2019.04.04.11
Files > Volume 4 > Vol 4 No 4 2019
REVISION/REVIEW
Secondary metabolites in plants: main classes, phytochemical analysis and pharmacological activities
Irina Francesca González Mera1, Daniela Estefanía González Falconí1, Vivian Morera Córdova1*.
Available from: http://dx.doi.org/10.21931/RB/2019.04.04.11
ABSTRACT
Plants are an essential source of chemical compounds with different biological properties that man can use to his advantage. These substances are mainly produced as a result of chemical conversions of secondary metabolism. This article reviews the main classes of secondary metabolites that synthesize plants as well as their characteristics and their biological functions. Examples are provided for each of the classes. Emphasis is placed on the methods of extracting secondary metabolites and phytochemical screening, as well as on the main pharmacological activities described for the MS.
Keywords: secondary metabolites, extraction, phytochemical screening, pharmacological activities
INTRODUCTION
Plants are autotrophic organisms. In addition to the primary metabolism present in all living beings, they have secondary metabolism that allows them to produce and accumulate compounds of a very diverse chemical nature. The compounds derived from secondary metabolism in plants are called secondary metabolites (SM) 1.
The SM of the plants constitute a large and varied group of organic compounds that are synthesized in small quantities; they have no direct function in essential processes such as photosynthesis, respiration, solute transport, protein synthesis, nutrient assimilation, and the differentiation or formation of carbohydrates, proteins, and lipids. They appear in plants as a result of chemical conversions and even when many of their functions are unknown, it is believed that SM are related to the defense of the plant against predators and pathogens, they also act as allelopathic agents that influence growth, survival, and reproduction of other plants, attract seed pollinators and serve to face adaptation to sudden changes in temperature, humidity, light intensity and drought 2,3,4. The SM of the plants have a differential distribution between taxonomic groups in the Kingdom of the plants, and therefore they are useful for Systematic Botany 5.
The study of biological functions and the structure of SM are of great importance because from this knowledge, it has been possible to use them in different industries. Many SM are used as aromas, resins, gums, flavor enhancers, as insecticides and herbicides 6,7,8,9,10. On the other hand, the majority of SM have found utility in the pharmaceutical industry, given a large number of pharmacological activities that are known about them 11. This article summarizes the main classes of SM in plants, some techniques for their extraction from natural sources and phytochemical screening, as well as the main pharmacological activities described for fundamental classes of SM.
Classes of SM in plants
Several criteria have been considered for the classification of SM: chemical structure (presence of rings or sugars), composition (containing nitrogen or not), their solubility in organic solvents or water, and the biosynthetic pathway. Of them, the most common criterion used for grouping the SM in plants has been the biosynthetic pathway. According to this, the SM in plants can be divided into three large groups: terpenes, phenolic compounds, and alkaloids 12.
Terpenes: they constitute the largest group of SM in plants to which more than 40,000 different molecules are allocated 12. From the chemical point of view, they are non-saponifiable lipids since fatty acids do not intervene in their formation. They are also known as isoprenoids, since the basic structural unit that forms them is the isoprene molecule 13. They are classified according to the number of isoprene units they contain. The most straightforward class of all is hemiterpenes with a single isoprene unit and five carbons in its structure. The best-known hemiterpene is isoprene, a volatile product that emerges from photosynthetically active tissues. With two groups, the terpenes are classified in monoterpenes, with three units in sesquiterpenes, with four in diterpenes, with six in triterpenes, with eight in tetraterpenes, and with more than 10 in polyterpenes 14,15 (Table 1).

Table 1. Classes of terpenes according to the number of isoprene units.
Many plants contain terpenes in their flowers and fruits as mixtures of volatile compounds with specific odors; among them, we can mention lemon, mint, eucalyptus, ginger, and great basil 24. Terpenes have several biological functions and participate in both the primary metabolism and the secondary metabolism of plants. In the central metabolism they are photosynthetic pigments (carotenes), electron carriers (ubiquinone and plastiquinone) regulators of plant growth and development (giberilins, strigolactones, brassinosteroids), are part of cell membranes (phytosterols) and participate in protein glycosylation 25. In secondary metabolism they participate as defense molecules, toxic compounds and food deterrents for insects. In some plants they are the responsible molecules for attracting pollinators, or they function as dispersers 26,27,28,29.
They are synthesized from primary metabolites by two pathways: that of mevalonic acid, active in the cytosol, in which three molecules of acetyl-CoA condense to form mevalonic acid that reacts to form isopentenyl diphosphate (IPP) or the pathway of methyleryritol phosphate (MEP) that functions in chloroplasts and also generates IPP 24.
Phenolic compounds: they are chemical compounds containing a hydroxyl group directly attached to an aromatic hydrocarbon. Chemically, phenolic compounds are a very diverse group of SM. The simplest representative of this class is phenol 30, 31, 32, 33. The most important criterion for classifying phenolic compounds is the number of carbons present in the molecule. According to this criterion, the phenolic compounds are classified into simple phenols, acidic phenols, acetophenones, and phenylacetic acids, hydroxycinnamic acids, coumarins, flavonoids, biflavonyls, benzophenones, xhantones, stilbenes, quinones and betacyanins (Table 2). Lignans, neolignans, tannins, and phlobaphenes also belong to this group. The latter are polymers and have more complex structures 34, 35.
