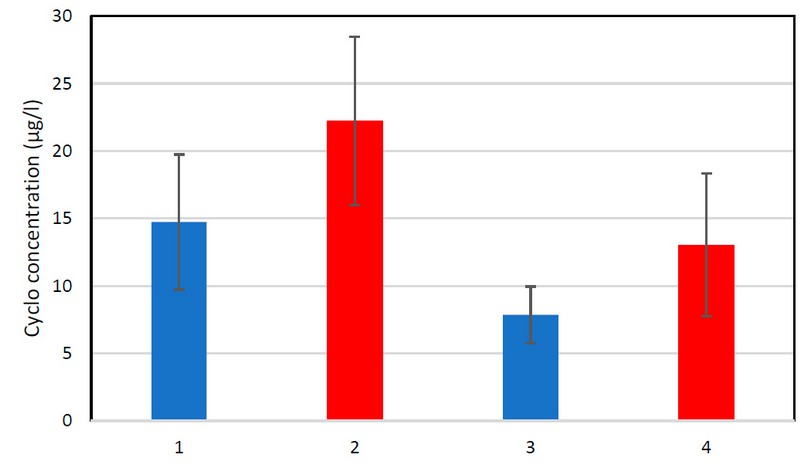
Figure 3. Cyclophosphamide drugs (urine, serum) after and before taking the drugs
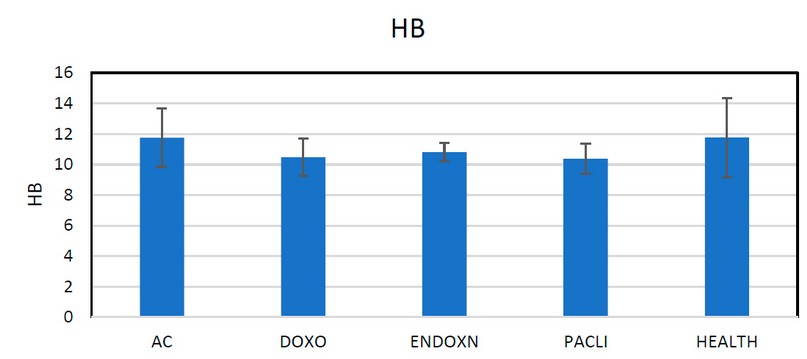
Figure 4. Shown patients receiving (AC) drugs, meaning (endoxan and DOX rubicin drugs) and influence or minimize the percentage of hemoglobin
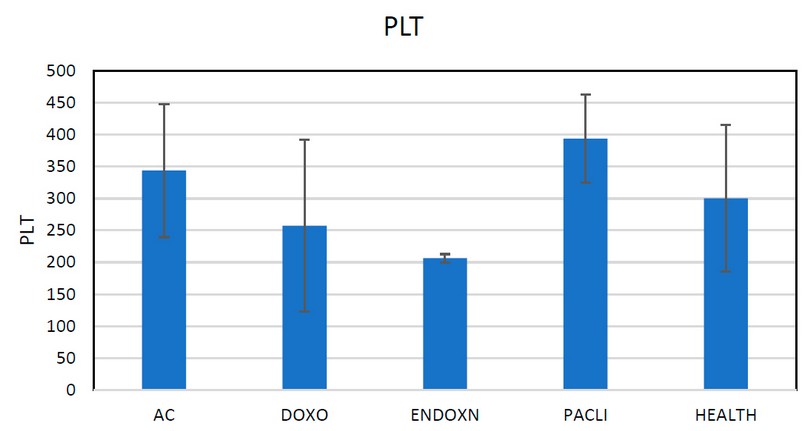
Figure 5. Shows the rate of platelet is increased in chemotherapy drugs
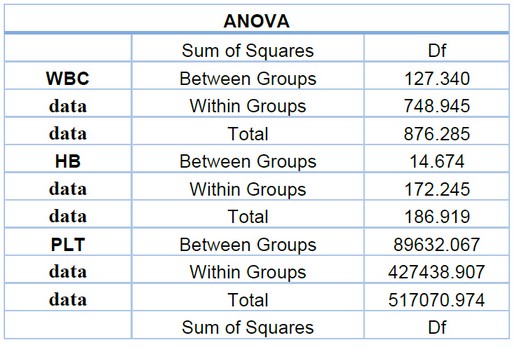
Table 1. Explain the sum and mean square between the WBC, HB, PLT
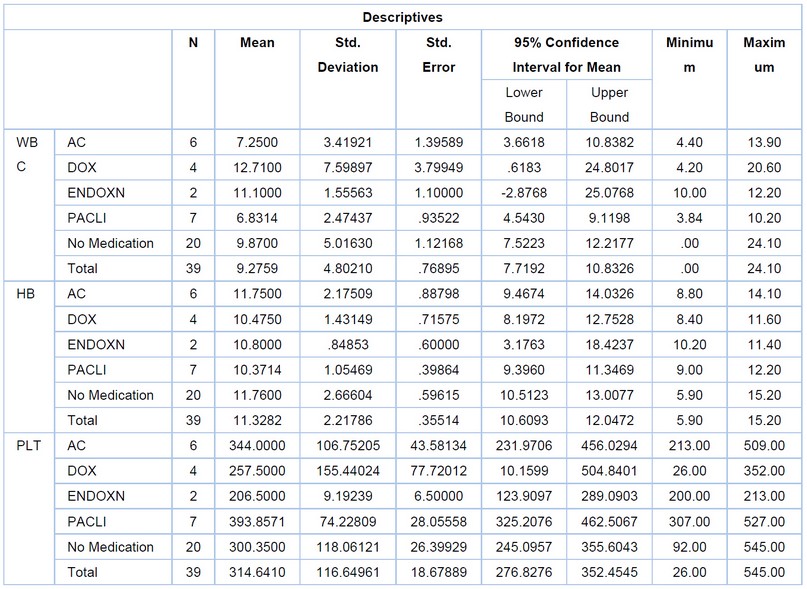
Table 2. Explains the descriptives statistics between the three drugs and other drugs, not chemotherapy
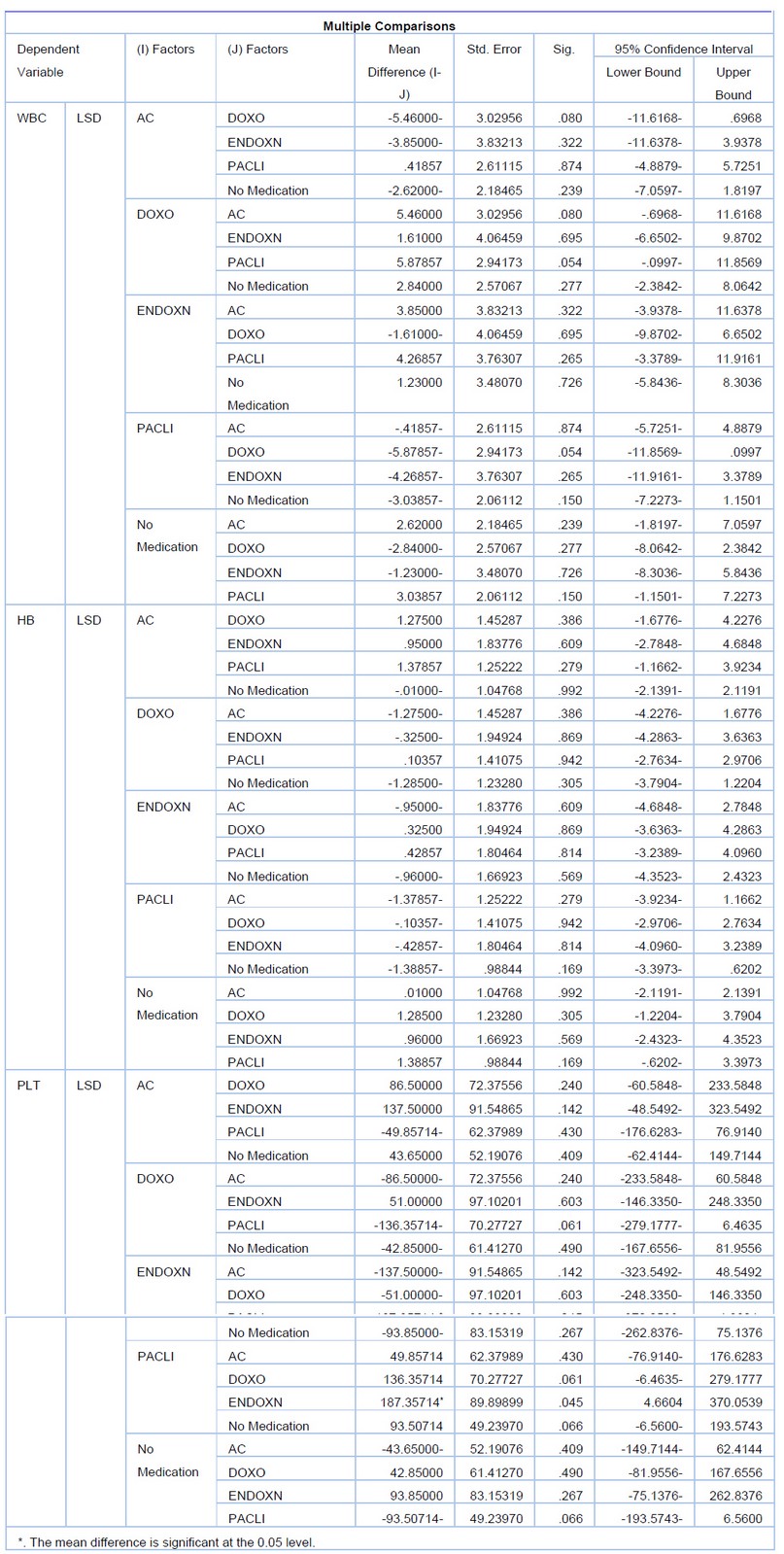
Table 3. The difference between the three drugs means the difference. *. The mean difference is significant at the 0.05 level.
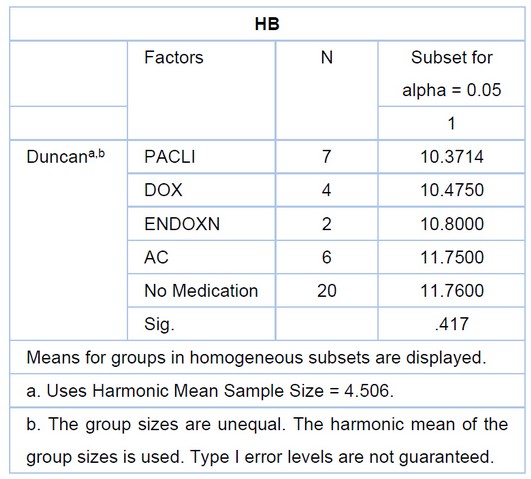
Table 4. Shows the rate of HB in three drugs
