2023.08.02.2
Files > Volume 8 > Vol 8 No 2 2023
Investigation of Crohn's Disease by Immunohistochemistry Technique in Iraqi Patients
1,2 Department of Biology, College of Science, University of Baghdad, Baghdad, Iraq.
3Gastroenterologist and Hepatologist, Gastrointestinal of the hospital, City of Medicine Baghdad, Iraq.
*Corresponding authors: Email: [email protected],
Available from: http://dx.doi.org/10.21931/RB/2023.08.02.2
Crohn's disease (CD) is one of the most common IBD types. CD necessitates an erratic immune response. Previous research has shown that inflammation of the intestines is elevated or continues due to inappropriate immune responses that result from the relationships between environmental factors, intestinal microbiota, and genetic factors. Induces intense transmural inflammation. This study aimed to investigate (i) CD detection by Histopathology and Immunohistochemistry (IHC) Markers that are Mycobacterium avium subspecies paratuberculosis MAP antibody and TWEAK/Fn14 antibody and their association with CD. (ii) Prove or disprove the hypothesis of MAP as a potential cause of CD. Tissue biopsies of 30 cases with a recognized diagnosis of CD and 20 cases as control presented without disease symptoms were collected. They are 20 males and 10 females for patients, and 13 males and 7 females for control with ages ranging from 9-55(±34.78) years. From 2019 - 2020, Biopsies were collected from Medical City Hospitals in Baghdad. One tissue section has been stained by the Hematoxylin & Eosin (H&E) for histopathology examinations. IHC stained the other two sections to the markers mentioned earlier in the IHC technique. The results of IHC for MAP showed a highly significant relationship in the ileal tissues of patients with disease CD with varying degrees according to the intensity of the immune reaction, which represents the intensity of the color, which is distributed between weak, moderate and strong, according to the (Aperio image Scope) program. Where it was 10% weak, 43.33% medium, and 46.67% strong. The P-value for patients vs. control was 0.0052 and 0.0001, respectively (P-value 0 ≤ 0.01). The result of IHC proves the hypothesis of MAP as a potential cause of CD. The other effects of IHC staining for TWEAK/Fn14 marker showed a highly significant relationship in the ileal tissues of patients with Crohn's Disease with varying degrees according to the intensity of the immune reaction, according to the Aperio image Scope program. It was 10% weak, 36.67% medium, and 53.33% strong. P-value for patients vs control were 0.0003 and 0.0001, respectively (P-value 0 ≤ 0.01).
Keywords: Inflammatory bowel disease, Crohn's disease, Mycobacterium avium subspecies paratuberculosis, tumor necrosis factor-like weak apoptosis inducer.
INTRODUCTION
CD can be defined as an inflammatory bowel disease (IBD) type that is quite common. The CD is a chronic, complicated condition primarily affecting the digestive system 1. This disease necessitates an erratic immune response, which results in severe inflammation. It primarily affects intestinal walls, particularly the ileum and portions of the colon, where inflated tissues thicken and swell, the inside becomes swollen, and ulceration may form on the digestive system's inner surfaces 2. CD usually appears between the ages of 10 and 20 but can occur at any age. Symptoms and signs reappear at various points in a person's life. The most typical symptoms of this disorder include persistent loss of appetite, diarrhea, stomachache, fever, and weight loss. As a result of inflamed bowel tissues, people with CD have blood in their stools; persistent bleeding can result in death. Sometimes, CD may as well cause inflammation in the eyes, ligaments and tendons, or skin 3,4. Intestinal obstruction is one of the common complications of CD. Obstructions in the intestinal walls can be caused by thickening or scar tissue buildup. Infected people frequently have fistulas or irregular contacts between intestines and other tissues. Fistulas form when the ulcers tear through the intestinal wall, and passages are formed between loops of intestines or between the intestine and the surrounding structures (like bladder, genitals, or skin) 5. Previous research has shown that inflammation of the intestines is elevated or persists due to associations between intestinal microbiota, external factors, and genetic factors, all contributing to IBD development.
Furthermore, the IBD prevalence and incidence vary from region to region and multiethnic 6,7. The CD is most prevalent in northern Europe, North America, and New Zealand, all of which are part of the Western developing world; it manifests between the ages of 15 and 60 and is more visible in cities than in rural areas. In 1990, Germany had the highest reported happening of IBD in the developed world, with 322 CD patients 322/ 100,000 people 8,9. And in North America, there are 319 / 100,000 people in the United States and Canada 10,9. Similarly, Europe and North America have the highest rates of IBD, though these percentages are stable or steadily decreasing (23.82 and 15.4 /100000 person-year for Crohn's Disease in Canada and Italy, respectively) 9. However, in Asia, particularly the Middle East, frequencies have steadily increased, reaching percentages of 5 / 100000 individual-year for Crohn's Disease 11. Northern Europeans and Jews of (Ashkenazi) origin have a high prevalence (3.2/1000), while South Americans, Africans, and Asians have a significantly unusual majority (10).
On the other hand, modern reports show a considerable increase in incidence in rapidly developing regions such as Australasia, Africa, and Asia 12. According to well-known data, the prevalence of CD in Saudi Arabia has been steadily increasing. According to 13, the prevalence and incidence of CD in Kuwait are rising at around the same rate as in European societies 14,15, and the prevalence of IBD appears to be rising in Egypt 16. Moreover, most CD in Iraq has increased over the last ten years 17. The histopathological examination of the patient's lesion begins with an intestinal crypt invasion. This causes ulceration to form first in the surface level of mucosa and then spread to the deeper layers. If the infection persists, non-caseating granulomas will develop in all intestinal wall layers. It may create a classic cobblestone mucosal appearance and miss lesions along the intestinal sparing areas' length with normal mucosa. When a Crohn's flare subsides, scarring substitutes the inflammatory intestine areas 18. Granuloma formation has been standard in CD; nonetheless, the absence of granuloma does not rule out the diagnosis. If the infection persists, non-caseating granulomas will develop in all intestinal wall layers. Continuous inflammation and scar tissue contribute to intestinal obstruction and fistula 19.
It is expected that new IBD-specific and responsive markers will be discovered. Such immunohistochemistry (IHC) markers could reduce utilizing the endoscopic and radiological assessments and encourage physicians to implement individualized strategies of treatment aimed at improving IBD patients' long-term prognosis 20. Anti-MAP antibodies were used to diagnose MAP and to demonstrate MAP involvement in CD pathogenesis, antibody titers to MAP-specific proteins increased in CD patients' sera 21. However, it is unknown whether MAP can invade humans 22. The most commonly used treatment methods for CD are steroids and monoclonal tumor necrosis factor (TNF) antibodies 23,24. TNF-like weak apoptosis inducer (TWEAK) is a TNF superfamily cytokine, promoting its impacts by binding to its specific receptor, fibroblast growth factor-inducible 14 (Fn-14) 25. TWEAK has been found in various cell types, including leukocytes, astrocytes, neurons 26,27, and several tumor cell lines. Fn-14 has been found at low levels in normal tissues. Some growth factors, however, are found in injured and unhealthy tissues 28. The intestines are one of the most commonly affected tissues by TWEAK/Fn14 during persistent inflammation 29. Following the breakdown of the mucosal barrier, continued activation of Fn14 on intestinal epithelial cells may contribute to excessive intestinal immunogenicity to commensal flora. Consequences include delayed regeneration, dysregulated tissue regeneration, and fibrosis 30. TWEAK/Fn14 activation aids tissue repair and regeneration, whereas extreme or persistent TWEAK/Fn-14 signals can result in severe chronic inflammation infiltration and tissue injury. In the current study, the Immunohistochemistry (IHC) technique is used to investigate both of the above immunological markers, which may increase the accuracy of the diagnosis and help doctors significantly track the stages of the disease. This study has aimed to ( i) investigate CD using Histopathology and Immunohistochemistry (IHC) Markers such as (MAP) antibody and (TWEAK/Fn14) antibody and their association with CD. (ii) Support or refute the hypothesis of MAP as a possible cause of CD.