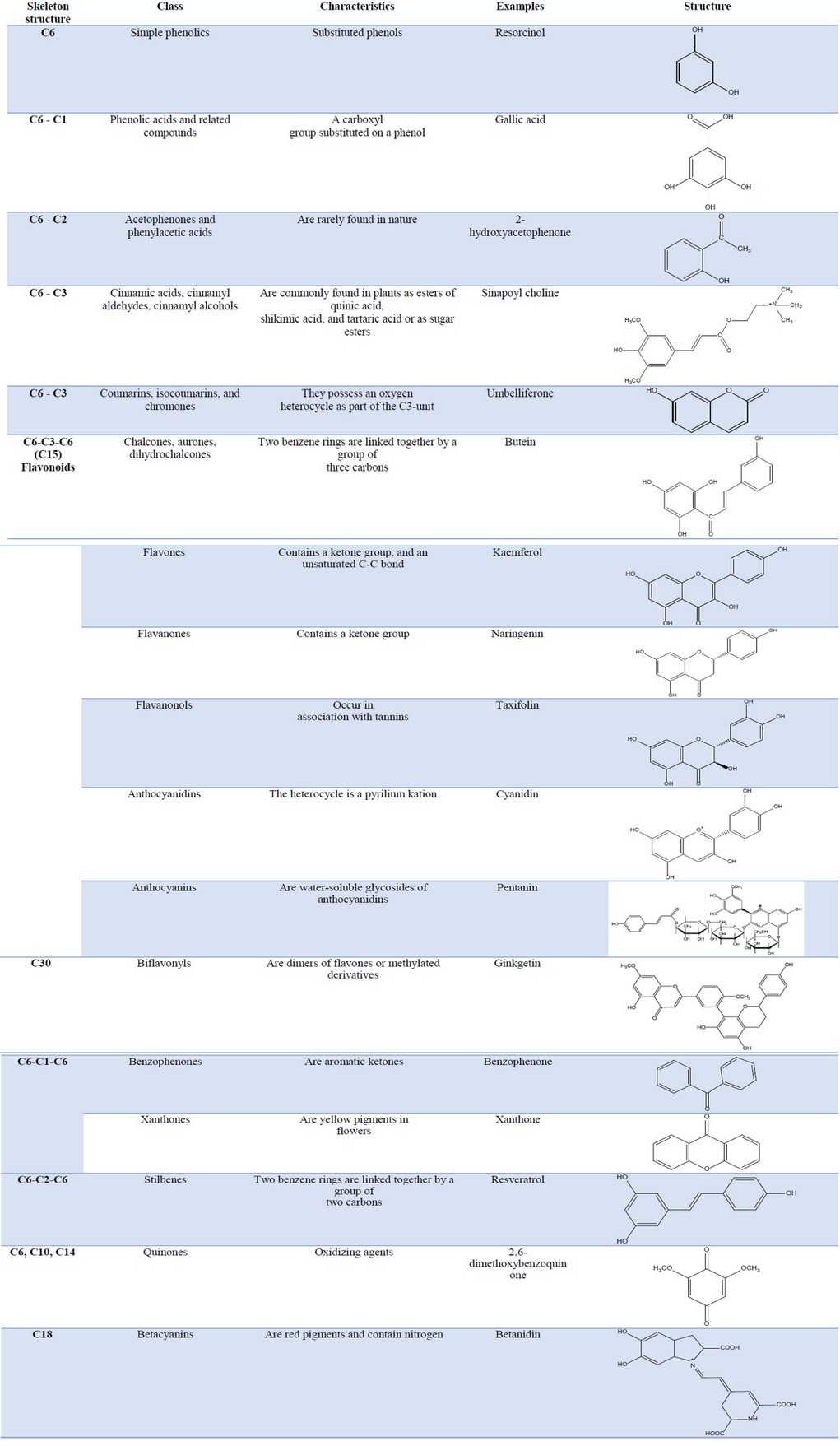
Table 2. Classes of phenolic compounds according to the number of carbons in the structure.
Phenolic compounds are synthesized in plant cells by the shikimic acid pathway or the malonate/acetate pathway (or both, for example, flavonoids) 36. The shikimic acid pathway provides the synthesis of phenylalanine and cinnamic acids and their derivatives (simple phenols, phenolic acids, coumarins, lignans, and phenyl propane derivatives) 37,38. The polyacetate pathway provides quinones and xanthones. The mixed pathways combine precursors of both the shikimic acid pathway and the polyacetate pathway. This is the case of flavonoids 39,40.
Phenolic compounds fulfill various functions in plants: they oxidize quickly and act as antioxidants 41,42,43, they act as plant growth inhibitors 44, seeds accumulate significant amounts of phenols that act as filter so that oxygen does not reach the embryo and inhibit its germination 45. Phenols also accumulate on surfaces of leaves, capturing up to 90% of UV radiation 46. Phenols confer aromas and colors to the fruits making them appetizing for herbivores, which favors the dispersion of seeds through feces 47. Plants compete with each other to preserve their territories, and in this process (allelopathy) the phenols participate 48. Plants also defend themselves against the attack of pathogens by synthesizing phytoalexins that are toxic to microorganisms and their presence prevents infections 49. Phenols also protect plants by generating bitter flavors or textures that are unpleasant for herbivores 50.
Alkaloids: alkaloids constitute another large and diverse group of SM that includes molecules isolated primarily from vascular plants 51. Plants generally produce a complex mixture of alkaloids, in which a significant constituent dominates 51. In a given plant the biosynthetic origin of the alkaloids present is common, even if their structures are slightly different 51. Another interesting observation is that the concentration of alkaloids varies considerably from one part to another of the same plant, and even in some parts it may not contain those at all 52. Alkaloids are also found in fungi, bacteria, and animals 53. They include an atom of nitrogen in their structure, are toxic compounds and respond to common precipitation reactions 54,55.
Even when there is no uniform classification of alkaloids, several criteria have been used in order to classify them: biosynthetic origin, presence of basic heterocyclic nucleus in the structure, pharmacological properties, and distribution in plant families 56. Among these criteria, the biosynthetic origin of the alkaloids has been used quite frequently. According to this criterion the alkaloids are classified as true alkaloids, protoalkaloids, and pseudoalkaloids 57. Pure alkaloids strictly comply with the fundamental characteristics of the alkaloids. The majority of the alkaloids found in plants belong to this group. They contain an intracyclic nitrogen, have basic character and are compounds of high reactivity, even in small quantities. In plants, they can be found free, although they predominate as salts. The precursor compounds of the true alkaloids are amino acids (L-ornithine, L-lysine, L-tyrosine, L-tryptophan, L-histidine, and L-arginine). Some pure alkaloids have been derived from anthranilic and nicotinic acids 57,58. The protoalcaloides constitute a smaller class in number. In this group, the nitrogen atom is not part of the heterocycle, and they derive from L-thyroid, L-tryptophan, and L-ornithine. They can also be considered aromatic amines 55. The pseudoalkaloids contain heterocyclic rings with nitrogen but are not derived from amino acids. They are formed by subsequent incorporation of nitrogen into compounds originally free of this element. To this group belong terpenic alkaloids 58.
Although the presence of alkaloids is not vital for the plant, there is evidence that indicates the roles that these substances play in vegetables. As for the functions they fulfill, at first, they were considered waste products of nitrogen metabolism, nitrogen reservoirs in the plant, and were even mentioned as growth regulators. Today it is accepted that the role they play is to defend the plant against insects and herbivores due to its toxicity and deterrent capacity. While some serve to protect the plant from predators or microorganisms (toxic or repellent substances), others do so to compete with other plant species in a given habitat (allelopathic substances) 59,60.
Alkaloids have remarkable physiological properties and toxicological that are exerted primarily on the nervous system central, with predominance in some of its levels (Table 3). For these reasons, they can be used as drugs. Prolonged use of any of these compounds produced in man accustoming, which constitute true drug addictions, with physical and psychic dependence and an increase in the tolerance 57,59. To date, around 15,000 alkaloids have been isolated from plants. If it is considered to have been examined less than 25% of the upper plant species of the planet, it is clear that there is still a wide field for his research. Because of its pharmacological and medicinal importance there is an excellent motivation to continue with the chemical-biological study of the alkaloids. This is one of the most important secondary metabolites of plants with therapeutic interest 60.
