2023.08.01.93
Files > Volume 8 > Vol 8 No 1 2023
The efficient procedure of embryogenic callus formation from anther in Capsicum pubescens Ruiz & Pav
Alexandra Jherina Pineda-Lázaro 1,* , Angel David Hernández-Amasifuen 1,*
, Angel David Hernández-Amasifuen 1,* and Hermila Belba Díaz-Pillasca 1
and Hermila Belba Díaz-Pillasca 1
1 Universidad Nacional José Faustino Sánchez Carrión, Laboratorio de Biotecnología Vegetal, Huacho, Perú.
* Correspondence: [email protected]
Available from: http://dx.doi.org/10.21931/RB/2023.08.01.93
ABSTRACT
The peppers or ajies (Capsicum spp.) are one of Peru's main productive crops, which is why it is among the top ten countries in production and exports. Among the species cultivated in Peru is Capsicum pubescens Ruiz & Pav., commonly called rocoto, and represents one of the most critical species in local and national gastronomy. The rocoto ecotype "Selva" or "de Monte" is characterized by its large size and is used to prepare rocoto relleno arequipeño. This crop presents the restriction to obtain homozygous lines for being self-incompatible. Using biotechnology, pure lines can be obtained from the anther culture, and homozygous lines can be obtained. The objective was to induce embryogenic callus formation from anther culture of Capsicum pubescens Ruiz & Pav. Ecotype "de Monte". In the results, 74.1% of embryogenic callus was induced in rocoto anthers with a longitudinal ratio of 1.04 mm; the induction was carried out using Murashige and Skoog culture medium added with 2.0 mg/L of 2,4-D. Likewise, the formation of somatic embryos in the globular stage was evidenced after 12 weeks in the culture medium.
Keywords: Aji; Biotechnology; Somatic embryos; Self-incompatible; Rocoto; Peppers.
INTRODUCTION
The peppers or ajies (Capsicum spp.) are a vegetable genus of great importance worldwide, with 31 species reported to date, five of which are cultivated (C. annuum L., C. chinense Jacq., C. frutescens L., C. baccatum L., C. pubescens Ruiz & Pav.) and 26 are wild species 1-3. Peru presents, cultivates and produces the five species of cultivated chili peppers, making it one of the top ten countries in producing and exporting fresh and processed chili peppers 4-6.
The Andean region of Peru is the origin of the rocoto (C. pubescens Ruiz & Pav.), with wild relatives still present in these areas, as well as in the highlands and high jungle 7. The rocoto is a plant species of great national relevance due to its use in gastronomy, based on its two most representative ecotypes 8. The ecotypes of rocoto in Peru are "Serrano" or "de Huerto" and "Selva" or "de Monte"; the first is the most distributed in the country due to its great adaptation to the different regions, and the second can only be found in the jungle 9. The Selva ecotype is distinguished by its large size and moderate pungency, which is why it is trendy in the preparation of one of Peru's most representative dishes, rocoto relleno arequipeño (stuffed red pepper) 10. The Serrano ecotype is smaller in size and has a high pungency, which is why it is widely used as a sauce in many national dishes 11.
The major problem affecting the rocoto crop is related to root rot diseases caused by species of the genus Fusarium, Phytoptora and Rhizoctonia 7,12,13. Because of this, rocoto germplasm banks have been implemented with accessions from all over Peru, among the most commercial ecotypes and genotypes with great agronomic potential. Based on the extensive collection of rocoto genetic material, genetic improvement programs have been initiated to obtain defined varieties. Currently, the rocoto crop does not have a specified variable; therefore, no certified seeds are available in the national market 10. For this reason, rocoto farmers resort to sources from previous crop seasons, obtaining heterogeneous fruits.
One of the main difficulties in the genetic improvement of rocoto is obtaining homozygous or pure lines because this species is considered self-incompatible; specifically, the genotypes of Peru, Bolivia and Argentina are reported as genotypes with gametophytic self-incompatibility 14. Likewise, the genetic control of self-incompatibility in rocoto has been identified and measured by the S locus, which is formed by multiple alleles. Therefore, pollen or, in some genotypes, the pollen tube can express enzymatic products that prevent their proliferation on the stigma 15.
