2020.05.04.18
Files > Volume 5 > Vol 5 No 4 2020
Phytochemical screening and in vitro anti-inflammatory activity of ethanolic extract of Epidendrum coryophorum leaves.
Irina Francesca González Mera1, Orestes Darío López Hernández2 and Vivian Morera Córdova1*.
Available from: http://dx.doi.org/10.21931/RB/2020.05.04.18
ABSTRACT
Epidendrum coryophorum belongs to the Orchidaceae family. Traditional uses of some species for this genus include infusions of the leaves used for kidney problems, treat influenza, conjunctivitis, liver pain, relieve kidney symptoms, and hypoglycemic effect. This work's objective was to determine the phytochemical profile of the ethanolic extract of Epidendrum coryophorum leaves and to evaluate the potential anti-inflammatory activity in vitro of the extract employing the erythrocyte membrane stabilization method. The phytochemical screening carried out in this work suggested phenols, coumarins, flavonoids, tannins, steroids, and sterols in the ethanolic extract of Epidendrum coryophorum leaves. Cardiotonic glycosides and carbohydrates were also found. The ethanolic extract's UV-Vis spectrum showed absorption maxima at 268 nm and 332 nm, which could correspond to flavonoids of the flavonoid classes, 3-OH substituted flavonols, or isoflavones. The quantitative determination of total phenols of the ethanolic extract was carried out using the Folin-Ciocalteu method. The total phenolic content expressed as mg Gallic acid equivalent (G.A.E.) per gram of extract was found to be 19,96 mgGAE/g of Epidendrum coryophorum. The ethanolic extract of Epidendrum coryophorum leaves showed hemolysis inhibition values of 18,19% at 1,0 mg/mL, 38,98% at 1,5 mg/mL and 40,94% at 2,5 mg/mL compared with aspirin (positive control) giving values of 65,33% at 1,0 mg/mL, 72,26% at 1,5 mg/mL and 73,75% at 2,5 mg/mL. The values obtained for inhibition of hemolysis with ethanolic extract, compared with the values obtained with a pure anti-inflammatory, are significant and demonstrate anti-inflammatory activity in Epidendrum coryophorum.
Keywords: Epidendrum coryophorum, total phenolic content, microencapsulation, anti-inflammatory activity
RESUMEN
Epidendrum coryophorum pertenece a la familia Orchidaceae. Los usos tradicionales de algunas especies para este género incluyen infusiones de las hojas que se utilizan para problemas renales, para tratar la influenza, para la conjuntivitis, para el dolor hepático, para aliviar los síntomas renales y por su efecto hipoglucemiante. El objetivo de este trabajo fue determinar el perfil fitoquímico del extracto etanólico de hojas de Epidendrum coryophorum y evaluar la potencial actividad anti-inflamatoria in vitro del extracto mediante el método de estabilización de membranas eritrocitarias. El cribado fitoquímico realizado en este trabajo sugirió la presencia de fenoles, cumarinas, flavonoides, taninos, esteroides y esteroles en el extracto etanólico de hojas de Epidendrum coryophorum. También se encontraron glucósidos cardiotónicos y carbohidratos. El espectro UV-Vis del extracto etanólico mostró máximos de absorción a 268 nm y 332 nm, que podrían corresponder a flavonoides de las clases flavonoides, flavonoles 3-OH sustituidos o isoflavonas. La determinación cuantitativa de fenoles totales del extracto etanólico se realizó mediante el método de Folin-Ciocalteu. Se encontró que el contenido fenólico total, expresado como mg de equivalente de ácido gálico (GAE) por gramo de extracto, fue 19,96 mgGAE/g de Epidendrum coryophorum. El extracto etanólico de hojas de Epidendrum coryophorum arrojó valores de inhibición de hemólisis de 18,19% a 1,0 mg/mL, 38,98% a 1,5 mg/mL y 40,94% a 2,5 mg/mL en comparación con la aspirina (control positivo) dando valores de 65,33% a 1,0 mg/mL, 72,26% a 1,5 mg/mL y 73,75% a 2,5 mg/mL. Los valores obtenidos de inhibición de la hemólisis con el extracto etanólico, en comparación con los valores obtenidos con un anti-inflamatorio puro, son significativos y demuestran la actividad anti-inflamatoria en Epidendrum coryophorum.
Palabras claves: Epidendrum coryophorum, contenido fenólico total, microencapsulación, actividad anti-inflamatoria
INTRODUCTION
Inflammation is a body's normal physiological response when it tries to protect itself from cellular damage caused by pathogens, irritants, or physical damage. The inflammation function is to remove damaged and necrotic tissues generated by the causative agent and initiate tissue repair. A not adequately cured inflammation is the basis of hypersensitivity reactions and chronic diseases1. Current treatments to alleviate the symptoms of acute and chronic inflammation cause adverse effects, and in many cases, the treatments have low effectiveness2. An alternative may be to develop drugs based on plants that have been used in traditional medicine for many years. The analysis of natural products through biochemical, phytochemical, and pharmacological studies has allowed the identification and characterization of various active compounds capable of counteracting the effects of inflammation, including phenolic compounds3-5.