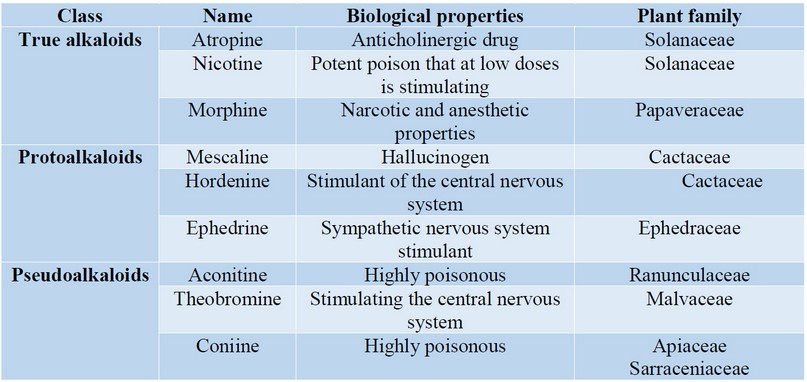
Table 3. Some biologically relevant plant-derived alkaloids.
Phytochemical analysis
Phytochemical studies generally are based on previous ethnobotanical and ethnopharmacological knowledge about plants and often constitute hypothesis-driven studies. The general methodology for studying SM from plants comprises several stages: extraction from natural sources, the phytochemical screening of extracts to determine qualitatively the main chemical classes of SM present in the plant, the purification of individual components and elucidation of their chemical structures, the biological activity studies through in vitro/in vivo assays and the toxicity-cytotoxicity studies on organisms or cells. The methodology involves a combination of different analytical techniques (Figure 1). In this methodology, the method of extracting secondary metabolites and their identification in phytochemical gait is crucial. These two aspects are reviewed below.

Figure 1. A brief summary of the general methodology for studying bioactive compounds from plants. SM-SM, MAE-Microwave Assisted Extraction, UAE-Ultrasound Assisted Extraction, ASE- Accelerated Solvent Extraction, SFE-Supercritical Fluid Extraction, TLC-Thin Layer Chromatography, HPLC-High Performance Liquid Chromatography, MS-Mass Spectrometry, FTIR-Fourier Transform Infrared Spectroscopy, 1H-NMR-proton Nuclear Magnetic Resonance and 13C-NMR-carbon Nuclear Magnetic Resonance.
Extraction
The initial step during extraction is the preparation of plant tissues. The extraction can be done on clean and ground leaves, barks, roots, fruits, and flowers, from fresh or dried plant material. In order to maintain the freshness of the samples and avoid possible chemical damage, it is recommended that the interval between harvest and the initiation of extraction does not exceed 3 hours since the plant tissue is fragile and tends to deteriorate faster than dry tissue 61. Otherwise it is preferable to dry the plant by air-drying, microwave-drying, oven-drying or lyophilization. Each of these methods has advantages and disadvantages that the researcher should consider 62,63,64,65. Another critical point to view during pre-treatment of the plant is the particle size of plant material. The smaller the particle size, the higher the area of contact between the plant material and the solvent, and consequently the more effective the extraction of the chemicals 66.
Extraction is the process that allows separating SM from the plant by using solvents of different polarity. As a result of the extraction remains two phases: a liquid phase containing solubilized metabolites and a solid containing the insoluble cell debris. Conditions as temperature and time are important factors to achieve high-quality extracts 67. The most common extraction methods are maceration, infusion, percolation, decoction, Soxhlet or continuous extraction, microwave-assisted extraction (MAE), ultrasound-assisted extraction (UAE), accelerated solvent extraction (ASE), and supercritical fluid extraction (SFE) 68.
Maceration is a solid-liquid extraction technique 69. The method consists of using a solvent or a mixture of solvents having different polarities and a particular affinity with compounds that are going to be extracted. The mixture (plant-solvent) is placed in a container with lid and let it rest for two or three days until the compounds could be transferred from vegetal tissues to the solvent. This method is widely used with soft vegetal material 70. The infusion is a maceration process too but uses shorter extraction times and the solvent usually is cold or boiling water. This method is used to obtain a diluted solution of compounds that are easily extracted 67. The decoction is a more convenient method for extracting water-soluble compounds from roots and barks that are stable at high temperatures and usually results in oil-soluble compounds compared to maceration and infusion 71. The decoction method is carried out boiling the vegetal material in water by 15 minutes, then cooling, filtering and adding water until it reaches the desired volume 67. Finally, percolation is an extraction method that shares similar fundamentals. The method uses a conical filtration camera open on both sides where the material is placed with the solvent. The camera is connected to a flask and once the material is inside the camera, the system is opened to let it strain. The solvent can be used several times to rinse the material until the saturation point 68. Another way to conduct the extraction of SM is using a Soxhlet apparatus. In this method, a Soxhlet extractor, a condenser, and a round bottom flask are used. The finely ground vegetal material is loaded into the thimble of a strong paper of cellulose and then placed in the Soxhlet extractor. The solvent goes in the round bottom flask, and it needs to be heated. The solvent vapors go into the thimble and then return to the flask after being condensed. The system is left, at least for sixteen hours 72. The main advantage of Soxhlet extractor is the use of smaller quantity of solvent compared to maceration. However, the exposure to hazardous and flammable organic solvents, with potential toxic emissions is high 68.
The microwave-assisted extraction (MAE) is another popular and easy technique in which the sample is heated using electromagnetic radiation. This method improves the extraction of intracellular compounds due to the rupture of the cellular wall. Increasing temperature, the humidity inside the cell is transformed into vapor; as a result the intracellular pressure increases and the lysis is provoked. This factor comes together with other effects in the solution that benefit the interaction of the compounds to be extracted with the solvent. The main disadvantage is the possible thermal degradation 73,74. The ultrasound-assisted extraction (UAE) facilitates partition of analytes with the occurrence the fragmentation of cell wall provoked by the collisions between the electromagnetic waves and the particles. There are two forms of applying it: in direct contact with the sample or using an ultrasound bath, where the contact is given through the walls of the bottle. In the first case the efficacy is 100 times higher than the second one. The procedure is simple, low cost and can be used in both small and larger-scale extraction 75.
In the method called accelerated solvent extraction (ASE), high temperatures and high pressures are applied to the samples. The time required to achieve the extraction is reduced to one hour, which is an advantage in comparison with other methods (48h or 72h). This is a method that separates efficiently analytes from the matrix. Since the nature of the solvent is an important fact in each method of extraction, the solvents used in this method determine the efficiency of the results. The solvents system, temperature, and time of action are determinants in accelerated solvent extraction. In the case of extraction of bixin the most efficient mixture of solvent was cyclohexane: acetone (6:4) at 50°C for 5 minutes 76.
The supercritical fluid extraction (SFE) involve a supercritical fluid. It is a substance that has both physical properties of gas and liquid in its critical point. Pressure and temperature are determinant factors to reach this critical point. The utility of the supercritical fluid is their gas behavior and solvating capacity of liquids. The most used solvent is CO2 due to its capacity to dissolve nonpolar analytes, it has low cost and low toxicity 77,78. This method is very selective, very fast, has a high yield percentage and the resultant product has high quality. It has been used in the coffee industry to decaffeinate coffee, or in other industries to extract essential oils 78.