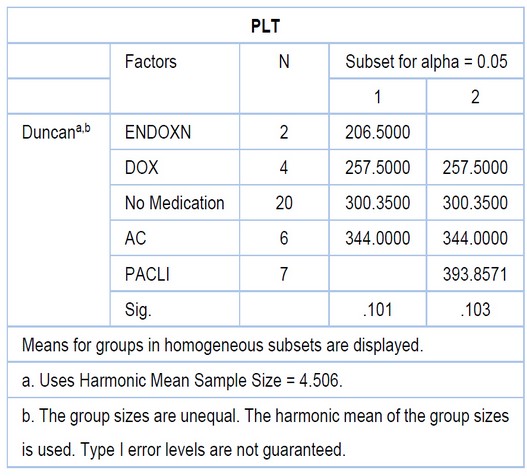
Table 5. Different rates of PLT between three drugs
DISCUSSION
The Ministry of Health should import cancer treatments from internationally accredited companies. With this study, it was possible to analyze cancer treatments to know their effect on patients and the degree of benefit to the patient after taking medicine. It also allowed us to see the influence of antitumor drugs and to reduce them by combining them with a special diet for patients to increase their immunity. Cancer patients should take the necessary preventive measures (wear masks and do not enter crowded places. The arrangement is made By comparing these concentrations with a set of experiments. The measured toxicity of the crossover was lower than predicted by the concentration addition model, indicating potentiating effects of CPCOOH toxicity. They revealed the genotoxic activity of CP and the mixture. UV irradiation degradation study of the samples containing (CP, cyclo, and DOX ) showed efficient degradation of the compounds and they remained toxic.The results of the current study were estimated, as they revealed the effect of these treatments on a decrease in WBC, as well as a decrease in PLT as a result of taking this treatment and its effect on the patient's immunity because it is classified as toxic solid chemotherapy as in the table of WBC, PLT, and HB.
CONCLUSIONS
This study suggests that no stable compounds with adverse effects were formed. This is the first study to describe the ecotoxicity and genotoxicity of the commonly used cytostatics CP, Cyclo and DOX, their known metabolites and their mixture. The results indicate the importance of toxicological evaluation and monitoring drug metabolites, as they may be more hazardous to humans than the parent compounds.