The period from 2019 - 2020, t cases with proven diagnoses of CD based on typical clinical symptoms, endoscopic, imaging, and histopathological criteria. Patients who had resections and those taking biological treatments for CD and others were excluded from this study, and 20 cases as control presented without disease symptoms (apparently healthy individuals); biopsies were collected from Medical City Hospitals Gastro-Enterology and Hepatology Hospital in Baghdad. They are 20 male and 10 female patients and 13 males and 7 females for control with ages ranging from 9-55 (±34.87) years; one endoscopic tissue biopsy was collected. H&E stained one tissue section for histopathology examinations, and IHC stained the other two to (MAP) antibody and (TWEAK/Fn14) antibody.
Immunohistochemically labeling images that were analyzed with Aperio Image Scope Soft wear (Aperio version 12.3.3.5048 Scope software. This program measures the reaction intensity of the Anti- (MAP) marker and Anti TWEAKR/Fn14 antibody marker in terms of "positivity" for a quantitative amount of specific color in tissue section Fig.1. The default of this system was set to input parameter according to color intensity degree which ranged from brown color, orange color, and yellow color, blue or white color for strong positive, optimistic, weak positive and negative colors respectively, according to Leica BioSystems Imaging, instructions 31.

Figure 1. Reaction intensity of the Aperio Image Scope program
Images were opened in the Aperio Image Scope application by selecting the "Open Image" option. The areas of interest were chosen using the "Pen Tool" option. The specified area was then evaluated by selecting the "Analysis" option. By choosing the "Annotations" option, the number of strongly positive, positive, and weakly positive pixels was calculated.The positivity value has been estimated by dividing the total number of the positive pixels by the total number of the pixels (N Total - N n) / (N Total), where N Total represents the total number of the pixels, (N n) represents the total number of negative pixels, and (N Total - N n) represents the total number of positive pixels. In its analysis, Aperio Image Scope software used the positive pixel method.
Statistical analyses
Statistical Analysis System- SAS (2012) software has been utilized for the detection of the effects of the different factors in the parameters of the study. T-test has been utilized for significant comparison between the mean values. Chi (-square-square test has been used for multiple comparisons between the percentages (0.05 and 0.01 probability in the present work. The allelic frequency and equilibrium of the studied genes were extracted according to the Hardy-Weinberg equation within the SAS.
RESULTS AND DISCUSSION
Histologically, the normal ileum structure is mucosa, lined by simple columnar epithelium and containing Peyer's patches, lamina propria and muscularis mucosae, submucosa, containing blood vessels, lymph nodes and the Meissner's plexus muscular consisting of an inner circular and outer longitudinal muscle layers and serosa, consisting of a simple squamous epithelium.
Pathologic principles in CD mucosal biopsies, even if superficial, can reveal characteristics suggestive of CD in the untreated situation. Mixed inflammatory infiltration and underlying lymphoid aggregation in sections of the ileum show moderate mononuclear cells infiltration with the proliferation of fibrous connective tissue between mucosal glands and neutrophils infiltration in the epithelial layer in the lumen of these glands that lead to destruct their epithelial layer Fig 2. In the damaged regions, architectural distortion is widespread, while nearby crypts might appear normal. In adults, granulomas are rare, but when they occur, they are usually badly formed, non-caseated, non-necrotizing, and linked with lymphocytic inflammations (Figs 3, 4 and 12). Sections in the ileum show marked mononuclear cell infiltration in the thickness lamina propria with hyperplasia and epithelial ulceration of epithelial Fig 5. Areas in the ileum show granuloma in the lamina propria with complete loss of epithelial layer Fig 6. The Section in the ileum shows severe mononuclear cells infiltration with the proliferation of fibrous connective tissue between mucosal glands and neutrophil infiltration in the lumen of these glands that lead to destruct their epithelial layer which helps differentiate CD from severe ulcerative colitis (UC).
In muscularis propria and submucosa with complete loss of villi Fig 7. At the same time, the Section in the ileum shows inflammatory reactions reaching the fatty tissues and crypts that are histologically normal Fig 8. The section in ileum shows marked mononuclear cells infiltration in the submucosa but do not present in the muscular mucosa Fig 9. In the submucosa of the ileum, a significant infiltration of neutrophils and peyer's patches is clearly present in Fig 10. In other cases, the Section in the ileum shows neutrophils and mononuclear cells infiltration between mucosal glands with cellular debris in the lumen of these glands Fig 11. Also, the Section in ileum shows non-caseated granuloma in the mucosa, in addition to neutrophils infiltration Fig 12. The Section in ileum shows hyperplasia of lymphoid tissue which as lymph nodes in the mucosal layer, and transmural inflammation areas, such as transmural lymphoid clusters with villi atrophy Fig 13. Inflammation can vary significantly in a single biopsy and across many biopsy fragments from one anatomic site. and occasionally with proliferation of fibrous connective tissue Fig 14. Additionally, Sections in ileum show severe neutrophils infiltration in the submucosa that forms micro abscess Fig 15. Other sections in ileum show granuloma that consists of the aggregation of the active macrophages and lymphocytes in sub epithelial layer Fig 16. Ulcers are frequently longitudinally orientated, separated with the histologically normal edematous mucosa. Sinuses, fistulas, fissures, and extensive inflammations, might be apparent. Transmural inflammations occur away from the deep ulcers Fig 17. And Section in ileum shows non-caseated granuloma in the lamina propria and proliferation of fibrous connective tissue between mucosal glands Fig 18.
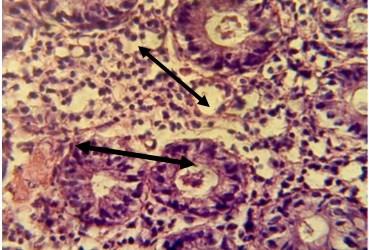
Figure 2. Section in ileum shows moderate mononuclear cell infiltration with proliferation of fibrous connective tissue between mucosal glands and neutrophils infiltration in the epithelial layer and in the lumen of these glands (H & E stain 40 x).
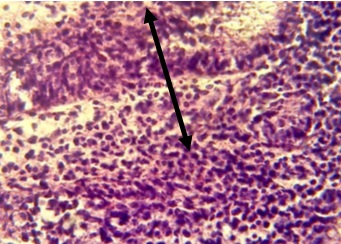
Figure 3. Sections in the ileum show granuloma in the lamina propria (H & E stains 40 x).