The orchids, besides being used as ornamentals and food, are also used for medicinal purposes. The orchid's extracts are prepared from different parts of the plant. It could be tubers, leaves, rhizomes, stems, pseudobulbs, roots, flowers, sheaths, bulbs, and in some cases, the whole plant. They are prepared as an infusion, decoction, dried and ground, paste, Yin tonic, tincture, and juice6,7. These extracts are used as diuretics, antirheumatics, anticancer, hypoglycemic, anti-aging, antimicrobial, anticonvulsants, antivirals, and anti-inflammatories8-13. The genus Epidendrum (Orchidaceae) is found in the Andes, represented by 360 species, of which 178 are native species to the Andean forest14,15. Traditional uses of some species for this genus include infusions of the leaves for kidney problems, treat influenza, conjunctivitis, and hepatic pain; other authors report the use of orchids of this genus with hypoglycemic effect and to alleviate renal symptoms16,17. Despite the broad representation of genus Epidendrum in the Ecuadorian Andes and the wide use of orchids for medicinal purposes in many regions of the world, there are only a few scientific publications concerning the chemical composition and pharmacological properties of this genus. Regarding Epidendrum coryophorum, there are no scientific reports at all on this species.
There are different and varied methods to evaluate anti-inflammatory activity. These methods are classified as in vivo (if using animal models) or in vitro (when performed in a controlled environment outside of a living organism). Each of these methods is associated with some peculiarities, advantages, and disadvantages18. Although the in vivo models correlate well with the clinical picture and help understand the mechanisms of inflammation better, they are more complex and expensive. Among the in vitro methods, the method based on the stabilization of the erythrocyte membrane has gained wide popularity among researchers since it is based on the similarity of the erythrocyte membrane with the lysosomal membrane and its stabilization by the secondary metabolites of the plant extract is considered a good experimental indicator of the anti-inflammatory activity of secondary metabolites; additionally, it is a relatively simple method and is inexpensive.
This work's objective was to determine the phytochemical profile of the ethanolic extract of Epidendrum coryophorum leaves and to evaluate the potential anti-inflammatory activity in vitro of the extract employing the erythrocyte membrane stabilization method.
MATERIAL AND METHODS
Leaves preparation
The Epidendrum coryophorum specimens were acquired at Ecuadorian company Ecuagenera, Guayaquil (http://www.ecuagenera.com). The plants were kept in pots until the leaves were separated for processing. Using a scalpel, leaves were cut from the base of the plant. Then, leaves were washed separately with common water followed with distilled water to remove dust and particles. Subsequently, the leave was drained and dried in the shade at 25°C. The dried tissues were pulverized using an electric grinder and stored at -20oC until they were used19.
Organic extraction
For the extraction of Epidendrum coryophorum leaves' phytochemicals, the solid-liquid extraction process was used with 70% ethanol as solvent20. The ratio of dry tissue powder and the organic solvent was 1:10 (w:v). Extraction was allowed to proceed for 48 hours with occasional manual stirring. Finally, the amber bottle with ground tissue, and the solvent was ultrasound for 1 hour at 25 °C. The solvent was filtered through filter paper with the vacuum's help, and the extract was stored in a new clean amber bottle, protected from light at 4 °C until use.
Phytochemical screening
Once the ethanolic extract from dried leaves was obtained, the preliminary phytochemical analysis applied simple, rapid, and selective phytochemical screening assays targeting the qualitative detection of secondary metabolites21,22.
UV-Vis analysis
The ethanolic extract was diluted to 1:20 (v:v) with the same solvent. The spectrum was registered from 200 nm to 800 nm using a UV-VIS NIR Lambda 1050 spectrophotometer.
Total phenolic content
To determine the total phenolic content (TPC) in ethanolic extract of Epidendrum coryophorum leaves, a dilution of 1:10 (v:v) in distilled water was made. TPC was determined by Folin-Ciocalteu spectrophotometric method23. Gallic acid was used as reference material in concentrations of 0,02 mg/mL, 0,04 mg/mL, 0,06 mg/mL, 0,08 mg/mL, 0,10 mg/mL, 0,12 mg/mL, 0,14 mg/mL, 0,20 mg/mL and 0,30 mg/mL. The solutions were kept protected from light. For standard solutions and ethanolic extract, to 300 µL of the solution was added 150 µL of 10% Folin-Ciocalteau reagent, then the mixture was let to react for 3 minutes at room temperature. Next to each tube, 120 µL of 7,5% sodium carbonate was added and let them rest for 30 minutes in a dark place. At the end of the reaction, the absorbance was measured at 765 nm using a UV-VIS NIR Lambda 1050 spectrophotometer. The absorbance's values were extrapolated in the standard calibration curve. The results were expressed as mg in equivalents of Gallic acid (G.A.E.) per grams of dry material. The determination was performed in triplicate, and the results were expressed as mean ± S.D.
Microencapsulation
The extract was concentrated in a rotary evaporator at 30 °C, which allowed the ethanol's distillation from the mixture. Then, the concentrated extract was weighed, labeled, and stored in an Eppendorf tube at 4 °C, avoiding the metabolites' degradation. Then, the extract was first dissolved in Sacha inchi oil until most of the extract homogenize in the oil. So, for the microencapsulated extract matrix, it was used maltodextrin, Arabic gum, and water in a proportion of 30:30:30 (v:v:v). The mixture was homogenized using an Ultra turrax Pt25 I.K.A. at 25,000 rpm for 1 minute. For the microencapsulation, the Mini Spray Dryer Buchi B290 was used, which was fed with the respective combination, once the equipment conditions were verified to be adequate (-50 mbar, 150 °C inlet and 90 °C outlet)24. Microencapsulated extract was dissolved in PBS to reach concentrations of 1,0 mg/mL, 1,5 mg/mL, and 2,5 mg/mL.