Phytochemical screening
The phytochemical screening is a fast and cheap procedure to determine the main classes of SM or groups of substances that a plant contains. Since each class or group of SM is related to specific biological activities, based on the results obtained in the preliminary phytochemical screening it is possible to guide further research to determine the biological activity of the species in question and the active principles involved. The phytochemical screening consists in executing chemical reactions on aliquots of the plant extracts. The reactions can be based on liquid-liquid partition with solvents, in chemical reactions that produce colorimetric changes, fluorescence, or precipitates of a specific color. Among the SM to be analyzed are alkaloids, anthraquinones, flavonoids, phenols, saponins, sterols, tannins, quinones, coumarins and terpenoids. There are numerous reviews summarizing the principles of chemical reactions and the qualitative changes that can be are observed 68,79,80,81. A summary of the experimental protocols for the phytochemical screening methods is shown in Table 4.
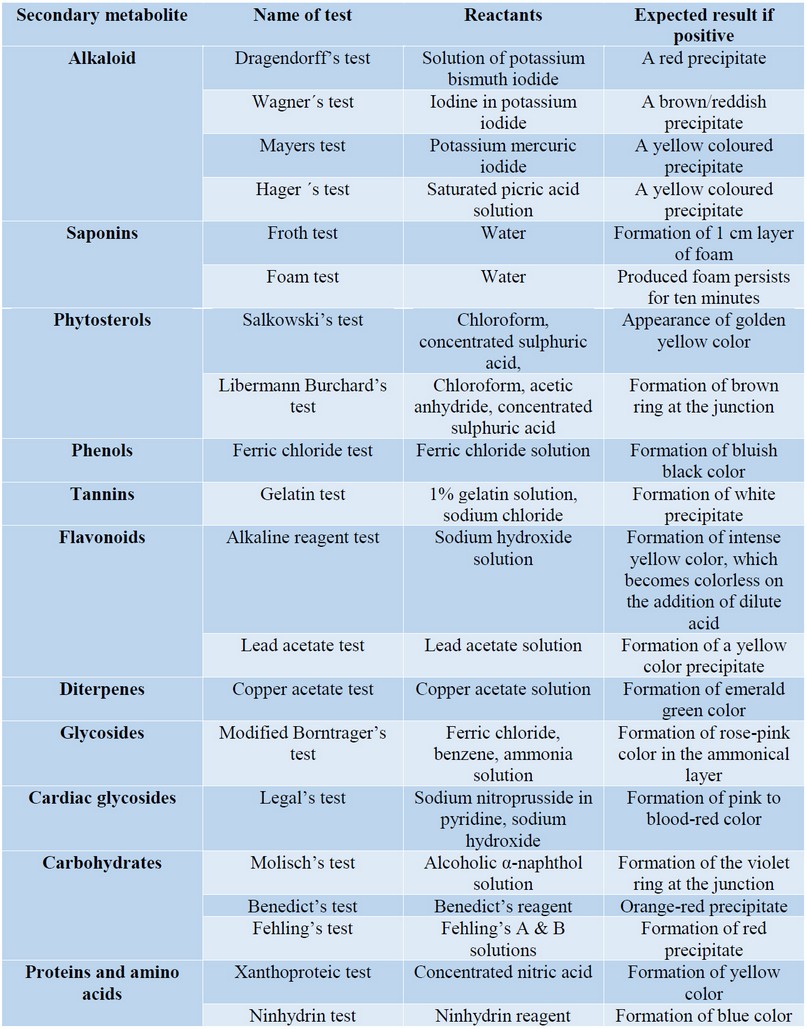
Table 4. A summary of the phytochemical screening methods.
Pharmacological activities of secondary metabolites
Plants have the ability to synthesize a vast and diverse group of SM. Many of them constitute bioactive substances that plants use as defense molecules. These molecules interact with specific targets in microorganisms or animal cells to exert some biological activity that neutralizes them. On the other hand, the diversity of metabolic pathways that plants use in the production of SM guarantees the existence in these defense molecules of specific structures useful to develop new drugs and medicinal products. That is why plants constitute an important source of substances that can be used for improving health and/or curing diseases. Among the beneficial pharmacological activities of the plants stand out antitumor, antioxidant, and antibacterial and activities 82-83.
Special attention has been devoted to the antitumor activity of SM. According to the World Health Organization, among the causes of death that most affect humanity today cancer is found 84. Even when there are numerous alternatives for cancer treatment, research is continuing today to find new molecules from natural sources with better treatment effectiveness or able to alleviate the toxic effects of treatments 85. Examples of isolated metabolites of plants with antitumor activity are lupeol, asiatic acid, celastrol, aurapten, ursolic acid, saidmanetin and indole-3-carbinol and hypericin. These substances have been shown to affect signaling to control cell growth and apoptosis, immune response and stromal microenvironment 85-95.
Another health problem that has been in focus on the action of medicinal plants is the antimicrobial resistance. It is estimated that around 25 thousand patients die per year in the European Union, due to infections caused by resistant bacteria 96. In the United States, it is estimated that resistant bacteria cause around 77 thousand deaths per year 97. These estimates give a clear idea that the search for new molecules with antimicrobial activity is a priority in basic research and necessity of the pharmaceutical industry. The antimicrobial activity of many plant extracts has demonstrated to be effective against Gram-positive and Gram-negative 98-99. Besides, several authors have pointed out the possible synergy between antibiotics and plant extracts 100. In the case of polyphenols, the antibacterial activity is based on the ability of these compounds to inhibit growth, reproduction, respiration, and any other vital function of microorganisms. This action is performed by the oxidation of specific enzymes, which inhibit some critical functions, such as breathing. It is also reported that polyphenols bind to DNA chains disrupting protein synthesis in microorganisms. Other authors suggest that some polyphenols can break the cell membranes of microorganisms, producing cell apoptosis 101,102. It is also known that monoterpenes can interact with the phospholipids of cell membranes of many microorganisms due to their lipophilic nature. As a result, the ordered structure of the membranes is interrupted, thus causing cell lysis 103,104.
The antioxidant activity has also been studied from plant extracts. It is mainly related to the presence of polyphenols or phenolic compounds. Flavonoids act primarily as buffers and capture free radicals to generate the flavinic radical, much less reactive since in their structure the missing electrons are more delocalized. Also, flavonols such as quercetin can chelate transition metal ions such as iron or copper, preventing the formation of reactive oxygen species 105,106.
CONCLUSIONS
Currently, phytochemical research is aimed at isolating and identifying compounds synthesized by plants with pharmacological activities of importance for the treatment of human diseases. The development of efficient methods of extraction and the battery of methods that exist for the scrutiny of the extracts of these medicinal plants allow more profound studies on the pharmacological activities of metabolites and their potential application in human health.
REFERENCES
1. Ruby Tiwari and C.S. Rana (2015) Plant secondary metabolites: a review. International Journal of Engineering Research and General Science, 3: 661-670.
2. Kroymann J. (2011) Natural diversity and adaptation in plant secondary metabolism. Current Opin Plant Biol. 14: 246–251. https://doi.org/10.1016/j.pbi.2011.03.021.
3. Ramakrishna A. and Ravishankar G.A. (2011) Influences of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav. 6: 1720–1731. https://doi.org/10.4161/psb.6.11.17613.