Within modern breeding programs, biotechnological tools are being applied through in vitro plant tissue culture 16,17. This allows working with a fragment of selected mother plants for the clonal propagation of these superior genotypes 18. Likewise, among the methods used in plant tissue culture, anther culture is presented, which allows taking advantage of and working with the male gametes of the plants of interest, thus inducing organogenesis or the formation of somatic embryos directly and indirectly (previous callus formation) for the regeneration of haploid plants, which can be induced to chromosomal duplication and thus obtain pure lines that can be used for crossbreeding between genotypes with a more significant commercial potential 19-21.
In this context, the objective of the present research was to induce the formation of embryogenic callus from anther culture of Capsicum pubescens Ruiz & Pav. Ecotype "de Monte".
MATERIALS AND METHODS
Plant material
Flower buds of rocoto of the "de Monte" ecotype were collected from rocoto seedlings in the agricultural zone of Santa Elena Norte, Barranca, Lima, Peru. Flower buds without opening or the presence of pest or disease damage were selected in the early morning hours. The flower buds were then transferred to the facilities of the Plant Biotechnology Laboratory of the Universidad Nacional José Faustino Sánchez Carrión, Huacho, Lima, Peru.
Determination of uninucleate microspores in rocoto anthers
The percentage of microspores in the uninucleate state was evaluated from anthers of rocoto flower buds, which were grouped by size difference and related between petal and sepal, based on the presence of microspores in the uninucleate state. For each sample per group collected, the anthers were extracted, from which the microspores were obtained and stained with 0.5% acetic orcein and observed under the optical microscope at 100X magnification to determine which group had the highest percentage of the indicated stage.
In vitro establishment of rocoto anthers
Once the morphological groups of rocoto flower buds were identified, we continued with the establishment under in vitro conditions, determining the optimal concentration of sodium hypochlorite (NaClO) for disinfection and presenting the lowest percentage of oxidation of the plant material. The treatments were set at 1.0%, 1.5%, 2.0%, 2.5%, 3.0%, 3.5%, 4.0%, 4.5% and 5.0% NaClO. Disinfection with the different concentrations of NaClO was carried out for 10 minutes and then washed in sterile distilled water, a process that was repeated three times. Once the flower buds were disinfected, they were dissected to separate the anthers from the rest of the plant material. All anthers were placed in semi-solid culture medium with basal salts Murashige and Skoog 22, and were kept in total darkness, 75% relative humidity and 24° C for two weeks. Evaluations were made after the first and second week of anther introduction into the culture medium to determine the treatment with minor contamination and anther necrosis.
Callus induction in rocoto anthers
For the induction of callus in rocoto anthers, culture medium with Murashige and Skoog 22 basal salts with the addition of vitamins (Table 1), 30 g/L sucrose, 6 g/L agar and treatments with different concentrations of the auxin 2,4-dichloro phenoxy acetic acid (2,4-D) (Table 2) were used. The conditions for maintaining the treatments were 24°C temperature, 75% relative humidity and total darkness. Evaluations were carried out every four weeks to determine the percentage of callus induction and the degree of callus induction 23 (Table 3), maintaining all treatments for a total of 12 weeks.
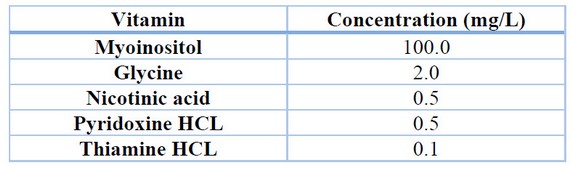
Table 1. Concentration of vitamins in culture medium.