Anti-inflammatory activity
Preparation of blood samples
The human red blood cell membrane stabilization method was used to study in vitro anti-inflammatory activity25. The human blood was obtained from two healthy volunteers who did not have used anti-inflammatory medications for at least a week and counted with a medical certificate to corroborate the health status. 3 mL of human blood was transferred to a conical tube and centrifuged for 10 min at 3000 rpm. The supernatant was taken out, and an equivalent volume of PBS was placed on the test tube to realize three consecutive washes to the erythrocyte suspension. After each extraction of plasma and the addition of PBS, the sample was centrifuged. 800 μL of the erythrocyte suspension was mixed with 1200 μL of PBS to form a suspension of 40% v/v in PBS.
Inhibition of heat-induced hemolysis
In 25 mL conical tubes were added 3 mL of the evaluated samples and 30 μL of 40% erythrocyte's suspension. The negative control was prepared using the same quantity of PBS instead of the sample. The microencapsulated extract and the commercial aspirin (positive control, from Sigma-Aldrich, USA) were evaluated at concentrations of 1,0 mg/mL, 1,5 mg/mL, and 2,5 mg/mL. All reaction mixtures were prepared in triplicates except the negative control. One conical tube of each reaction mixture was incubated for 20 min at 54 °C, and the other was left at room temperature. The negative control was incubated too. After 20 min of incubation, all test tubes were centrifuged at 3000 rpm for 10 min. The absorbance of supernatants was measured at λ = 560 nm25. The percentage of hemolysis inhibition was calculated according to the following expression:
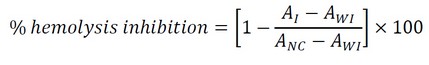
Where: A.I. represents the absorbance of the sample with incubation, A.W.I. represents absorbance of the sample without incubation, and A.N.C. represents the absorbance of the negative control.
Statistical analysis
Statistical analysis was performed by calculating the mean and standard deviation of the percentages of hemolysis inhibition for the extract's three concentrations. The used program was STATGRAPHICS Centurion XV, Version 15.2.05. The data were processed through a one-way analysis of variance (ANOVA), and subsequently, Duncan's test was performed, considering significant differences of at least p <0.05.
RESULTS AND DISCUSSION
The phytochemicals produced by plants have useful pharmacological activities for treating various diseases, including those that occur with inflammation. There are numerous studies and reviews on plants containing phytochemicals, specifically flavonoids, triterpenes, tannins, phenolic compounds, coumarins, and steroids; anti-inflammatory activity has been attributed26-33. However, very little has been investigated about these phytochemicals and their possible anti-inflammatory activity, especially in Epidendrum (Orchidaceae). In the ethanolic extract of Epidendrum coryophroun leaves, we found that phenols, coumarins, flavonoids, tannins, and anti-inflammatory activity were demonstrated. This work constitutes the first report on the phytochemical screening and anti-inflammatory activity of Epidendrum coryophorum.
Phytochemical screening
Phytochemical screening is one of the initial stages of phytochemical research, which allows the to determining qualitatively the main chemical groups present in the plant and guide the extraction and/or fractionation of the extracts, isolation and characterization of specific metabolites34. The phytochemical screening carried out in this work suggested phenols, coumarins, flavonoids, tannins, steroids and sterols in the ethanolic extract of Epidendrum coryophorum leaves. There were also found cardiotonic glycosides and carbohydrates. Anthocyanins, anthraquinones, flobatanins, saponins, proteins and free amino acids were not detected (Table 1). The presence of secondary metabolites in a given extract depends on the used solvent. For a solute (secondary metabolite) to be dissolved in a solvent, it is necessary that the solvent disaggregate the molecules and thus facilitate their solvation; this process depends on both the dielectric constant of the solvent and the solute's (secondary metabolites) and solvent's polarity. Generally, in ethanolic extracts, tannins, phenols, flavanols, terpenoids, sterols and alkaloids have been detected35, which coincides with the type of metabolites detected in the ethanolic extract of Epidendrum coryophorum leaves (Table 1).