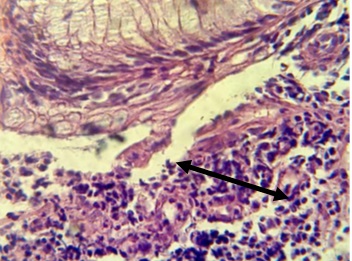
Figure 4. Section in ileum shows granuloma in the lamina propria in addition to neutrophils infiltration (H & E stains 40 x).
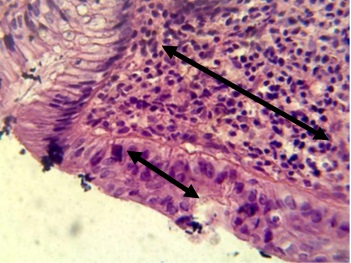
Figure 5. Section in ileum shows marked mononuclear cells infiltration in lamina propria with hyperplasia (H & E stains 40 x).
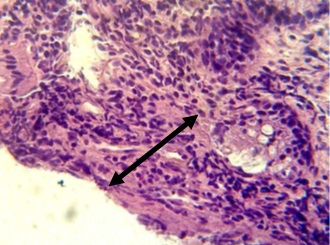
Figure 6, Section in ileum shows marked mononuclear cells infiltration in lamina propria with hyperplasia (H & E stains 40 x).
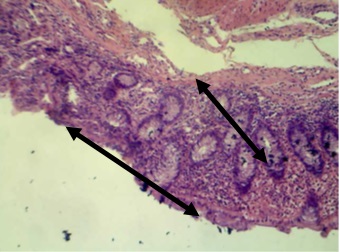
Figure 7. Section in ileum shows marked mononuclear cells infiltration in the mucosa with complete loss of villi (H & E stains 10 x).
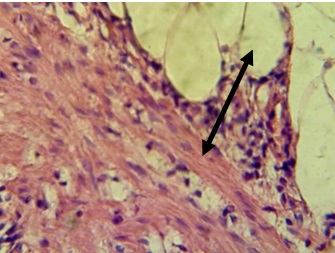
Figure 8. Section in ileum shows inflammatory reaction reach the adipose tissues (H & E stains 40 x).
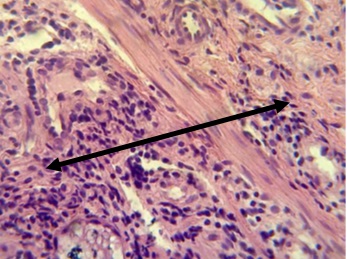
Figure 9. Section in ileum shows marked mononuclear cell infiltration in the submucosa (H & E stains 40 x).
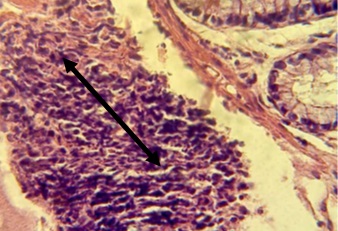
Figure 10. Section in ileum shows marked Payer's patches (H & E stains 40 x).
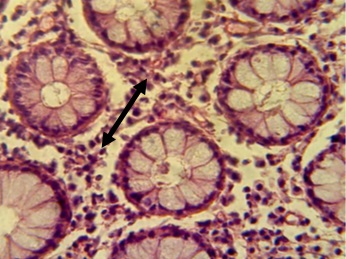
Figure 11. Section in ileum shows neutrophils and mononuclear cells infiltration between mucosal glands with cellular debris in the lumen of these glands (H & E stains 40 x).
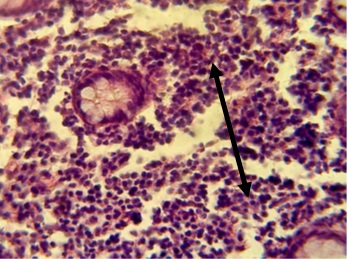
Figure 12. The Section in ileum shows granuloma in the mucosa and neutrophil infiltration (H & E stains 40 x).
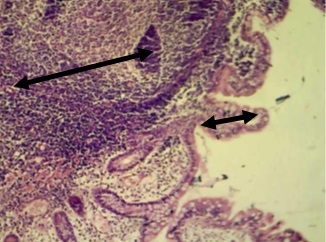
Figure 13. Section in ileum shows hyperplasia of lymphoid tissue in the mucosal layer and atrophy of villi (H & E stains 10 x).
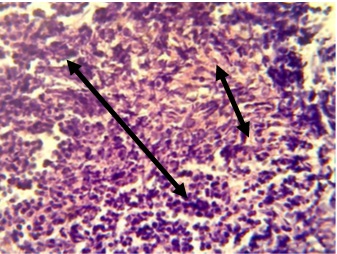
Figure 14. Section in ileum shows granuloma in the lamina propria in addition to neutrophils infiltration and proliferation of fibrous connective tissue (H & E stains 40 x).
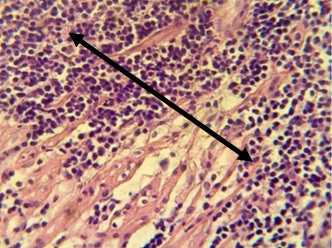
Figure 15. Section in ileum shows severe neutrophil infiltration in the submucosa, forming micro abscesses (H & E stains 40 x).
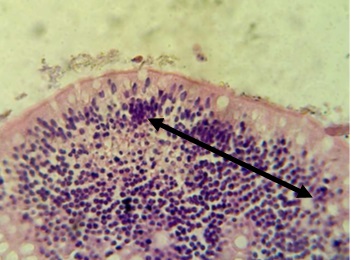
Figure 16. The Section in the ileum shows granuloma that consists of the aggregation of the active macrophages and lymphocytes in the subepithelial layer (H & E stains 40 x).
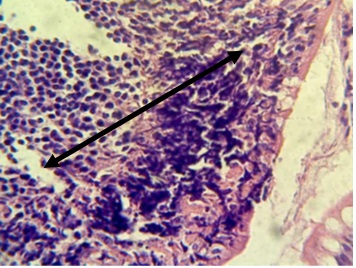
Figure 17. Section in ileum shows marked neutrophils and mononuclear cell infiltration in the lamina propria with ulceration of epithelial layer (H & E stains 40 x).
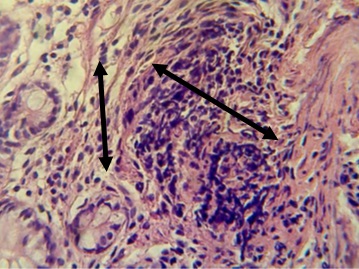
Figure 18. Section in ileum shows non-caseated granuloma in the lamina propria and proliferation of fibrous connective tissue between mucosal glands (H & E stains 40 x).
The results of immunohistochemistry staining for (MAP), showed a highly significant relationship in ileal tissues of patients with CD with varying degrees according to the intensity of the immune reaction, which represents the intensity of the color, which is distributed between weak, moderate and strong, according to the (Aperio image Scope) program. Where it was 10% weak, 43.33% medium, and 46.67% strong. P-values (control vs. patients) were 0.0052 and 0.0001 (P-value 0 ≤ 0.01) Table (1) Figs. (19, 20, and 21).