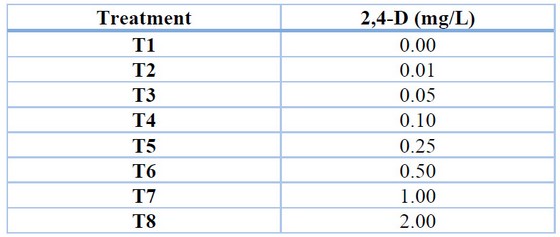
Table 2. Treatments for callus induction in Capsicum pubescens anthers.
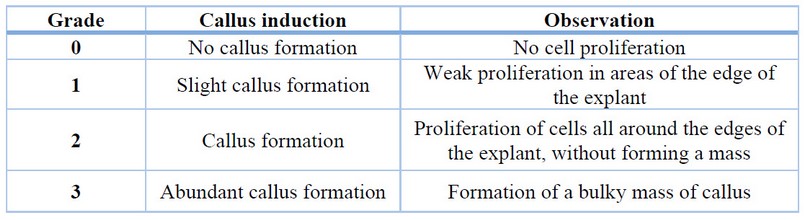
Table 3. Callus induction grades for evaluation in Capsicum pubescens anthers.
Experimental design and statistical analysis
A completely randomized design (CRD) was used to determine uninucleate microspores, in vitro establishment and callus induction in rocoto anthers. Statistical analysis was performed by analysis of variance (ANOVA) and comparison of means with Tukey's test (p ≤ 0.05). All comments were processed in the R program (version 4.1.3 for Windows) and Rstudio graphical interface (version 2022.07 for Windows).
RESULTS
Determination of uninucleate microspores in rocoto anthers
Five groups of flower buds were obtained by longitudinal relationship (mm) between petal and sepal (Figure 1), and the flower bud group with the highest percentage of uninucleated microspores was also determined (Table 4). Flower buds that presented openings, physical damage and oxidation were discarded for this part of the research and subsequent sections. Flower buds that were grouped by a 1.04 mm sepal to petal length ratio presented 87.31% of microspores in the uninucleate state, representing the appropriate group and, therefore, selected for the next phase of the research focused on callus induction.

Figure 1. Flower buds of Capsicum pubescens grouped by the longitudinal relationship between sepal and petal.
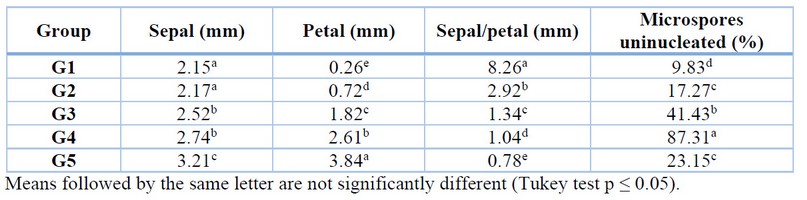
Table 4. Longitudinally related flower bud clusters with uninucleate microspores.
In vitro establishment of rocoto anthers
The disinfection of flower buds showed significant differences between treatments for the evaluations of the percentage of contamination and rate of necrosis or oxidation of the explants (Table 5). The NaClO concentrations that presented the lowest percentages of bacterial or fungal contamination were 4.5% and 5.0%, with 11.53% and 8.45% contamination, respectively; these concentrations did not show significant differences between them. In evaluating the percentage of necrosis per explant, the 1% to 4.0% NaClO concentrations presented 0.00%, representing the best treatments to avoid explant oxidation.
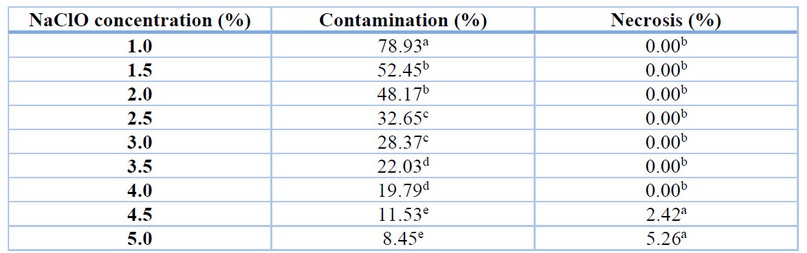
Table 5. Sodium hypochlorite concentrations in flower bud disinfection.