4. Berini J.L., Brockman S.A., Hegeman A.D., Reich P.B., Muthukrishnan R., Montgomery R.A. and Forester J.D. (2018) Combinations of abiotic factors differentially alter production of plant secondary metabolites in five woody plant species in the boreal-temperate transition zone. Front Plant Sci. 9: 1257-1273. https://doi.org/10.3389/fpls.2018.01257.
5. Anuj Tyagi, Balwinder Singh, Naveen K., Billekallu Thammegowda Niraj and K. Singh (2019) Shotgun metagenomics offers novel insights into taxonomic compositions, metabolic pathways and antibiotic resistance genes in fish gut microbiome. Archives of Microbiology 201: 295–303. https://doi.org/10.1007/s00203-018-1615-y.
6. Hall R.D., Brouwer I.D. and Fitzgerald M.A. (2008) Plant metabolomics and its potential application for human nutrition. Physiol Plant. 132: 162–175. https://doi.org/10.1111/j.1399-3054.2007.00989.x.
7. Freeman B.C. and Beattie G.A. (2008) An overview of plant defenses against pathogens and herbivores. Plant Health Instr. 94-DOI: 10.1094/ phi-i-2008-0226-01.
8. Caputi, Lorenzo and Aprea, Eugenio (2011) Use of Terpenoids as Natural Flavouring Compounds in Food Industry. Recent Patents on Food, Nutrition & Agriculture 3: 9-16.
9. O. V. Zillich, U. Schweiggert‐Weisz, P. Eisner and M. Kerscher (2015) Polyphenols as active ingredients for cosmetic products. International Journal of Cosmetic Science 37: 455–464. https://doi.org/10.1111/ics.12218.
10. Thomas Vanhercke, Craig C. Wood, Sten Stymne, Surinder P. Singh and Allan G. Green (2013) Metabolic engineering of plant oils and waxes for use as industrial feedstocks. Plant Biotechnology Journal 11: 197–210. https://doi.org/10.1111/pbi.12023.
11. Ilya Raskin, David M. Ribnicky, Slavko Komarnytsky, Nebojsa Ilic, Alexander Poulev, Nikolai Borisjuk, Anita Brinker, Diego A. Moreno, Christophe Ripoll, Nir Yakoby, Joseph M. O’Neal, Teresa Cornwell, Ira Pastor and Bertold Fridlender (2002) Plants and human health in the twenty-first century. Trends Biotechnol. 20: 522–531. https://doi.org/10.1016/S0167-7799(02)02080-2.
12. Adolfo Ávalos García y Elena Pérez-Urria Carril. (2009) Metabolismo secundario de plantas. Reduca (Biología). Serie Fisiología Vegetal. 2: 119-145.
13. Vranová E., Coman D. and Gruissem W. (2012) Structure and dynamics of the isoprenoid Pathway Network. Mol. Plan. 5: 318-333. DOI: 10.1093/mp/sss015.
14. Paul M. Dewick (2009). Medicinal natural products: a biosynthetic approach. John Wiley and Sons. ISBN 9780470741689.
15. Taiz, L. and Zeiger, E. (2010) Plant Physiology. 5th Edition, Sinauer Associates Inc., Sunderland, 782 p.
16. Sara Emilia Giraldo, Javier Rincón, Pilar Puebla, Mariel Marder y Cristina Wasowski (2010) Isovaleramida, principio anticonvulsivo aislado de Valeriana pavonii. Biomédica 30: 245-250. ISSN: 0120-4157.
17. W. Chen and A.M. Viljoen. (2010) Geraniol - A review of a commercially important fragrance material. South African Journal of Botany 76: 643–651. https://doi.org/10.1016/j.sajb.2010.05.008.
18. Dubey, V.S. and Luthra, R. (2001) Biotransformation of geranyl acetate to geraniol during palmarosa (Cymbopogon martinii, Roxb. wats. var. motia) inflorescence development. Phytochemistry 57: 675–680. https://doi.org/10.1016/S0031-9422(01)00122-4.
19. Azanchi, T., Shafaroodi, H. and Asgarpanah, J. (2014) Anticonvulsant activity of Citrus aurantium blossom essential oil (neroli): Involvment of the GABAergic system. Nat. Prod. Commun. 9: 1615–1618.
20. Amir Pourfarzad and Gisou Raouf Mehrpour (2017) Health Benefits of Hazelnut. EC Nutrition 8.3: 101-105.
21. G. P. Ghimire, H. T. Nguyen, N. Koirala, and J. K. Sohng (2016) Advances in biochemistry and microbial production of squalene and its derivatives. Journal of Microbiology and Biotechnology 26: 441–451. https://doi.org/10.4014/jmb.1510.10039.
22. Cutzu, R., Annalisa Coi, Fulvia Rosso, Laura Bardi, Maurizio Ciani, Marilena Budroni, Giacomo Zara, Severino Zara and Ilaria Mannazzu (2013) From crude glicerol to carotenoids by using a Rhodotorula glutinis mutant. World Journal of Microbiology and Biotechnology 29: 1009–1017. DOI:10.1007/s11274-013-1264-x.
23. Bharat Singh and Ram A. Sharma (2015) Plant terpenes: defense responses, phylogenetic analysis, regulation and clinical applications. Biotech 5: 129–151. https://doi.org/10.1007/s13205-014-0220-2.
24. Tiago Olivoto, Maicon Nardino, Ivan Ricardo Carvalho, Diego Nicolau Follmann, Vinícius Jardel Szareski, Mauricio Ferrari, Alan Junior de Pelegrin and Velci Queiróz de Souza (2017) Plant secondary metabolite and its dynamical systems of induction in response to environmental factors: A review. African Journal of Agricultural Research 12: 71-84. https://doi.org/10.5897/AJAR2016.11677.
25. Loreto F., Dicke M., Schnitzler J.P. and Turlings T.C.J. (2014) Plant volatiles and the environment. Plant Cell Environ 37:1905–1908. https://doi.org/10.1111/pce.12369.
26. Trapp S. and Croteau R. (2001) Defensive Resin Biosynthesis in Conifers. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 52: 689-724. DOI: 10.1146/annurev.arplant.52.1.689.
27. Veitch G.E., Boyer A. and Ley S.V. (2008) The Azadirachtin Story. Angew. Chem. Int. Ed. 47: 9402-9429. https://doi.org/10.1002/anie.200802675.
28. Soriano I.R., Rilye I.T., Potter M.J. and Bowers W.S. (2004) Phytoecdysteroids: A Novel Defense Against Plant-Parasitic Nematodes. J. Chem. Ecol. 30: 1885-1899. https://doi.org/10.1023/B:JOEC.0000045584.56515.11.
29. Franceschi V.R., Krokene P., Christiansen E. and Krekling T. (2005) Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol. 167: 353-376. https://doi.org/10.1111/j.1469-8137.2005.01436.x.
30. Lattanzio V., Kroon P.A., Quideau S. and Treutter D. (2008) Plant phenolics – Secondary metabolites with diverse functions. In: Daayf F, Lattanzio V (eds) Recent advances in polyphenol research, vol 1. Wiley-Blackwell, Oxford, pp 1–35.