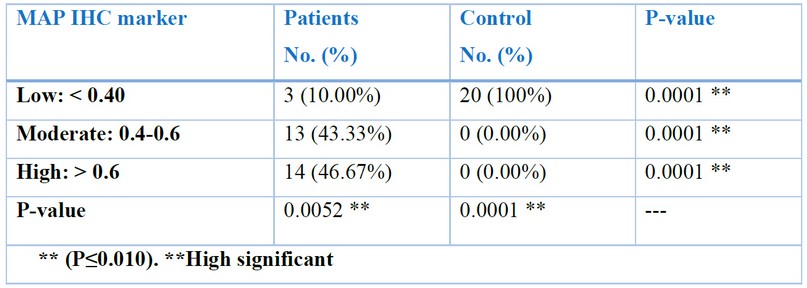
Table 1. Distribution of sample study according to MAP IHC marker in patients and control groups
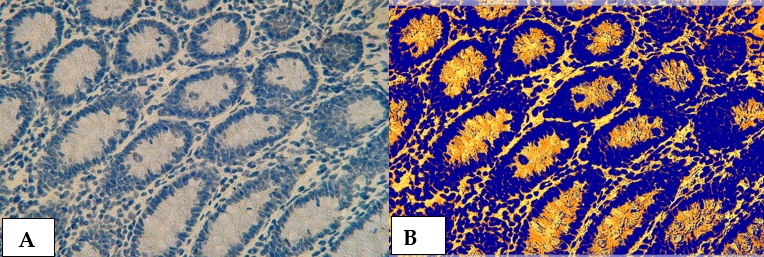
Figure 19. Cross section in ileum of CD patient with Anti-Mycobacterium tuberculosis antibody marker by IHC. Showing (A) Weak reaction 40X (B): Weak reaction analysis by Aperio Image Scope 40X
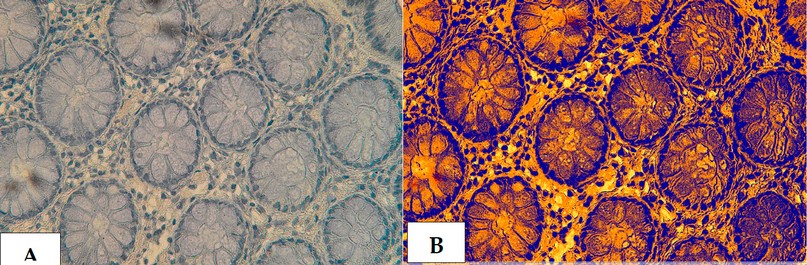
Figure 20. The cross section in the ileum of CD patient with Anti-Mycobacterium tuberculosis antibody marker by IHC. Showing (A) Moderate reaction 40X (B): Moderate reaction analysis by Aperio Image Scope 40X.
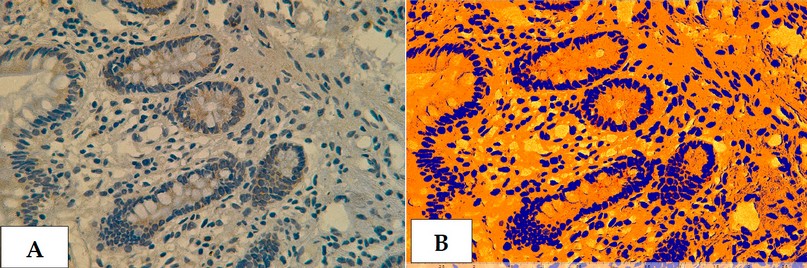
Figure 21. Cross section in ileum of CD patient with Anti-Mycobacterium tuberculosis antibody marker by IHC. Showing (A) Strong reaction 40X (B): Strong reaction analysis by Aperio Image Scope 40X.
The results of immunohistochemistry staining for (TWEAK/Fn14) , showed a highly significant relationshipin the ileal tissues of patients who have CD with varying degrees according to the intensity of the immune reaction, which represents the intensity of the color, which are distributed between weak, moderate and strong, according to the (Aperio image Scope) program. Where it was 10% weak, 36.67% medium, and 53.33% strong. P-value (patients vs control) were 0.0003 and 0.0001 (P-value 0 ≤ 0.01) Table (3-5) Figs. (22, 23 and 24).
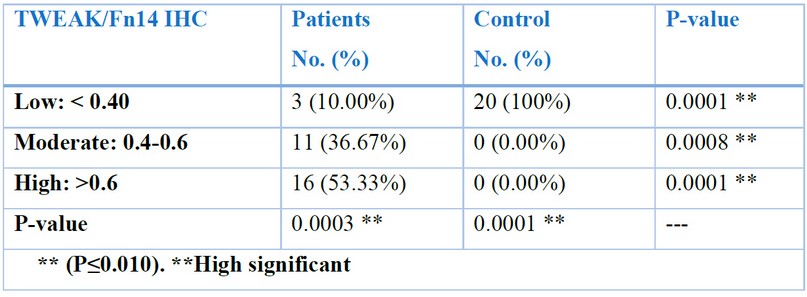
Table 2. Distribution of sample study according to (TWEAK/Fn14) IHC. Marker in patients and control groups .
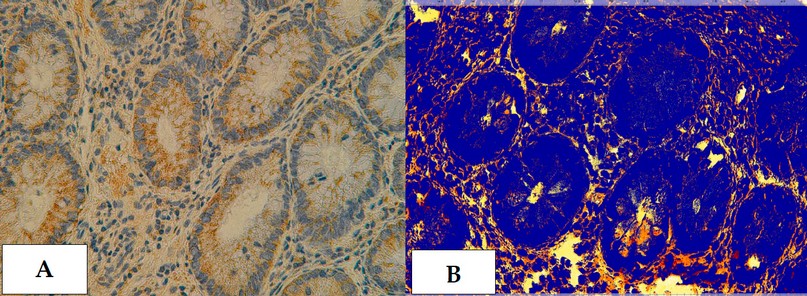
Figure 22. Cross section in ileum of CD patient with Anti-(TWEAK/Fn14) antibody marker by IHC. Showing (A) Weak reaction 40X (B): Weak reaction analysis by Aperio Image Scope 40X.
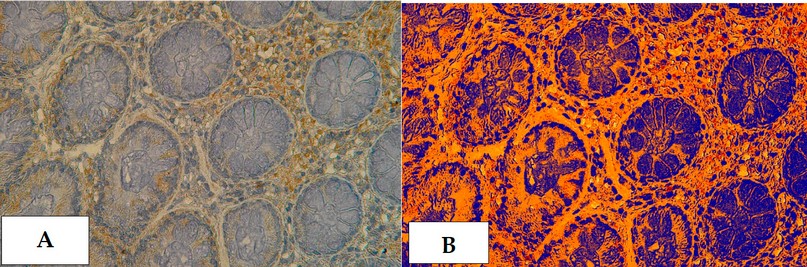
Figure 23. Cross section in ileum of CD patient with Anti-(TWEAK/Fn14) antibody marker by IHC. Showing (A) Moderate reaction 40X (B): Moderate reaction analysis by Aperio Image Scope 40X.