Callus induction in rocoto anthers
The response of rocoto anthers in culture medium with the addition of different auxin 2.4-D induced the formation of embryogenic callus (Figure 2) in various degrees of proliferation. In the last evaluation corresponding to week 12, after introducing the anthers into the culture media, the formation of somatic embryos in the globular stage from the embryogenic callus could be recorded (Figure 2). Treatments T7 and T8 induced the highest embryogenic callus formation, with 61.3% and 74.1%, respectively (Table 6). In the degrees of the proliferation of embryogenic callus, the highest percentages were obtained in grade 3 with treatments T7 and T8, with 38% and 59%, respectively (Table 6).
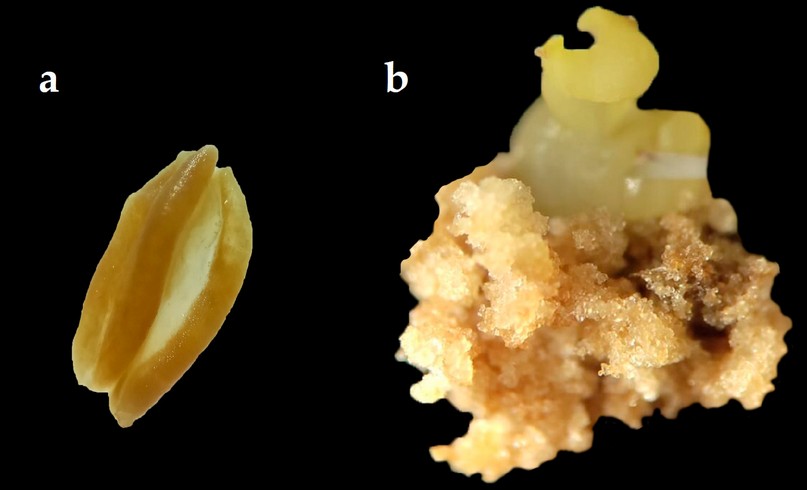
Figure 2. Anther culture of Capsicum pubescens ecotype "de Monte"; (a) Anther without response to callus formation. (b) Anther with embryogenic callus formation at twelve weeks of culture and somatic embryo formation.
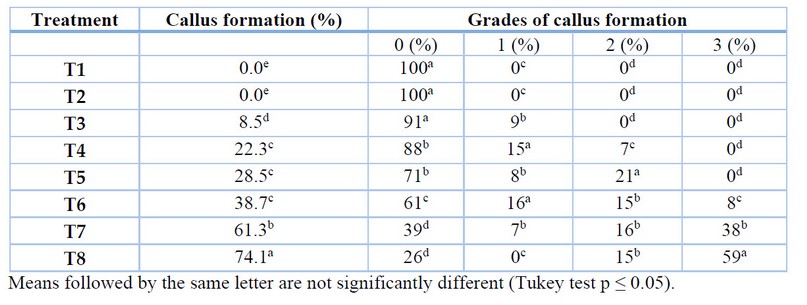
Table 6. Treatments for callus induction in Capsicum pubescens anthers.
DISCUSSION
The group of flower buds G4 had the highest percentage of uninucleate microspores with 87.31% and a ratio of 1.04 sepal/petal. These results validate what was stated by de Vaulx et al. 24, who established that in flower buds of Capsicum annuum L. the longitudinal size ratio of sepals and petals should be similar to one because in this size ratio, the highest percentages of uninucleated microspores are presented. Compared with the research on anthers of rocoto ecotype "Serrano" 25, the researchers obtained 81.3% of uninucleated microspores in flower buds with a morphological ratio of sepal and petal of 1.09, results lower than those presented in the present research.
In the disinfection of rocoto anthers, the best treatments were 4.5% and 5.0% concentrations of sodium hypochlorite, obtaining contaminations of less than 12%. These results are similar to those obtained by Barroso et al. 26, who, for the disinfection of flower buds of Capsicum annuum L. used a 2% sodium hypochlorite solution with three drops of Tween 20 for 10 minutes. Koleva et al. 27 disinfected nine varieties of Capsicum annuum L. using 70% ethanol for thirty seconds and 5% calcium hypochlorite with two to three drops of Tween 20 for 10 minutes and three washes of sterile distilled water.