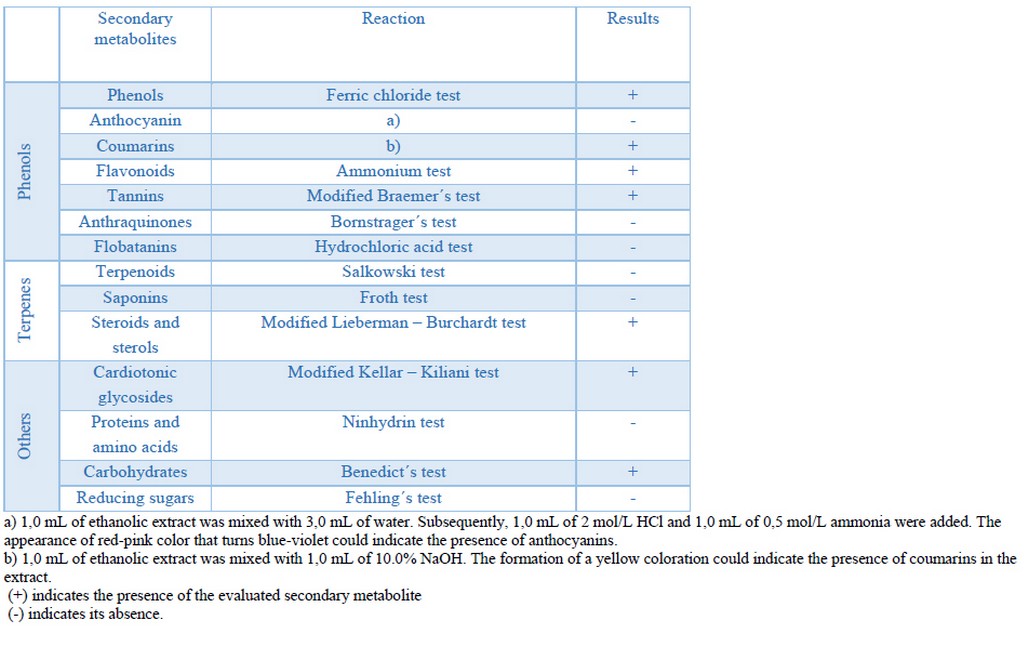
Table 1. Secondary metabolites tested and detected by chemical reactions in the ethanolic extract of Epidendrum coryophorum leaves.
The phytochemical screening of ethanolic extract of Epidenddrum coryophorum leaves constitutes the first report on the presence of specific secondary metabolites in this species. However, phytochemical screening of other species belonging to the genus Epidendrum has been performed before. Cerna et al. evaluated the phytochemical content of ethanolic extracts of the species Epidendrum blepharoclinium, Epidendrum blepharoclinium, Epidendrum cochlidium sp1, Epidendrum cochlidium sp2, Epidendrum jamiesonis, Epidendrum medusae, Epidendrum nocturnum, Epidendrum paniculatum, Epidendrum porphyreum, Epidendrum secundum, Epidendrum secundum. Even though these authors' extraction conditions are not identical to those used in our work, they also found flavonoids in 5 species, saponins in 11 species, and tannins in 7 of the 11 studied36.
UV-Vis analysis
UV-Vis spectroscopy is a technique that allows the detection of compounds in which can occur electronic transitions of the type π → π * and n → π * as a result of interaction with light in the ultraviolet and visible regions of the electromagnetic spectrum. The absorption maxima can be correlated with compounds containing π bonds and lone pairs of electrons37. In the UV-Vis spectrum of ethanolic extract of Epidendrum coryophorum leaves, the absorption maxima were recorded at 268 nm, 332 nm, 410 nm, 468 nm, 504 nm, 536 nm, 606 nm, and 664 nm. Of these maxima, the most intensive peaks (Peak 1-268 nm and Peak 2-332 nm, Figure 1A) could correspond to flavonoids of flavone, flavonols 3-OH substituted, or isoflavones classes. Flavonoids are natural pigments, especially abundant in plants' leaves, and their primary function is to protect them from ultraviolet rays. The flavonoids have in their structure a standard diphenylpyran skeleton (C6-C3-C6), composed of two phenyl rings (A and B) linked through a pyran C ring (heterocyclic). This basic structure allows for a multitude of substitution patterns and variations in the C ring38. UV-Vis spectroscopy is a useful technique in the differentiation of flavonoids since the bands' position in the UV-Vis spectrum depends on the extent of conjugation and, therefore, on the nature of the C ring and the position of the phenyl group called ring B (C-2, C-3 or C-4 substituent). For these compounds, the UV-Vis spectrum presents two absorption maxima in the ranges from 310 to 360 nm and from 245 to 280 nm corresponding to bands I and II of rings B and A, respectively, according to the class of flavonoids (Figure 1B)39.
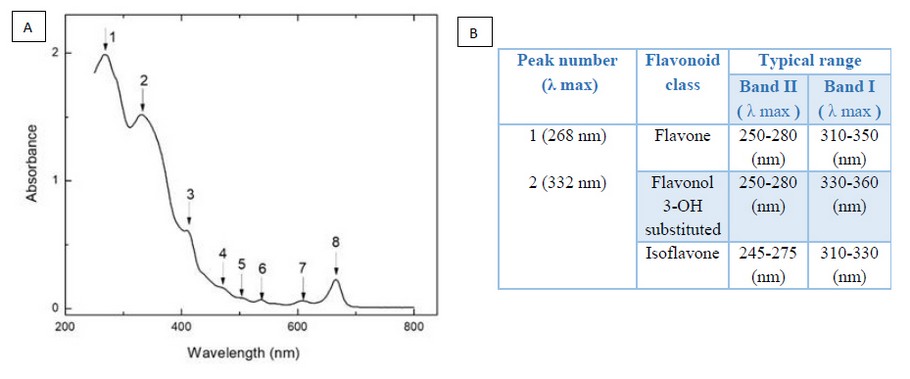
Figure 1. UV-Vis analysis of an ethanolic extract of Epidendrum coryophorum leaves. A: in the spectrum, the observed absorption maxima are indicated with arrows and numbers. B: correlation between the wavelengths of observed absorption maxima in the spectrum and typical ranges of absorption maxima wavelengths for some flavonoid classes.