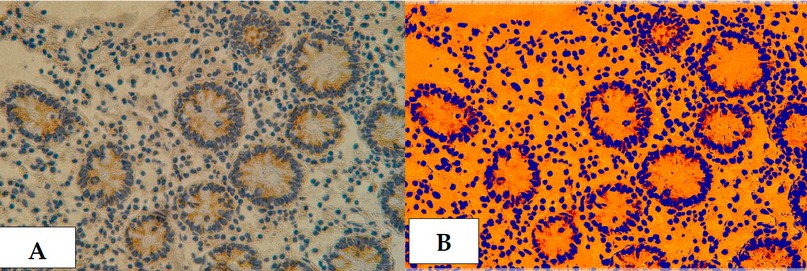
Figure 24 . Cross section in ileum of CD patient with Anti-(TWEAK/Fn14) antibody marker by IHC. Showing (A) Strong reaction 40X (B): Strong reaction analysis by Aperio Image Scope 40X.
Inflammatory bowel disease IBD is histologically diagnosed by looking at four key characteristics, which are: (a) mucosal design, (b) submucosal cellularity and lamina propria, (c) neutrophil granulocyte infiltration, (d) epithelial irregularities 32. Therefore, understanding the normal histology of the GI mucosa is essential for accurate analysis of biopsy results. I - Cryptographic architectural distortion refers to the morphologic aspects of mucosal architecture modifications. Normal crypts are straight and parallel, extending from the surface to just above muscularis mucosae. IBD crypt architectural distortions are well-defined by unevenly organized, dilated, branching, and/or truncated crypts. Which may be one of the symptoms of chronic inflammation or of renewal process 33. The crypts in the normal rectum, on the other hand, can have slight irregularities suggesting architectural deformation 34. II - Atypical lamina propria cellularity indicates a steadily increasing and diverse distribution of normally occurring types of cells. In the colorectal lamina propria, lymphocytes and plasma cells are typically observable. The proportion of inflammatory cells in normal colons ranges according to anatomical location. The cecum and right colon have been defined as the most cellular in overall, with a gradual decline in cellularity from right to left sides. Furthermore, there is a focally increased number of lymphoid cells near the lymphoid tissues of a typical gut-associated lymphoid tissue (GALT) 35. In healthy individuals, eosinophil granulocytes vary significantly 34, 35.
III - Neutrophil granulocyte infiltration characterizes the active disease. The neutrophils may be found in lamina propria or infect crypt surface epithelium and the lumen of the crypts, resulting in crypt abscesses. The neutrophils aren't commonly found in normal mucosa 36. IV- Epithelial anomalies that are seen in the IBD include depletion of the Mucin, surface epithelial destruction and metaplastic alterations. The reduction of the number of goblet cells or a reduction in intra-cellular mucin amounts has characterized the depletion of the Mucin. The Pyloric gland metaplasia (which is one of the common indicators of chronicity in the ileal Crohn's Disease involvement) and Paneth's cell metaplasia have been defined as metaplastic alteration examples. Surface epithelial damage, like the flattening, localized cell loss, erosions, and ulcers, indicate the activity of the disease. None of those signs have been definitively disease-specific, and they may happen in the UC, Crohn's Disease, or other types of colitis 36.
The histological results for IBD differ according to the disease's clinical stages as well as levels of inflammatory activities. The IBD has been categorized into "Chronic inactive," "Chronic active," or "Active" (chronicity symptoms are absent) 34. Histological features like crypt architectural deformations, widespread mixed lamina propria inflammation, crypt atrophy, basally located lymphoid clusters, basal plasmacytosis, and Paneth's cell metaplasia define chronicity (independent of "activity"). The presence of neutrophils confirms the presence of inflammatory activity. Neutrophilic cryptitis, bleeding, crypt abscesses, erosions, necrosis, and ulceration characterize active inflammation. The pathology report has to describe histological chronicity and activity aspects in detail and, if possible, grade the activity level as mild/moderate/severe. Grading has to begin with the most affected biopsy, and when possible, samples from different colon portions should be assigned a different grade. Because of the need for grading, many scoring systems or histology indices have been developed. There is no gold standard for activity grading, but the Nancy Index 37, which was endorsed by ECCO in 2020 as suitable for daily clinical grading of IBD, is one example 36. The Nancy Index has 5 grades that range from 0 to 4. A normal biopsy with no (or very little) chronic inflammations and no active inflammations has been referred to as a grade zero biopsy. The presence of chronic inflammations but no active inflammations has been indicated by a grade one (chronic inflammation). Only a few neutrophils are visible in grade two ("mild activity"), and no ulceration exists. The term "ulceration" in grade four refers to superficial epithelial ulceration 37.
Due to the disease's patchy inflammatory patterns, endoscopic Crohn's Disease biopsies may exhibit varying inflammation degrees in one biopsy and between biopsies from one segment of the bowel. This is why the presence of focal microscopic alterations like chronic inflammations and focal crypt architectural distortion near normal crypts may be utilized for the diagnosis of Crohn's Disease. The inflammatory infiltrations in Crohn's Disease could be transmural. Deep fissures, fibrosis and ulcers have resulted from the transmural lymphoid aggregates and inflammatory cells infiltrating submucosa and even lamina muscularis. Transmural alterations may only be characterized in the resection due to the fact that the biopsies are more superficial. The existence of the non-caseating epithelioid granulomas results in promoting the diagnosis of Crohn's Disease; however, only in the case where they're characterized with no associations with the ruptured crypts, and identification of the granuloma isn't required strictly for the establishment of the diagnosis 34. The epithelioid cell granuloma can be defined as epithelioid cell granuloma (i.e. the active histiocytes) with or with no multi-nucleated giant cells. Necrosis is not common in the granulomas of Crohn's Disease that are commonly ill-defined. In addition to that, the granulomas are more widespread in children compared to it in adults 38. The uneven villous architecture in the terminal ileum biopsies is one of the Crohn's Disease symptoms; however, when it's continuous with the proximal colitis, it may as well be a backwash ileitis marker in the UC. Pyloric gland metaplasia can occur and is seen in up to 25% of ileal biopsies from CD patients (and quite more often in the ileal samples after the surgical resections); however, only seldom in the patients who have the UC 33.
Histopathological evaluation is also useful in detecting early signs of dysplasia and preventing cancer growth. The histopathological examination report must include the results that are suggestive of chronic colitis (such as basal lymphoid aggregates, basal plasmacytosis, crypt architectural/atrophic changes, metaplasia and mucin depletion) in addition to disease activity indication based upon neutrophil infiltration extent (in the lamia propria, crypt abscesses and cryptitis) and the epithelial damages for the symbol (i.e., the erosions). Even though histological remissions are not (yet) utilized as one of the treatment targets in routine clinical practices, it may be helpful for physicians in making decisions in the case of the establishment of a diagnosis and selection of the treatment. For the optimum implementations of histologic index and assessments of histological remissions in clinical settings, consensus agreement involving the clinicians/endoscopists and pathologists is necessary. If possible, a standardized endoscopic biopsy process has to be developed so that the samples for the histological evaluation are consistent across patients and medical facilities.