Funding: This research received no external funding
Institutional Review Board Statement: In this section, "The study was conducted according to the guidelines (or Ethics Committee) of the university of Kufa (protocol code 58799and 2021/12/16
Informed Consent Statement: Informed consent was obtained from all subjects involved in the study.
Data Availability Statement: At the tumors hospital in the middle Euphrates. Iraq
Acknowledgments: In this study, three types of chemotherapy were used, which are Doxorubicin, paclitaxel and cyclophosphamide. The devices include (ultraviolet rays and centrifuges), and samples include blood, urine, and sewage.
Conflicts of Interest: The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results".
REFERENCES
1- International Agency for Research on Cancer . On the International Agency for Research on Cancer classification of glyphosate as a probable human carcinogen. European Journal of Cancer Prevention.2018, 27(1), 82-87.) 2
2- Cancer I A R, Goodman, J. E., Mayfield, D. B., Becker, R. A., Hartigan, S. B., & Erraguntla, N. K.. Recommendations for further revisions to improve the International Agency for Research on Cancer (IARC) Monograph program. Regulatory Toxicology and Pharmacology.2020, 113, 104639).
3- Sun J, Von Tungeln LS, Hines W, Beger RD. Identification of metabolite profiles of the catechol-Omethyl transferase inhibitor tolcapone in rat urine using LC/MS-based metabonomics analysis. J Chromatogr B Analyt Technol Biomed Life Sci 2009;877:2557–2565
4- Aitken,Knapp EA, Fink AK, Goss CH, Sewall A, Ostrenga J, Dowd C, Elbert A, Petren KM, Marshall BC. Teh Cystic Fibrosis Foundation patient registry: design and methods of a national observational disease registry. Ann Am Thorac Soc 2016;13:1173–1179.
5- Aitken and Kleinrock,.Murray Aitken, executive director, Ernst R. Berndt., Medicines use and spending in the US—a review of 2015 and outlook to 2020. Parsippany (NJ): The Institute; 2015. IMS Institute for Healthcare Informatics.
6- Cheung-Ong .Cystic Fibrosis Foundation. Cystic Fibrosis Foundation patient registry: 2013 annual report.
7- Xie. Bethesda, MD: Cystic Fibrosis Foundation; 2015. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: A randomized trial. JAMA. 2012;307:1383–93.
8- Al-Enzy, A. F. M., Saed, Z. J. M., Naser, A. S., Mohammed, Th. T., Abdulateef, S. M., Al- Khalani, F. M. H. & Abdulateef, F. M. The role of adding sodium chloride in broiler chicks diets to improve production performance and antioxidant status during heat stress. Annals of Tropical Medicine and Public Health. 2020, 23(16): 231- 612. Doi: 10.36295/ASRO.2020.231612
9- Huang and Li, H.T., Kathrein, K.L., Barton, A., Gitlin, Z., Huang, Y.H., Ward, T.P., Hofmann, O., Dibiase, A., Song, A., Tyekucheva, S., Hide, W., Zhou, Y., and Zon, L. 2013
10- Emadi, A., Jones, R. J., and Brodsky, R. A. Cyclophosphamide and Cancer: golden Anniversary. Nat. Rev. Clin. Oncol. 6 (11), 638–647. doi:10.1038/nrclinonc.2009.146
11- Lawley P.D. and Phillips,D.H. Mutat. Res., 355, 13–40.1996
12- Bagley A prospective study of 22,707 men in Taiwan. Lancet;2:1129 –33. 1973
13- Lopes, MD Serrao, SM Gressert Ussery, RG Hall II , etal: Evaluation of chemotherapy-induced severe myelosuppression incidence in obese patients with capped dosing J Clin Oncol 7: 13– 17,2011 Google Scholar (Williston Park. 2009.
14- Kümmerer, Nakamura, E. F., & Kessler, R. C. Epidemiology of mental disorders in children and adolescents. Dialogues in Clinical Neuroscience.2010, 11, 7.
Received: July 20th 2022 / Accepted: October 15th 2022 / Published:15 November 2022
Citation: Al-Shibli Q, Al-Haidarey M. Chemotherapy's impact on a few blood parameters.Revis Bionatura 2022;7(4) 7. http://dx.doi.org/10.21931/RB/2022.07.04.7