The induction of callus in rocoto anthers allowed obtaining up to 74.1% in the Murashige and Skoog culture medium added with 2.0 mg/L of 2,4-D, with 59% of callus formation in grade 3 (formation of a voluminous mass of callus). These results are superior to those obtained in rocoto ecotype "Serrano" 25, where 45.75% of callus were obtained in rocoto anthers using Murashige and Skoog culture medium added with 1 mg/L of kinetin and 1 mg/L of 2,4-D. Similarly, the results obtained were superior to those obtained by Koleva et al. 27 from their research on anthers of nine varieties of Capsicum annuum L., obtained the highest percentages in the varieties 58.88% Feferona variety in Nitch medium supplemented with 1.0 mg/L indole-3-acetic acid (IAA) and 48.90% Slatko Luta variety in Murashige and Skoog medium supplemented with 1.0 mg/L kinetin, 0.01 mg/L 2,4-D and 0.001 mg/L IAA. In the case of, Barroso et al. 26, they evaluated the response of anthers of Capsicum annuum L. varieties UFPB 134, UFPB 390 and hybrids between both varieties, where they obtained greater results up to 22.0% embryogenic callus in the genotype F2 of hybrids of Capsicum annuum L. varieties UFPB 134 and UFPB 390 in Murashige and Skoog culture medium added with 4.65 µM kinetin and 5.73 µM naphthaleneacetic acid (NAA); while for the same genotype, they obtained 14.1% and 20.0% for genotype UFPB 134 using Murashige and Skoog culture medium added with 4.65 µM kinetin and 4.52 µM 2,4-D.
The auxin 2,4-D effectively induces embryogenic callus formation in anthers of rocoto ecotype "de Monte." The growth regulator 2,4-D is added to the culture media to induce the proliferation of undifferentiated cells, forming the characteristic cell mass called callus 28. The 2,4-D has a rapid induction effect initially at the edges of the initial explant until it covers the entire structure, depending on the concentrations, combinations with other growth regulators, initial explant and the species with which the research is being carried out 29-31. From this embryogenic callus, it was possible to observe the formation of somatic embryos after 12 weeks in the culture medium, concerning the fact that some species have the characteristic of inducing the formation of somatic embryos after the formation of callus (indirect somatic embryogenesis), and likewise, remaining in the same culture medium without changing it or refreshing the medium 32.
CONCLUSIONS
The formation of embryogenic callus was induced from anther culture of Capsicum pubescens Ruiz & Pav. Ecotype "de Monte," using Murashige and Skoog culture medium added with 2.0 mg/L of 2,4-D, obtaining an induction percentage of 74.1% of embryogenic callus, of which 59% represent grade 3 callus, that is, with the voluminous formation of the callus mass. Likewise, the appearance of somatic embryos in the globular stage was evidenced after 12 weeks in the culture medium.
Author Contributions: Conceptualization, AJPL and ADHA; methodology, AJPL; software, ADHA; validation, HBDP; formal analysis, ADHA; investigation, AJPL; resources, HBDP; data curation, ADHA; writing—original draft preparation, AJPL; writing—review and editing, ADHA; visualization, HBDP; supervision, HBDP All authors have read and agreed to the published version of the manuscript.
Funding: This research received no external funding.
Conflicts of Interest: The authors declare no conflict of interest.
REFERENCES
1. Tripodi, P.; Kumar, S. The Capsicum Crop: An Introduction. The Capsicum Genome 2019, 1, 1-8.
2. Meckelmann, S.; Riegel, D.; van Zonneveld, M.; Rios, L.; Pena, K.; Ugas, R.; Quinonez, L.; Mueller-Seitz, E.; Petz, M. Compositional Characterization of Native Peruvian Chili Peppers (Capsicum spp.). Journal of Agricultural and Food Chemistry 2013, 61(10), 2530-2537.