Furthermore, agreeing on a specific scoring criterion would make comparing facilities easier. Recent findings in CD ileocecal resection specimens suggested that it can be advantageous to routinely report on active inflammations, plexitis, and granulomas in the margins of resection for advising the clinical follow-up post-surgery 39. The latest advancements in using the histologic indexes in clinical trials and their ability to assist as a predictive factor for guiding disease management even after diagnosis was established visibly prove that histopathology is still one of the significant factors in controlling the IBD 40.
The present statistical results of immunohistochemistry for the marker of Mycobacterium avium paratuberculosis (MAP), showed that there was a highly significant association with Crohn's patients according to the p-value that was 0.0052 and 0.0001(patients vs. control) (P-value 0 ≤ 0.01) table (1). And the results were distributed between weak, medium and strong, according to the program (Aperio image Scope). Where 10% were weak, 43.33% moderate, and 46.67% strong because of the severity of the infection, disease stage, taking antibiotics, immune-enhancing drugs and because of the severity of the infection as well as external environmental factors such as food, heat and sterilization conditions. This is consistent with the MAP Marker's relationship with CD 41. As the twenty-first century arrived, a growing body of parallel studies had characterized 2 main bacteria groups as candidates for gross inflammatory disease pathogenesis of intestines in Crohn's Disease. This is a typical intestinal flora group 42. An abnormal gut flora, like the adherent invasive E. coli (AIEC) 43. Furthermore, given the global spread of Crohn's Disease and its serious implications for public safety in addition to the cumulative individual suffering, scientists and clinicians in the research area must recognize that accurate evidence that has been obtained from every one of the 3 evidence forms is convergent and that there aren't any conflicts between them. Based on experimental and clinical evidence, there isn't any reason to suspect that bacteria from normal gut microbes community can infect and inflame the gut wall, and this occurs in Crohn's Disease.
Nonetheless, without another distinct activating factor, the unexpected emergence and increase of Crohn's Disease in human societies worldwide due to an epidemic of normal intestinal flora appear highly suspicious. The gut bacteria serves as a nursery for horizontal gene transfer 44. We've seen the pathological effects of this modification in common gut bacteria like E. coli that may be enterohaemorrhagic, enteropathogenic, enteroaggregative, enterotoxigenic, and, more lately, the entire adherent and infective AIEC 45. Those changes are typically the result of some sort of external selection pressure. Recent studies reveal that typical gut flora, like E. coli, may be significant predictors 46. MAP's status as a known multi-host inflammatory bowel pathogen identifies it from all other harmful microorganisms as the primary cause of Crohn's Disease. MAP has been shown in numerous animals, including primates, to have the unique capability for initiating and sustaining chronic inflammation of small bowel of various histological types. MAP infection causes both local and systemic immunological dysfunction in animals. In addition to that, it is neuropathogenic as well, particularly to the non-myelinated neurons, and small intestine disease has been linked to chronic enteric neuropathy 47. Despite its widespread pathogenicity, MAP infection in animals can last for years without inevitably progressing into clinical illness.
When tried-and-true methods were used, the majority of the individuals who have Crohn's Disease have been found infected with MAP 48. In layman's terms, most individuals with chronic inflammatory bowel disease (CD) have been infected with the Mycobacterium, one of the known IBD causes.
MAP infects the gut extensively in Crohn's Disease and can be found in the more normal-looking digestive tract as well as highly inflamed and infected parts of the intestine 49. Immunological responses of CD-4 T cell lines derived from patients' intestines appear to be dominated by MAP antigens 50. Bacterial intracellular death is inhibited by mannans produced by MAP 51.
MAP infection results in causing primary microscopic inflammation, specific immunological dysregulation and enteric neuropathy 49. Mucosal integrity, as well as other critical intestinal functions, are jeopardized. The visible components of gross chronic inflammation disease are caused by a dysregulated neuro-immune response to secondary gut flora penetration into the gut wall, including the normal intestinal bacteria and the ones that have undergone changes that lead to more invasive phenotypes, such as AIEC. Even though MAP has been discovered in human gut granulomas 47. The occurrence or lack of those and other characteristics of the CD's variable histopathological image is determined primarily by big-scale responses to secondary co-pathogens like MAP, which is commonly recovered in the culture from the Crohn's Disease tissues 52. As a result, the three current lines of research intersect in a two-tiered co-operative disease pathogenesis. The evidence for the link between MAP pathogens and CD is solid and effective and agrees with the previous investigation 41,53. The present study utilized immunohistochemistry, which reflects true and specific immune reactions between anti-MAP and MAP using the Aprio imaging Scope program. In conjunction with other approved methods, this type of diagnosis can be one of the most dependable methodologies for specific diagnostic CD.
This immunohistochemistry investigation results show that the expression of TWEAK and its receptor Fn14 has been elevated in enteritis or (inflammatory bowel disease) tissue, particularly Crohn's disease. TWEAK protein expression was significantly higher in the Crohn's disease group than in the seemingly healthy group, as demonstrated by rigorous statistical analysis based on Aperio scope images, Where it was 10% weak, 36.67% medium, and 53.33% strong. P-value (patients vs control) were 0.0003 and 0.0001 (P-value 0 ≤ 0.01), and these differences in the proportions between patients may be due to the different stage of the disease and its severity, or Marker-inhibiting drugs, the person's immune status, and external factors. According to TWEAK and Fn-14, expression typically increases in response to stresses, tissue damage, or remodeling 54. TWEAK has been expressed widely by monocytes, natural killer (NK) cells, and dendritic cells, with macrophages/monocytes being the main soluble TWEAK (sTWEAK) source in inflammatory body tissue 55. In the current study, Fn14 expression has been found to be much higher in Enteritis patients than in seemingly healthy and healthy tissues; in comparison, Fn14 expression was relatively weak in the control. However, it can be strongly induced in injured and sick tissues by a range of different growth factors 28. Fn14 can be found in many tissues, which include the colon, skin, small intestine, heart, brain, kidney, striated muscle, and pancreas 26, 27. TWEAK and Fn14 expression in healthy tissue is quite minimal. This is proven by the fact that the most frequently used CD treatments rely on steroids as well as the monoclonal antibodies against the TNF 24. Overexpression of TWEAK During chronic inflammation, the digestive system is one of the tissue types most affected by the TWEAK/Fn-14 29. Following mucosal barrier collapse continued Fn-14 stimulation on the intestinal epithelial cells may result in increased gut immune responses against the commensal flora. As a result, healing is slowing down, tissue repair is compromised, and fibrosis develops 56. Although TWEAK/Fn-14 has been shown to have similar effects in models of enteritis caused by recurrent acute damage, the role of TWEAK/Fn14 in a more immunologically driven inflammation is unknown. The current study supports previous findings that showed increased expressions of TWEAK and Fn-14 in the Crohn's Disease tissue lesions compared to healthy controls 57. Finally, to validate the current findings, we looked at the expressions of Fn-14 TWEAK in the ileal tissues of the IBD and non-IBD patients. TWEAK and Fn14 were significantly overexpressed during active inflammation in ileal biopsy specimens from CD patients compared to healthy humans. These findings support the theory that the TWEAK/Fn-14 signaling pathways have been activated as a defense mechanism during acute and chronic inflammation. However, over-activation in IBD could result in increased gut immune responses against intestinal flora. As a result, healing is slowed, tissue repair is compromised, and fibrosis develops 58, 59. Our findings differ from those of Kawashima and his team 55, TWEAK/Fn-14 levels have been higher in patients with active UC but not in CD patients or healthy controls for two reasons. First, only four CD patients have been included in the studies, compared to 30 in the current report. Second, the current studies primarily used small intestine tissue specimens, whereas previous studies only looked at colonic tissues. TWEAK/Fn-14 over-expression was discovered in tissue lesions obtained from patients with active CD, according to the current study. 56. To summarize, the current findings show significant TWEAK/Fn-14 overexpression in CD patients' small intestine tissue lesions, as well as a mechanistic role for Fn-14 signaling in CD pathogenesis. With the introduction of new TWEAK/Fn14-targeting medications 26.