Total phenolic content
Considering that several secondary metabolites were detected in the extract of Epidendrum coryophorum leaves classified as phenolic compounds40, the TPC was determined using the Folin-Ciocalteu method as previously described. The value was calculated using a calibration curve with Gallic acid, whose equation resulted in y = 8.8282x + 0.0328 with R2 = 0.9987. The TPC in the ethanolic extract was 19.96 mgGAE/g of Epidendrum coryophorum (Table 2). Values of TPC of Epidendrum coryophorum have not been reported before in the literature or other genus Epidendrum species.
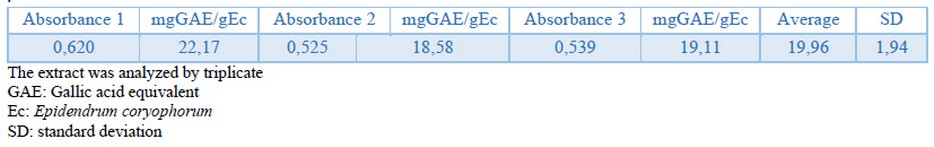
Table 2. Total phenolic content of ethanolic extract from Epidendrum coryophorum leaves
Anti-inflammatory activity
The erythrocyte membrane stabilization test in the presence of microencapsulated ethanolic extract in an isotonic medium was positive and showed concentration-dependent anti-inflammatory activity. An increase in the percentage of hemolysis inhibition was observed as the amount of microencapsulated extract increased. The microcapsules of ethanolic extract of Epidendrum coryophorum leaves gave values of hemolysis inhibition of 18,19% at 1,0 mg/mL, 38,98% at 1,5 mg/mL, and 40,94% at 2,5 mg/mL in comparison with aspirin (positive control) giving values of 65,33% at 1,0 mg/mL, 72,26% at 1,5 mg/mL, and 73,75% at 2,5 mg/mL (Table 3). The results were statistically evaluated using the statistical method of ANOVA and presented statistical significance; that is, the treatments used were significantly different (p <0.05). Therefore, the data were analyzed using Duncan's Test, with which we were able to corroborate the obtained results. The concentration of 1,00 mg/mL of the extract showed a significant difference concerning the concentrations of 1,5 mg/mL and 2,5 mg/mL of the extract itself and a lower percentage of hemolysis inhibition. However, the concentrations of 1,5 and 2,5 mg/mL of extract did not present significant differences between them and presented similar values of percentage of hemolysis inhibition. Similar behavior occurred in the case of aspirin (Figure 2). The erythrocyte membrane stabilization test is considered as an in vitro measure of the anti-inflammatory activity of drugs or extracts of plants41. The erythrocyte membrane is very similar to the membrane lysosomal so the effect of drugs on the stabilization of the erythrocyte membrane could be extrapolated to the stabilization of the lysosomal membrane; its stability is crucial since it can limit the inflammatory response, inhibiting the release of inflammation mediators as proteases and bactericidal enzymes42.
Additionally, this test is a convenient, rapid, and inexpensive method to be applied for preliminary investigations on the anti-inflammatory activity of crude extracts of plants. Numerous investigations associate the presence of phenolic compounds in plants with the anti-inflammatory activity evaluated by this method43. Thus, we could assume that the observed anti-inflammatory activity can result from certain phenols' individual action in the extrac or of synergy between them44. Considering the coarse texture of the ethanolic extract after drying and to ensure the bioavailability of its active principles during the hemolysis inhibition test, it was microencapsulated using natural water-soluble polymers. During the microencapsulation procedure of the ethanolic extract of Epidendrum coryophorum leaves, the homogenization step is essential for forming the emulsion. This guarantees the formation of microcapsules with a polynuclear internal structure, which allows the dispersion of the microcapsules in water, forming a nanoemulsion on contact with the environment in which the cells are found and better dispersion of the active principles in the medium45.
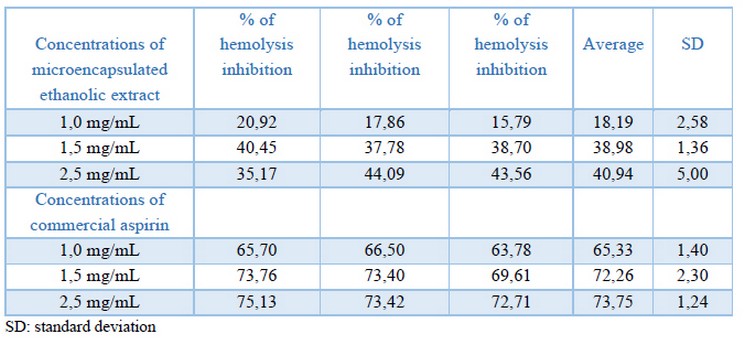
Table 3. Percentage of inhibition of hemolysis of the erythrocyte membrane for the three tested extract and control drug concentrations.