3. Vera-Guzmán, A.M.; Chávez-Servia, J.L.; Carrillo-Rodríguez, J.C.; López, MG Phytochemical evaluation of wild and cultivated pepper (C. annuum and C. pubescens) from Oaxaca, Mexico. Chilean Journal of Agricultural Research 2011, 71, 578-585.
4. Rojas, J.; Lugo, B.; Pineda, A.; Aguilar, G.; Argüelles, A.; Díaz, H. Establecimiento de un método eficiente de germinación in vitro de arnaucho supano (Capsicum chinense Jacq.). Big Bang Faustiniano 2020, 9(4), 4-7.
5. Hernández, A.; Pineda, A.; Díaz, H. Efecto de la luz y del ácido giberélico en la germinación in vitro de Capsicum annuum L. cv.‘Papri King’. Biotecnología Vegetal 2019, 19(3), 165-170.
6. Hernández, A.; Pineda, A.; Rojas, J.; Díaz, H. Regeneración in vitro de arnaucho (Capsicum chinense Jacq.) a partir de yemas apicales. Manglar 2021, 18(1), 71-75.
7. Valdez, I. Caracterización fenotípica de quince accesiones de germoplasma de rocoto (Capsicum pubescens Ruiz & Pavón.) en la estación INIA Santa Rita Arequipa. Bachelor Degree, Universidad Nacional de San Agustín, Arequipa, Peru. 2017.
8. Flores, N. Elaboración de una salsa a base de huacatay (Tagetes minuta) y rocoto (Capsicum pubescens) evaluando sus características fisicoquímicas y sensoriales. Bachelor Degree, Universidad Nacional de Cajamarca, Cajamarca, Peru. 2019.
9. Espinoza, D. Caracterización morfológica de los ajíes de la costa del Perú. Bachelor Degree, Universidad Nacional Agraria la Molina, Lima, Peru. 2017.
10. Sardón, E. Fortalecimiento de la cadena de valor del rocoto fresco (Capsicum pubescens) de la selva central para el mercado de Lima. Bachelor Degree. Universidad Nacional Agraria La Molina, Lima, Peru. 2015.
11. Sánchez, J. Relación de color y características fisiológicas y fisicoquímicas del rocoto (Capsicum pubescens). Bachelor Degree. Universidad Nacional de Trujillo, Trujillo, Perú. 2015.
12. Lucana, C. Respuesta de 5 especies de Capsicum spp. A Phytophthora capsici Leon, bajo condiciones de invernadero, en los laboratorios de fitopatología de la UNALM – Lima. Bachelor Degree. Universidad Nacional de San Antonio Abad del Cusco, Cusco, Peru. 2012.
13. Hernández, A.; Pineda, A.; Díaz, H. Aislamiento e identificación de Fusarium oxysporum obtenidos de zonas productoras de "ají paprika" Capsicum annumm L. (Solanaceae) en el distrito de Barranca, Perú. Arnaldoa 2019, 26(2), 689-698.
14. Saborío, M.; Da Costa, C. Autoincompatibilidad en Caspsicum pubescens. Agronomía Costarricense 1992, 16(2), 279-286.
15. Bo, M.; Cosa, M.; Carrizo, C. Autoincompatibilidad y funciones sexuales en Caspsicum pubescens. XXXIII Jornadas Argentinas de Botánica 2011, 1, 34.
16. Pineda, A.; Argüelles, A.; Rojas, J. Díaz, H. Respuesta en el establecimiento y regeneración in vitro de rocoto (Capsicum pubescens Ruiz & Pav.). Aporte Santiaguino 2021, 14(1), 31-42.
17. Hernández-Amasifuen, A.D.; Pineda-Lázaro, A.J.; Díaz-Pillasca, H.D. In vitro micropropagation of sour orange (Citrus aurantium L.) from nodal segments. Revista Bionatura 2021, 6(4), 2216-2221.