CONCLUSIONS
The current findings support the notion that pharmacological inhibition of Fn-14 signaling can be one of the beneficial and feasible methods of preventing inflammatory responses in CD patients,
Funding: Self-Funding.
Acknowledgments:
Conflicts of Interest: The authors declare no conflict of interest.
1- Lightner AL, McKenna NP, Alsughayer A, Loftus Jr EV, Raffals LE, Faubion WA, Moir C. Anti-TNF biologic therapy does not increase postoperative morbidity in pediatric Crohn's patients. Journal of pediatric surgery. 2019; 54(10):2162-2165.
2- Marazuela GP, López-Jurado A, Vicente BA. Acute abdominal pain in patients with Crohn's disease: what urgent imaging tests should be done? Radiologia. 2019;61(4):333–336.
3- Aksan A, Farrag K, Stein J. An update on the evaluation and management of iron deficiency anemia in inflammatory bowel disease. Expert Rev Gastroenterol Hepatol . 2019;13(2):95–97.
4- Hwang JH, Yu CS. Depression and resilience in ulcerative colitis and Crohn's disease patients with ostomy. Int Wound J. 2019; 16 (Suppl 1):62–70
5- Khan S, Rupniewska E, Neighbors M, Singer D, Chiarappa J, Obando C. Real-world evidence on adherence, persistence, switching and dose escalation with biologics in adult inflammatory bowel disease in the United States: A systematic review. J Clin Pharm Ther. 2019; 44(4):495–507.
6- Thia KT, Loftus EV Jr, Sandborn WJ, Yang S-K. An update on the epidemiology of inflammatory bowel disease in Asia. Am J Gastroenterol . 2008;103(12):3167–3182.
7- de Souza HSP, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol . 2016;13(1):13–27.
8- Hein R, Köster I, Bollschweiler E, Schubert I. Prevalence of inflammatory bowel disease: estimates for 2010 and trends in Germany from a large insurance-based regional cohort. Scand J Gastroenterol. 2014;49(11):1325–1335
9- Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017; 390(10114):2769–2778.
10- Coward S, Clement F, Benchimol EI, Bernstein CN, Avina-Zubieta JA, Bitton A, Carroll MW, Hazlewood G, Jacobson K, Jelinski S, Deardon R. Past and future burden of inflammatory bowel diseases based on modeling of population-based data. Gastroenterology.2019;156(5):1345-1353.e4.
11- Molodecky NA, Soon S, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46-54.
12- Ghersin I, Khteeb N, Katz LH, Daher S, Shamir R, Assa A. Trends in the epidemiology of inflammatory bowel disease among Jewish Israeli adolescents: a population-based study. Aliment Pharmacol Ther . 2019;49(5):556–563.
13- Al-Nooh BM, Alaslani MH, Almaghamsi A, Basehi M, Mufti F. Crohn's Disease Prevalence and Causes among Saudi Arabia Population. Int J Med Res Prof. 2018; 4(1):254–257.
14- Siddique I, Alazmi W, Al-Ali J, Al-Fadli A, Alateeqi N, Memon A, Hasan F. Clinical epidemiology of Crohn's disease in Arabs based on the Montreal Classification. Inflamm Bowel Dis. 2012;18(9):1689–1697.
15- Siddique I, Alazmi W, Al-Ali J, Longenecker JC, Al-Fadli A, Hasan F, Memon A. Demography and clinical course of ulcerative colitis in Arabs–a study based on the Montreal classification. Scandinavian Journal of Gastroenterology. 2014 ; 49(12):1432-1440.
16- Esmat S, El Nady M, Elfekki M, Elsherif Y, Naga M. Epidemiological and clinical characteristics of inflammatory bowel diseases in Cairo, Egypt. World J Gastroenterol .2014; 20(3):814–821.
17- Hamasur KS. Prevalence of Oral Manifestations of Inflammatory Bowel Disease in Patients Admitted to Sulaymaniyah teaching hospital – Iraq. Al-Kindy Col Med J . 2020;16(1):47–53.
18- Greuter T, Piller A, Fournier N, Safroneeva E, Straumann A, Biedermann L, Godat S, Nydegger A, Scharl M, Rogler G, Vavricka SR. Upper gastrointestinal tract involvement in Crohn's disease: frequency, risk factors, and disease course. Journal of Crohn's and Colitis. 2018 ;12(12):1399-1409.
19- Brennan GT, Melton SD, Spechler SJ, Feagins LA. Clinical implications of his- tologic abnormalities in ileocolonic biopsies of patients with Crohn's disease in remission. J Clin Gastroenterol. 2017;51(1):43-e48.
20- Mitsuyama K, Niwa M, Takedatsu H, Yamasaki H, Kuwaki K, Yoshioka S, Yamauchi R, Fukunaga S, Torimura T. Antibody markers in the diagnosis of inflammatory bowel disease. World J Gastroenterol [Internet]. 2016;22(3):1304–1310.
21- Nakase H, Nishio A, Tamaki H, Matsuura M, Asada M, Chiba T, Okazaki K. Specific antibodies against recombinant protein of insertion element 900 of Mycobacterium avium subspecies paratuberculosis in Japanese patients with Crohn's disease. Inflammatory bowel diseases. 2006 ;12(1):62-69.
22- Biet F, Gendt L, Anton E, Ballot E, Hugot J-P, Johanet C. Serum antibodies to Mycobacterium avium subspecies paratuberculosis combined with anti-Saccharomyces cerevisiae antibodies in Crohn's disease patients: prevalence and diagnostic role. Dig Dis Sci .2011; 56(6):1794–1800.
23- Feldman P, Wolfson, D. and Barkin, J. Medical management of Crohn's disease. Clin Colon Rectal Surg.2007; 20:269–281.
24- Lirhus SS, Hoivik ML, Moum B, Melberg HO. Regional differences in anti-TNF-alpha therapy and surgery in the treatment of inflammatory bowel disease patients: a Norwegian nationwide cohort study. Scand J Gastro- enterol. 2018; 53:952–957.
25- Burkly LC. TWEAK/Fn14 axis: the current paradigm of tissue injury-inducible function in the midst of complex- ities. Semin Immunol. 2014; 26:229–236.