31. Metcalf, R.L. Plant volatiles as insect attractants. CRC Crit. Rev. Plant Sci.1987, 5, 251-301. https://doi.org/10.1080/07352688709382242.
32. Velderrain-Rodríguez G.R., Palafox-Carlos H., Wall-Medrano A.;, AyalaZavala J.F., Chen C.-Y.O., Robles-Sanchez M., Astiazaran-Garcí, H., Alvarez-Parrilla, E. and González-Aguilar G.A. (2014) Phenolic compounds: Their journey after intake. Food Funct. 5: 189–197. DOI: 10.1039/c3fo60361j.
33. Sales P.M., Souza P.M., Simeoni L.A., Magalhães P.O. and Silveira, D. (2912) α-Amylase Inhibitors: A Review of Raw Material and Isolated Compounds from Plant Source. J. Pharm. Pharm. Sci. 15: 141–183.
34. Seabra R.M., Andrade P.B., Valentão P., Fernandes E., Carvalho F. and Bastos M.L. (2006) In Biomaterials from Aquatic and Terrestrial organisms; Fingerman, M., Nagabhushanam, R., Eds.; Science Publishers: Enfield, NH, USA, 115-174.
35. Jin Dai and Russell J. Mumper (2010) Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 15: 7313-7352. https://doi.org/10.3390/molecules15107313.
36. J.E. Beart, T.H. Lilley and E. Haslam (1985) Plant polyphenols—secondary metabolism and chemical defence: Some observations. Phytochemistry 24: 33-38. https://doi.org/10.1016/S0031-9422(00)80802-X.
37. Randhir R., Lin Y.T. and Shetty, K. (2204) Stimulation of phenolics, antioxidant and antimicrobial activities in dark germinated mung bean sprouts in response to peptide and phytochemical elicitors. Process Biochem. 39: 637–646.
38. Vattem D.A., Randhir R. and Shetty K. (2005) Cranberry phenolics-mediated antioxidant enzyme response in oxidatively stressed porcine muscle. Process. Biochem. 40: 2225–2238. https://doi.org/10.1111/j.1745-4514.2005.00007.x.
39. Lin D.R., Hu L.J., You H., Sarkar D., Xing B.S. and Shetty, K. (2010) Initial screening studies on potential of high phenolic-linked plantclonal systems for nitrate removal in cold latitudes. J. Soils Sediment. 10: 923–932. ISSN: 1439-0108. DOI: https://doi.org/10.1007/s11368-010-0214-6.
40. Véronique Cheynier, Gilles Comte, Kevin M. Davies, Vincenzo Lattanzio and Stefan Martens (2013) Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiology and Biochemistry 72: 1-20. https://doi.org/10.1016/j.plaphy.2013.05.009.
41. Martins N., Barros L., and Ferreira I.C. (2016) In vivo antioxidant activity of phenolic compounds: Facts and gaps. Trends in Food Science & Technology 48: 1-12. https://doi.org/10.1016/j.tifs.2015.11.008.
42. Sumczynski D., Kotásková E., Družbíková H. and Mlček J. (2016) Determination of contents and antioxidant activity of free and bound phenolics compounds and in vitro digestibility of commercial black and red rice (Oryza sativa L.) varieties. Food Chemistry 211: 339-346. https://doi.org/10.1016/j.foodchem.2016.05.081.
43. Surco-Laos F., Valle Campos M., Loyola E., Dueñas M., and Santos C. (2016). Actividad antioxidante de metabolitos de flavonoides originados por la microflora del intestino humano. Revista de la Sociedad Química del Perú, 82: 29-37. ISSN: 1810-634X. Disponible en: https://www.redalyc.org/articulo.oa?id=3719/371946049004.
44. C Sudha Rani and C Sudhakar (2018) Effect of plant growth retardants on growth, yield and economics of kharif pigeonpea (Cajanus cajan. L). International Journal of Chemical Studies 6: 1495-1498. P-ISSN: 2349–8528. E-ISSN: 2321–4902.
45. Stanisław Weidner, Sebastian Chrzanowski, Magdalena Karamać, Angelika Król, Anna Badowiec, Agnieszka Mostek and Ryszard Amarowicz. (2014) Analysis of Phenolic Compounds and Antioxidant Abilities of Extracts from Germinating Vitis californica Seeds Submitted to Cold Stress Conditions and Recovery after the Stress. Int. J. Mol. Sci. 15: 16211-16225; https://doi.org/10.3390/ijms150916211.
46. D. Verdaguer, M.A.K. Jansen, L. Llorens, L.O. Morales and S. Neugart (2017) UV-A radiation effects on higher plants: Exploring the known unknown. Plant science 255: 72-81. https://doi.org/10.1016/j.plantsci.2016.11.014.
47. Kim Valenta, Ryan J. Burke, Sarah A. Styler, Derek A. Jackson, Amanda D. Melin and Shawn M. Lehman. (2013) Colour and odour drive fruit selection and seed dispersal by mouse lemurs. Sci. Rep. 3: 2424. https://doi.org/10.1038/srep02424.
48. Jacob John and S. Sarada (2012) Role of phenolics in allelopathic interactions. Allelopathy Journal 29: 215-230.
49. Ishita Ahuja, Ralph Kissen and Atle M. Bones (2012) Phytoalexins in defense against pathogens. Trends in Plant Science 17:73-90. https://doi.org/10.1016/j.tplants.2011.11.002.
50. Karen J. Marsh, Ian R. Wallis, Carsten Kulheim, Robert Clark, Dean Nicolle, William J. Foley and Juha-Pekka Salminen. (2019) New approaches to tannin analysis of leaves can be used to explain in vitro biological activities associated with herbivore defence. New Phytologist https://doi.org/10.1111/nph.16117.
51. Roberts M.F. and Michael Wink. Alkaloids: Biochemistry, ecology, and medicinal applications. Plenum Press, New York, USA, 1998: 1-7. Editors: Roberts, Margaret F. (Ed.). ISBN 978-1-4757-2905-4.
52. Ng Y.P. and Or, T.C.; Ip, N.Y. Plant alkaloids as drug leads for Alzheimer’s disease. Neurochem. Int. 2015, 89, 260–270. https://doi.org/10.1016/j.neuint.2015.07.018.
53. Bérdy J. (2005) Bioactive microbial metabolites. The Journal of Antibiotics. 58: 1-26.
54. Matsuura H.N. and Fett-Neto A.G. (2017) Plant Alkaloids: Main Features, Toxicity, and Mechanisms of Action. In: Gopalakrishnakone P., Carlini C., Ligabue-Braun R. (eds) Plant Toxins. Toxinology. Springer, Dordrecht. ISBN 978-94-007-6463-7.
55. Babbar, N. (2015) An introduction to alkaloids and their application in pharmaceutical industry. Pharma Innovation Journal, 4: 74-75. ISSN: 2277- 7695.