18. Pineda, A.; Hernández, A.; Díaz, H. Multiplicación y reducción del crecimiento in vitro de papa chaucha (Solanum tuberosum L. grupo Phureja). Manglar 2021, 18(2), 123-128.
19. Germaná, M.A. Anther culture for haploid and doubled haploid production. Plant Cell Tissue Organ Cult 2011, 104, 283-300.
20. Seguí-Simarro, J.; Corral-Martínez, P.; Parra-Vega, V.; González-García, B. Androgenesis in recalcitrant solanaceous crops. Plant Cell Reports 2011, 30(5), 765-778.
21. Parra-Vega, V.; González-García, B.; Seguí-Simarro, J.M. Morphological markers to correlate bud and anther development with microsporogenesis and microgametogenesis in pepper (Capsicum annuum L.). Acta Physiol Plant 2013, 35, 627-633.
22. Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 1962, 15, 473-497.
23. Santana, N.B. Determinación de un medio adecuado para la obtención de callos en variedades de caña de azúcar (Saccharum spp) in vitro. Cultivos Tropicales 1982, 4(3), 48-52.
24. de Vaulx, R.D; Chambonnet, D.; Pochard, E. Culture in vitro d’anthères de piment (Capsicum annuum L.): amèlioration des taux d'obtenction de plantes chez différents génotypes par des traitments à +35°C. Agronomie 1981, 1(10), 859-864.
25. Hernández-Amasifuen, A.D.; Pineda-Lázaro, A.J.; Díaz-Pillasca, H.D. In vitro anther culture of rocoto (Capsicum pubescens Ruiz & Pav.). Idesia 2022, 40(1), 115-121.
26. Barroso, P.; Rêgo, M.; Rêgo, E.; Soares, W. Embryogenesis in the anthers of different ornamental pepper (Capsicum annuum L.) genotypes. Genetics and molecular research 2015, 14(4), 13349-13363.
27. Koleva-Gudeva, L.; Spasenoski, M.; Trajkova, F. Somatic embryogenesis in pepper anther culture: the effect of incubation treatments and different media. Scci Hortic 2007, 11, 114-119.
28. Argüelles, A.; Hernández, A.; Cortez, A.; Díaz, H. Callogénesis in vitro de paprika (Capsicum annuum L.) cv. Papri King a partir de tallos. Big Bang Faustiniano 2020, 9(1), 4-7.
29. Hernández-Amasifuen, A.D.; Argüelles-Curaca, A.; Cortez-Lázaro, A.A.; Díaz-Pillasca, H.B. In vitro induction of callus from foliar explants in rocoto (Capsicum pubescens Ruiz & Pav.). La Granja 2021, 32(2), 127-135.
30. Hernández, A.; Argüelles, A.; Cortez, A.; Díaz, H. Effect of 2,4-dichlorophenoxyacetic concentration on in vitro callus induction using cotyledons of rocoto (Capsicum pubescens Ruiz & Pav.). The Biologist 2020, 17(2), 327-334.
31. Hernández-Amasifuen, A.D.; Cortez-Lázaro, A.A.; Argüelles-Curaca, A.; Díaz-Pillasca, H.B. In vitro callogenesis of peach (Prunus persica L.) var. Huayco rojo from leaf explants. Ciencia Tecnologia Agropecuaria 2021, 23(1), e2032.
32. Zhang, M.; Wang, A.; Qin, M.; Qin, X.; Yang, S.; Su, S.; Sun, Y.; Zhang, L. Direct and Indirect Somatic Embryogenesis Induction in Camellia oleifera Abel. Front. Plant Sci. 2021, 12, 644389.
Received: October 23, 2022 / Accepted: January 15, 2023 / Published:15 February 2023
Citation: Pineda-Lázaro , A.J.; Hernández-Amasifuen , A.D.; Díaz-Pillasca , H.B. The efficient procedure of embryogenic callus formation from anther in Capsicum pubescens Ruiz & Pav. Revis Bionatura 2023;8 (1)29. http://dx.doi.org/10.21931/RB/2023.08.01.93