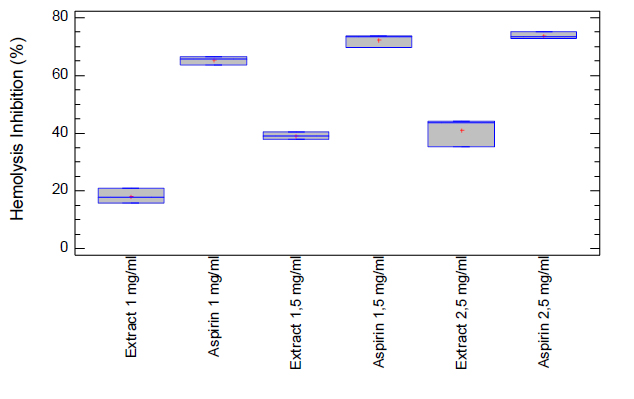
Figure 2. Percentages of hemolysis inhibition of the ethanolic extract of Epidendrum coryophorum leaves. Commercial aspirin was used as a positive control.
p < 0.05 was considered statistically significant in all analyses.
CONCLUSIONS
The phytochemical screening carried out in this investigation suggested the presence of phenols, coumarins, flavonoids, and tannins in the ethanolic extract of Epidendrum coryophorum leaves. The ethanolic extract's UV-Vis spectrum showed absorption maxima at 268 nm and 332 nm, which could correspond to flavonoids of the flavonoid classes, 3-OH substituted flavonols, or isoflavones. The ethanolic extract of Epidendrum coryophorum leaves contains phenolic compounds. The total phenolic content was found to be 19.96 mgGAE/g of Epidendrum coryophorum. The extract showed an erythrocyte membrane stabilization effect. The values obtained of hemolysis inhibition with ethanolic extract (crude extract) in comparison with those values obtained with a pure anti-inflammatory drug are significant and demonstrate anti-inflammatory activity in Epydendrum coryophorum. However, it should be mentioned that this study constitutes a preliminary study on the anti-inflammatory effect of this extract; therefore, it is suggested to expand the study of the anti-inflammatory effect using other methods and to consider the use of experimental animal models.
Acknowledgment
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflictos de interés
Los autores declaran no tener conflictos de interés.
REFERENCES
1. Cronkite D.A. and Strutt T.M. (2018) The Regulation of Inflammation by Innate and Adaptive Lymphocytes. Journal of Immunology Research, vol. 2018, Article ID 1467538, 14 pages, 2018. https://doi.org/10.1155/2018/1467538.
2. Recio M.C., Andujar I. and Rios, J.L. (2012) Antiinflammatory Agents from Plants: Progress and Potential. Current Medicinal Chemistry 19: 2088–2103. https://doi.org/10.2174/092986712800229069.
3. Maione F., Russo R., Khan H. and Mascolo, N. (2016). Medicinal plants with anti-inflammatory activities. Natural Product Research 30: 1343–1352. https://doi.org/10.1080/14786419.2015.1062761.
4. Duangjai Tungmunnithum, Areeya Thongboonyou, Apinan Pholboon and Aujana Yangsabai (2018) Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 5: 93-108. doi: 10.3390/medicines5030093.
5. Middleton E. and Kandaswami C. (1992) Effects of flavonoids on immune and inflammatory cell functions. Biochem. Pharmacol. 43: 1167–1179. doi: 10.1016/0006-2952(92)90489-6.
6. Gutiérrez R.M.P. (2010) Orchids: A review of uses in traditional medicine, its phytochemistry and pharmacology. Journal of Medicinal Plants Research 4: 592-638. DOI: 10.5897/JMPR10.012.
7. Vibha S., Hebbar Sushmitha S, Mahalakshmi, S.N. and Prashith Kekuda, T.R. (2019) A comprehensive review on ethnobotanical applications and pharmacological activities of Acampe praemorsa (Roxb.) Blatt. & McCann (Orchidaceae). Journal of Drug Delivery & Therapeutics 9: 331-336. DOI: http://dx.doi.org/10.22270/jddt.v9i1.2224.
8. Mamta Arora, Anupama Mahajan and Jaspreet K. Sembi (2017) A REVIEW ON PHYTOCHEMICAL AND PHARMACOLOGICAL POTENTIAL OF FAMILY ORCHIDACEAE. International Research Journal of Pharmacy 8: 9 – 24. DOI: 10.7897/2230-8407.0810176.
9. Arora M., Mahaian A. and Sembi, J.K. (2017) A review on phytochemical and pharmacological potential of family Orchidaceae. International Research Journal of Pharmacy 8: 9-24. DOI: 10.7897/2230-8407.0810176.
10. Attri L.K. (2016) Therapeutic potential of orchids. World Journal of Pharmaceutical Sciences 5: 438-446. DOI: https://doi.org/10.22270/jddt.v9i1.2224.
11. Chinsamy M., Finnie J.F. and Van Staden J. (2014) Anti-inflammatory, antioxidant, anti-cholinesterase activity and mutagenicity of South African medicinal orchids. South African Journal of Botany 91: 88–98. DOI: 10.1016/j.sajb.2013.12.004.
12. Mohammad M.H. (2011) Therapeutic orchids: traditional uses and recent advances — An overview. Fitoterapia 82: 102–140. DOI: 10.1016/j.fitote.2010.09.007.
13. Hossain M.M. (2011) Therapeutic orchids: traditional uses and recent advances - An overview. Fitoterapia 82:102-140. DOI: 10.1016/j.fitote.2010.09.007.
14. Jørgensen P.M. and León-Yánez S. (1999) Catalogue of the vascular plants of Ecuador. Monographs in Systematic Botany from the Missouri Botanical Garden 75: i-viii, 1-1182.