56. Kumar, S. (2014) Alkaloidal drugs: A review. Asian Journal of Pharmaceutical Sciences & Technology 4: 107-119.
57. Tadeusz Aniszewski (2007) Alkaloids – secrets of life. Alkaloid chemistry, biological significance, applications and ecological role. Joensuu, Finland. Ed: Elsevier, Amsterdam • Boston • Heidelberg • London • New York • Oxford • Paris. San Diego • San Francisco • Singapore • Sydney • Tokyo.
58. Pelletier, S. W. (1983) The nature and definition of an alkaloid. In: Alkaloids. Chemical and Biological Perspectives. Vol. One (Pelletier, S. W., ed.), pp. 1–31. New York: John Wiley & Sons.
59. V.G. Kartsev. (2004) Natural compounds in drug discovery. Biological activity and new trends in the chemistry of isoquinoline alkaloids. Med Chem Res 13: 325-336.
60. Shakhnoz Azimova, Yunusov Marat. Natural Compounds-Alkaloids. Springer Science Business Media New York 2013.
61. Vongsak B., Sithisarn P., Mangmool S., Thongpraditchote S., Wongkrajang Y., et al. (2013) Maximizing total phenolics, total flavonoids contents and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method Ind. Crops Prod 44: 566-571. DOI: 10.1016/j.indcrop.2012.09.021.
62. Kaufmann B. and Christen P. (2002) Recent extraction techniques for natural products: microwave-assisted extraction and pressurized solvent extraction. Phytochem. Anal 13: 105-113.
63. Mediani F.A., Khatib A., Tan C.P. (2013) Cosmos Caudatus as a potential source of polyphenolic compounds: Optimisation of oven drying conditions and characterisation of its functional properties. Molecules 18: 10452-10464. https://doi.org/10.3390/molecules180910452.
64. Abdullah S., Shaari A.R. and Azimi A. (2012) Effect of Drying Methods on Metabolites Composition of Misai Kucing (Orthosiphon stamineus) Leaves. APCBEE Procedia 2: 178-182. https://doi.org/10.1016/j.apcbee.2012.06.032.
65. Methods Optimization in Accelerated Solvent Extraction in Technical note (2013) 208: 1-4.
66. Borhan M.Z., Ahmad R., Rusop M. Mohd and Abdullah S (2013) Impact of Nano powders on Extraction Yield of Centella asiatica. Adv. Mater. Res 667: 246-250.
https://doi.org/10.4028/www.scientific.net/AMR.667.246.
https://doi.org/10.4028/www.scientific.net/AMR.667.246.
67. Pandey A., Tripathi S. and Pandey C.A. (2014) Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. J Pharmacogn Phytochem JPP. 115: 115–119.
68. Azwanida NN. () A Review on the Extraction Methods Use in Medicinal Plants, Principle, Med Aromat Plants 2015, 4:3. http://dx.doi.org/10.4172/2167-0412.1000196.
69. Verde-star M.J. and García-González S. Metodología científica para el estudio de plantas medicinales. 2016; 1–39.
70. J. B. Harborne. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis. second ed., Chapman and Hall, New York, USA. Chapmer and Hall. 1984.
71. Rathi B.S., Bodhankar S.L. and Baheti A.M. (2006) Evaluation of aqueous leaves extract of Moringa oleifera Linn for wound healing in albino rats. Indian J Exp Biol 44: 898-901.
72. James Redfern, Malcolm Kinninmonth, Dariel Burdass and Joanna Verran. (2014) Using Soxhlet Ethanol Extraction to Produce and Test Plant Material (Essential Oils) for Their Antimicrobial Properties 15:45–46. doi:10.1128/jmbe.v15i1.656.
73. Ingle K.P., Deshmukh A.G., Padole D.A., Mahendra S., Moharil M.P. and Khelurkar V.C. (2017) Phytochemicals : Extraction methods, identification and detection of bioactive compounds from plant extracts. 6: 32–36.
74. Trusheva B., Trunkova D. and Bankova V (2007) Different extraction methods of biologically active components from propolis: a preliminary study. Chem Cent J 1: 3. doi: 10.1186/1752-153X-1-13.
75. Terigar, B.G., Balasubramanian, S., Sabliov, C.M. and Lima, M., Boldor, D. (2011) Soybean and rice bran oil extraction in a continuous microwave system: from laboratory to pilot scale. J. Food Eng. 104: 208–217. https://doi.org/10.1016/j.jfoodeng.2010.12.012.
76. Rahmalia W., Fabre J.F. and Mouloungui Z. (2015) Effects of Cyclohexane/Acetone Ratio on Bixin Extraction Yield by Accelerated Solvent Extraction Method. Procedia Chem. 14: 455–464. http://dx.doi.org/10.1016/j.proche.2015.03.061.
77. Azwanida N.N. (2015) A Review on the Extraction Methods Use in Medicinal Plants, Principle,
Strength and Limitation. Medicinal & Aromatic Plants 4: 3–8. DOI:10.4172/2167-0412.1000196.
78. Duque A.M.R., Londoño-Londoño J., Álvarez D.G., Paz Y.B. y Salazar B.L.C. (2013) Comparación del aceite de aguacate variedad Hass cultivado en Colombia, obtenido por fluidos supercríticos y métodos convencionales: Una perspectiva desde la calidad. Rev Lasallista Investig. 9:151–61. Retrieved October 16, 2019, from http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S1794-44492012000200016&lng=en&tlng=.
79. Prashant Tiwari, Bimlesh Kumar, Mandeep Kaur, Gurpreet Kaur and Harleen Kaur. (2011) Phytochemical screening and Extraction: A Review. Internationale Pharmaceutica Sciencia 1: 98-106.
80. S. Sasidharan, Y. Chen, D. Saravanan, K.M. Sundram and L. Yoga Latha. (2011) Extraction, isolation and characterization of bioactive compounds from plant’s extracts. Afr J Tradit Complement Altern Med. 8: 1-10.
81. Mukherjee P.K., Maity N., Nema N.K. and Sarkar BK (2011) Bioactive compounds from natural resources against skin aging. Phytomedicine 19: 64-73. doi: 10.1016/j.phymed.2011.10.003.
82. Chen Q.L., Chen X.Y., Zhu L., Chen H.B., Ho H.M., Yeung W.P., Zhao Z.Z. and Yi T (2016) Review on Saussurea laniceps, a potent medicinal plant known as ‘‘snow lotus’’: botany, phytochemistry and bioactivities. Phytochem Rev. doi: 10.1007/s11101-015-9452-y.
83. Cragg G.M., Grothaus P.G. and Newman DJ (2014) New horizons for old drugs and drug leads. J Nat Prod 77:703–723. doi: 10.1021/np5000796.
84. World health statistics overview 2019: monitoring health for the SDGs, sustainable development goals. Geneva: World Health Organization; 2019 (WHO/DAD/2019.1). Licence: CC BY-NC-SA 3.0 IGO.
85. Helder Ombui Nyaboke, Martha Mora, Leonidah Kerubo Omosa, Armelle T. Mbaveng, Nchiozem-Ngnitedem Vaderament-Alexe, Veronicah Masila, Evans Okemwa, Matthias Heydenreich, Thomas Efferth and Victor Kuete. (2018) Cytotoxicity of Lupeol from the Stem Bark of Zanthoxylum gilletii against Multifactorial Drug Resistant Cancer Cell Lines. Investigational Medicinal Chemistry and Pharmacology 1:10. This article is available at https://investchempharm.com/.
86. Yan Liu, Tingting Bi, Gang Wang, Wei Dai, Guoliang Wu, Liqiang Qian and Quangen Gao (2015) Lupeol inhibits proliferation and induces apoptosis of human pancreatic cancer PCNA-1 cells through AKT/ERK pathways. Naunyn-Schmiedeberg's Arch Pharmacol 388: 295. https://doi.org/10.1007/s00210-014-1071-4.
87. Hsu, Y.L.; Kuo, P.L.; Lin, L.T.; Lin, C.C. (2005) Asiatic acid, a triterpene, induces apoptosis and cell cycle arrest through activation of extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways in human breast NSCLC cells. J. Pharmacol. Exp. Ther. 313: 333–344. https://doi.org/10.1124/jpet.104.078808.
88. Tiancong Wua, Ji Geng, Wenjie Guo, Jing Gao and Xixu Zhua. (2017) Asiatic acid inhibits lung cancer cell growth in vitro and in vivo by destroying mitochondria. Acta Pharmaceutica Sinica B 7: 65 – 72. https://doi.org/10.1016/j.apsb.2016.04.003.
89. Idris, A.I.; Libouban, H.; Nyangoga, H.; Landao-Bassonga, E.; Chappard, D. and Ralston, S.H. (2009) Pharmacologic inhibitors of IkB kinase suppress growth and migration of mammary carcinosarcoma cells in vitro and prevent osteolytic bone metastasis in vivo. Mol. Cancer Ther. 2009, 8, 2339–2347. DOI: 10.1158/1535-7163.MCT-09-0133.
90. Gali-Muhtasib, H., Hmadi, R., Kareh, M., Tohme, R. and Darwiche, N. (2015) Cell death mechanisms of plant-derived anticancer drugs: Beyond apoptosis. Apoptosis 20: 1531–1562. doi: 10.1007/s10495-015-1169-2.
91. Motlagh F.M. and Gholami O. (2017) Comparison of Umbelliprenin and Auraptene in cytotoxic effects and myeloid cell leukemia Type-1 (Mcl-1) gene expression. Indian J Pharm Sci. 78:827–833. DOI: 10.4172/pharmaceutical-sciences.1000189.
92. Yeh, C.T.; Wu, C.H. and Yen, G.C. (2010) Ursolic acid, a naturally occurring triterpenoid, suppresses migration and invasion of human breast NSCLC cells by modulating c-jun n-terminal kinase, akt and mammalian target of rapamycin signaling. Mol. Nutr. Food Res. 54: 1285–1295. https://doi.org/10.1002/mnfr.200900414.
93. Jendzelovska Z., Jendzelovsky R., Hilovska L., Koval J., Mikes J. and Fedorocko, P. (2014) Single pre-treatment with hypericin, a st. John’s wort secondary metabolite, attenuates cisplatin- and mitoxantrone-induced cell death in a2780, a2780cis and hl-60 cells. Toxicol. In Vitro 28: 1259–1273. doi: 10.1016/j.tiv.2014.06.011.
94. Yong Y., Matthew S., Wittwer J., Pan L., Shen Q., Kinghorn A.D., Swanson S.M. and De Blanco, E.J. (2013) Dichamanetin inhibits cancer cell growth by affecting ros-related signaling components through mitochondrial-mediated apoptosis. Anticancer Res. 33: 5349–5355.
95. Caruso J.A., Campana R., Wei C., Su C.H., Hanks A.M., Bornmann,W.G. and Keyomarsi, K. (2014) Indole-3-carbinol and its n-alkoxy derivatives preferentially target eralpha-positive breast NSCLC cells. Cell Cycle 13: 2587–2599. doi: 10.4161/15384101.2015.942210..
96. Matthias Michael Fischer and Matthias Bild. (2019) Hospital use of antibiotics as the main driver of infections with antibiotic-resistant bacteria - a reanalysis of recent data from the European Union. BioRxiv. https://doi.org/10.1101/553537.
97. US CDC. Sepsis: Making Health Care Safer. Aug 2017. https://www.cdc.gov/media/releases/2017/p0831-sepsis-recognitiontreatment.html.
98. Katz L. and Baltz R.H. (2016) Natural product discovery: past, present, and future. J Ind Microbiol Biotechnol. 43:155-76. doi: 10.1007/s10295-015-1723-5.
99. Othman L., Sleiman A. and Abdel-Massih R.M. (2019) Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Front Microbiol. 10: 911-921. doi: 10.3389/fmicb.2019.00911.
100. Mikulasova M., Chovanova R. and Vaverkova S. (2016) Synergism between antibiotics and plant extracts or essential oils with efflux pump inhibitory activity in coping with multidrugresistant staphylococci. Phytochem Rev. doi:10.1007/s11101-016-9458-0.
101. Martelli G. and Giacomini D. (20118) Antibacterial and antioxidant activities for natural and synthetic dual-active compounds. Eur J Med Chem. 5: 91-105. doi: 10.1016/j.ejmech.2018.09.009.
102. Tocci, N., Weil, T., Perenzoni, D., Narduzzi, L., Madriñán, S., Crockett, S., . . . Mattivi, F. (2018). Phenolic profile, chemical relationship and antifungal activity of Andean Hypericum species. Industrial Crops and Products, 112, 32-37.
103. Eric Concha, Hermann J. Heipieper, Lukas Y. Wick, Gustavo A. Ciudad and Rodrigo Navia (2018) Effects of limonene, n-decane and n-decanol on growth and membrane fatty acid composition of the microalga Botryococcus braunii. AMB Expr (2018) 8: 189. https://doi.org/10.1186/s13568-018-0718-9.
104. N.G.Vasconcelos, J.Croda and S.Simionatto. () Antibacterial mechanisms of cinnamon and its constituents: A review. Microbial Pathogenesis 120: 198-203 https://doi.org/10.1016/j.micpath.2018.04.036.
105. Tian-yang Wang, Qing Li and Kai-shun Bi. (2018) Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian Journal of Pharmaceutical Sciences 13: 12-23. https://doi.org/10.1016/j.ajps.2017.08.004.
106. Shivraj Hariram Nile, Young Soo Keum, Arti Shivraj Nile, Shivkumar S. Jalde and Rahul V. Patel. (2018) Antioxidant, anti‐inflammatory, and enzyme inhibitory activity of natural plant flavonoids and their synthesized derivatives. Journal of Biochemical and Molecular Toxicology. https://doi.org/10.1002/jbt.22002.
Received: 20 September 2019
Accepted: 25 October 2019
Irina Francesca González Mera1, Daniela Estefanía González Falconí1, Vivian Morera Córdova1*.
1 Yachay Experimental Technology Research University. School of Chemical Sciences and Engineering. San Miguel de Urcuquí. Hacienda San José s/n. Imbabura, Ecuador.
* Correspondence author: [email protected]