15. Endara L., Williams N. and León-Yánez S. (2009) Patrones de endemismo de orquídeas endémicas ecuatorianas: perspectivas y prioridades para la conservación. In: Pridgeon A M, Suarez JP (eds) Proceedings of the Second Scientific Conference on Andean Orchids. Universidad Técnica Particular de Loja, Loja, Ecuador, pp 63-70. https://doi.org/10.1117/12.2028244.
16. Tene V., Malagon O., Finzi P.V., Vidari G., Armijos Ch. and Zaragoza T. (2007) An ethnobotanical survey of medicinal plants used in Loja and Zamora-Chinchipe, Ecuador. Journal of Ethnopharmacology 111: 63–81. doi: 10.1016/j.jep.2006.10.032.
17. Novaes A.P., Rossi C., Poffo C., Junior E.P., Oliveira A.P., Schlemper V., Niero R., Cechinel-Filho V. and Burger C. (2001) Preliminary evaluation of the hypoglycemic effect of some Brazilian medicinal plants. Therapie. 56: 427‐430.
18. Ifeanyi F.E., Philip F.U., Ikechukwu P., Bonaventure C.O. and Osadebe P.O. (2019) In vitro and In vivo Models for Anti-inflammation: An Evaluative Review. INNOSC Theranostics and Pharmacological Sciences 2: 3-15. DOI: 10.36922/itps.v2i2.775.
19. Jhansi K., Venkatesh R. and Khasim, S.M. (2019) A Study on Phytochemical and Anticancer Activitiesvof Epiphytic Orchid Aerides odorata Lour. European Journal of Medicinal Plants 28: 1-21. https://doi.org/10.9734/ejmp/2019/v28i330135.
20. Belwal T., Dhyani P., Bhatt I.D., Rawal R.S. and Pande V. (2016) Optimization extraction conditions for improving phenolic content and antioxidant activity in Berberis asiatica fruits using response surface methodology (R.S. M). Food Chem. 207: 115–124. DOI: 10.1016/j.foodchem.2016.03.081.
21. Trease G. E. and Evans W. C. (1989) Trease and Evan's Textbook of Pharmacognosy. 13th Edition. Cambridge University Press, London. 546.
22. Behrooz Alizadeh Behbahani, Fakhri Shahidi, Farideh Tabatabaei Yazdi, Seyed Ali Mortazavi and Mohebbat Mohebbi. (2017) Antioxidant activity and antimicrobial effect of tarragon (Artemisia dracunculus) extract and chemical composition of its essential oil. Food Measure 11: 847–863. https://doi.org/10.1007/s11694-016-9456-3.
23. Shanaida M., Golembiovska O., Hudz N. and Wieczorek PP (2018) Phenolic compounds of herbal infusions obtained from some species of the Lamiaceae family. Current Issues in Pharmacy and Medical Sciences 31: 194–199. https://doi.org/10.1515/cipms-2018-0036.
24. Jyothi N.V.N., Prasanna P.M., Sakarkar S.N., Prabha K.S., Ramaiah P.S. and Srawan G.Y. (2010) Microencapsulation Techniques. Factors Influencing Encapsulation Efficiency. J. Microencapsul. 27: 187–197. https://doi.org/10.3109/02652040903131301.
25. Anosike C.A., Obidoa O. and Ezeanyika L.U. (2012) Membrane Stabilization as a Mechanism of the Anti-inflammatory Activity of Methanol Extract of Garden Egg (Solanum Aethiopicum). DARU, J. Pharm. Sci. 20: 1–7. https://doi.org/10.1186/2008-2231-20-76.
26. Adebayo S.A., Dzoyem J.P., Shai L.J. and Eloff J.N. (2015) The anti-inflammatory and antioxidant activity of 25 plant species used traditionally to treat pain in southern African. B.M.C. Complement Altern Med. 15: 159. doi: 10.1186/s12906-015-0669-5.
27. Adebayo S.A., Dzoyem J.P., Shai L.J. and Jacobus N.E. (2015) The anti-inflammatory and antioxidant activity of 25 plant species used traditionally to treat pain in southern African. B.M.C. Complement Altern Med 15: 159. https://doi.org/10.1186/s12906-015-0669-5.
28. Sowjanya R., Shankar M., Sireesha B., Naik A.E., Yudharaj P. and Priyadarshini R.R. (2017) An overview on inflammation and plant havinganti inflammatory activity. International journal of phytopharmacy research 7: 25-32. http://dx.doi.org/10.22270/jddt.v9i3.2906.
29. Lock O., Perez E., Villar M., Flores D. and Rojas R. (2016) Bioactive Compounds from Plants Used in Peruvian Traditional Medicine. Nat Prod Commun. 11: 315–37.
30. Sobeh M., El-Raey M., Rezq S., Abdelfattah M.A.O., Petruk G., Osman S., El-Shazly A.M., El-Beshbishy H.A., Mahmoud M.F. and Wink M. (2019) Chemical profiling of secondary metabolites of Eugenia uniflora and their antioxidant, anti-inflammatory, pain killing and anti-diabetic activities: A comprehensive approach. J Ethnopharmacol. 240: 111939. doi: 10.1016/j.jep.2019.111939.
31. Falcão T.R., de Araújo A.A., Soares L.A.L., de Moraes Ramos R.T., Bezerra I.C.F., Ferreira M.R.A., de Souza Neto M.A., Melo M.C.N., de Araújo R.F. and de Aguiar Guerra, A.C.V. (2018) Crude extract and fractions from Eugenia uniflora Linn leaves showed anti-inflammatory, antioxidant, and antibacterial activities. BMC Complement Altern. Med. 18: 84–90. DOI: 10.1186/s12906-018-2144-6.
32. Abima Shazhni J.R., Renu A. and Vijayaraghavan P. (2018) Insights of antidiabetic, anti-inflammatory and hepatoprotective properties of antimicrobial secondary metabolites of corm extract from Caladium x hortulanum. Saudi Journal of Biological Sciences 25: 1755–1761. https://doi.org/10.1016/j.sjbs.2018.03.013.
33. Napagoda M., Gerstmeier J., Butschek H., Lorenz S., Kanatiwela D., De Soyza S.D., Qader M., Nagahawatte A., Wijayaratne G.B., Svatoš A., Jayasinghe L., Koeberle A. and Werz O. (2018) Lipophilic extract of Leucas zeylanica, a multi-purpose medicinal plant in the tropics, inhibits key enzymes involved in inflammation and gout. J. Ethnopharmacol. 224: 474–481. DOI: 10.1016/j.jep.2018.04.042.
34. Łukasz Cies and Ruin Moaddela (2016) Comparison of analytical techniques for the identification of bioactive compounds from natural products Nat Prod Rep. 33: 1131–1145. doi:10.1039/c6np00016a.
35. S. Sasidharan, Y. Chen, D. Saravanan, K.M. Sundram and L. Yoga Latha (2011)Afr J Tradit Complement Altern Med. 8: 1-10.
36. Cerna M., Mencias F., Salazar T. and Gutiérrez S. Estudio Fitoquímico, Actividad Antioxidante de Especies de Orquídeas de Los Generos Epidendrum, Onicidium y Caucaea. BIONATURA 2018, 1 (1). https://doi.org/10.21931/RB/CS/2018.01.01.8.
37. Dhivya S.M. and Kalaichelvi K. (2017) UV-Visible Spectroscopic and FTIR Analysis of Sarcostemma brevistigma, Wight. and Arn. Int. J. Curr. Pharm. Res. 9: 46-49. https://doi.org/10.22159/ijcpr.2017.v9i3.18890.
38. Duangjai Tungmunnithum, Areeya Thongboonyou, Apinan Pholboon and Aujana Yangsabai. (2018) Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 5: 93-108 doi:10.3390.
39. Markham K.R. (1982) Techniques of flavonoids identification. Biological Techniques Series: Series editors: J.E. Treheme and P.H. Rubery. Academic Press, London p. 144.
40. Lattanzio V., Kroon P.A., Quideau S. and Treutter D. (2008) Plant phenolics – Secondary metabolites with diverse functions. In: Daayf F, Lattanzio V (eds) Recent advances in polyphenol research, vol 1. Wiley-Blackwell, Oxford, pp 1–35.
41. Debnath P.C., Das A., Islam A., Islam M.A., Hassan M.M., Gias Uddin S.M. (2013) Membrane stabilization – A possible mechanism of action for the anti-inflammatory activity of a Bangladeshi medicinal plant: Erioglossum rubiginosum (Bara Harina). Pharmacogn J. 5:104–107. DOI 10.1016/j.phcgj.2013.04.001.
42. Gadamsetty G., Maru S., Tyagi A. and Chakravarthula S.N. (2013) Anti-Inflammatory, Cytotoxic and Antioxidant Effects of Methanolic Extracts of Drypetes sepiaria (Euphorbiaceae). Afr J Tradit Complement Altern Med. 10: 274–82. DOI: 10.4314/ajtcam.v10i5.9.
43. Fabian I.E., Philip F.U., Ikechukwu P., Bonaventure C.O. and Patience O.O. (2019) In vitro and In vivo Models for Anti-inflammation: An Evaluative Review. INNOSC Theranostics and Pharmacological Sciences 2: 3-15. DOI: 10.36922/itps.v2i2.775.
44. Lijuan Z., Virgousb C. and Sia H. (2019) Synergistic anti-inflammatory effects and mechanisms of combined phytochemicals. 69: 19-30. https://doi.org/10.1016/j.jnutbio.2019.03.009.
45. López O.D., Nuñez Y., Menéndez R.A., Nogueira A., Reyes M.I., Toledo C., Pérez E. y Agüero S. (2010) Influencia del Proceso de Microencapsulación sobre el Efecto Farmacológico de los Lípidos Presentes en las Semillas de Cucurbita pepo L. Latin American Journal of Pharmacy 29 (4): 612-616.
Received: 22 September 2020
Accepted: 12 October 2020
Irina Francesca González Mera1, Orestes Darío López Hernández2 and Vivian Morera Córdova1*.
1 Yachay Experimental Technology Research University. School of Chemical Sciences and Engineering. San Miguel de Urcuquí. Hacienda San José s/n. Imbabura, Ecuador.
2 Technical University of Ambato. Faculty of Food Science and Engineering. Biochemical Engineering Career. Ambato, Ecuador.
* Correspondence author: [email protected]
Irina Francesca González Mera https://orcid.org/0000-0001-7013-3923
Orestes Darío López Hernández https://orcid.org/0000-0002-3217-9493
Vivian Morera Córdova https://orcid.org/0000-0003-3026-9716