26- Wajant H. The TWEAK-Fn14 system as a potential drug target: TWEAK/Fn14 targeting. Br J Pharmacol .2013; 170(4):748–764.
27- Chen J, Wei L, Xia Y. Roles of tumour necrosis factor-related weak inducer of apoptosis/fibroblast growth factor-inducible 14 pathway in lupus nephritis: A TWEAK/Fn14 pathway in lupus nephritis. Nephrology (Carlton). 2017; 22(2):101–106.
28- Burkly LC, Michaelson JS, Hahm K, Jakubowski A, Zheng TS. TWEAKing tissue remodeling by a multifunctional cytokine: role of TWEAK/Fn14 pathway in health and disease. Cytokine .2007;40(1):1–16.
29- Dohi T, Borodovsky A, Wu P, Shearstone JR, Kawashima R, Runkel L, Rajman L, Dong X, Scott ML, Michaelson JS, Jakubowski A. TWEAK/Fn14 pathway: a nonredundant role in intestinal damage in mice through a TWEAK/intestinal epithelial cell axis. Gastroenterology .2009;136(3):912–923.
30- Kawashima R, Kawamura YI, Oshio T, Son A, Yamazaki M, Hagiwara T, Okada T, Inagaki–Ohara K, Wu P, Szak S, Kawamura YJ.Interleukin-13 damages intestinal mucosa via TWEAK and Fn14 in mice-a pathway associated with ulcerative colitis. Gastroenterology .2011; 141(6):2119-2129.e8.
31- Firas, Riyadh & Muhammad, & Abdulammer, Hayder & Abdul-Ameer, Hayder. Immunohistochemical and morphometric study of early alveolar bone regeneration using Anti-Periostin antibody and xenograft in rabbit.2020.
32- Magro F, Langner C, Driessen A, Ensari AR, Geboes K, Mantzaris GJ, Villanacci V, Becheanu G, Nunes PB, Cathomas G, Fries W. European Society of Pathology (ESP), & European Crohn's and Colitis Organisation (ECCO) (2013). Journal of Crohn's & colitis. 7(10):827–851.
33- Langner C, Magro F, Driessen A, Ensari A, Mantzaris GJ, Villanacci V, Becheanu G, Borralho Nunes P, Cathomas G, Fries W, Jouret-Mourin A. The histopathological approach to inflammatory bowel disease: a practice guide. Virchows Archiv. 2014; 464:511-527.
34- Fenton TM, Jørgensen PB, Niss K, Rubin SJ, Mörbe UM, Riis LB, Da Silva C, Plumb A, Vandamme J, Jakobsen HL, Brunak S. Immune Profiling of Human Gut-Associated Lymphoid Tissue Identifies a Role for Isolated Lymphoid Follicles in Priming of Region-Specific Immunity. Immunity. 2020. 17(3):557-570.
35- Fenton TM, Jørgensen PB, Niss K, Rubin SJ, Mörbe UM, Riis LB, Da Silva C, Plumb A, Vandamme J, Jakobsen HL, Brunak S. Immune profiling of human gut-associated lymphoid tissue identifies a role for isolated lymphoid follicles in priming of region-specific immunity. Immunity. 2020 Mar 17;52(3):557-70.
36- Magro F, Doherty G, Peyrin-Biroulet L, Svrcek M, Borralho P, Walsh A, et al. ECCO position paper: Harmonization of the approach to ulcerative colitis histopathology. J Crohns Colitis. 2020; 14(11):1503–1511.
37- Marchal-Bressenot A, Scherl A, Salleron J, Peyrin-Biroulet L. A practical guide to assess the Nancy histological index for UC. Gut . 2016;65(11):1919.2-1920.
38- Hong SW, Yoon H, Shin CM, Park YS, Kim N, Lee DH, Kim JS. Clinical significance of granulomas in Crohn's disease: A systematic review and meta‐analysis. Journal of Gastroenterology and Hepatology. 2020; 35(3):364-373.
39- Ananthakrishnan AN, Deshpande V. It is all in the fine print: A call for a histopathology checklist for IBD. Clin Gastroenterol Hepatol . 2021;19(3):446–447.
40- Kellermann L, Riis LB. A close view on histopathological changes in inflammatory bowel disease, a narrative review. Dig Med Res. 2021; 4:3–3.
41- Xia A, Stempak JM, Grist J, Bressler B, Silverberg MS, Bach H. Effect of inflammatory bowel disease therapies on immunogenicity of Mycobacterium paratuberculosis proteins. Scand J Gastroenterol .2014;49(2):157–163.
42- Martinez-Medina M, Aldeguer X, Lopez-Siles M, González-Huix F, López-Oliu C, Dahbi G, Blanco JE, Blanco J, Garcia-Gil JL, Darfeuille-Michaud A. Molecular diversity of Escherichia coli in the human gut: new ecological evidence supporting the role of adherent-invasive E. coli (AIEC) in Crohn's disease. Inflammatory bowel diseases. 2009 ;15(6):872-882.
43- Kurokawa K, Itoh T, Kuwahara T, Oshima K, Toh H, Toyoda A, Takami H, Morita H, Sharma VK, Srivastava TP, Taylor TD. Comparative metagenomics revealed commonly enriched gene sets in human gut microbiomes. Dna Research. 2007 ;14(4):169-181.
44- Darfeuille-Michaud A, Neut C, Barnich N, Lederman E, Di Martino P, Desreumaux P, Gambiez L, Joly B, Cortot A, Colombel JF. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn's disease. Gastroenterology. 1998 ;115(6):1405-1413.
45- Tagkopoulos I, Liu YC, Tavazoie S. Predictive behaviour within microbial genetic networks. Science. 2008;320:1313–137.
46- Zamani S, Zali MR, Aghdaei HA, Sechi LA, Niegowska M, Caggiu E, Keshavarz R, Mosavari N, Feizabadi MM. Mycobacterium avium subsp. paratuberculosis and associated risk factors for inflammatory bowel disease in Iranian patients. Gut pathogens. 2017 ;9:1-10.
47- Scanu AM, Bull TJ, Cannas S, Sanderson JD, Sechi LA, Dettori G, Zanetti S, Hermon-Taylor J. Mycobacterium avium subspecies paratuberculosis infection in cases of irritable bowel syndrome and comparison with Crohn's disease and Johne's disease: common neural and immune pathogenicities. Journal of clinical microbiology. 2007 ;45(12):3883-3890.
48- Olsen I, Lundin KE, Sollid LM. Increased frequency of intestinal CD4+ T cells reactive with mycobacteria in patients with Crohn's disease. Scandinavian journal of gastroenterology. 2013;48(11):1278-85.
49- Mpofu CM, Campbell BJ, Subramanian S, Marshall–Clarke S, Hart CA, Cross A, Roberts CL, McGoldrick A, Edwards SW, Rhodes JM. Microbial mannan inhibits bacterial killing by macrophages: a possible pathogenic mechanism for Crohn's disease. Gastroenterology. 2007;133(5):1487-1498.
50- Bull TJ, McMinn EJ, Sidi-Boumedine K, Skull A, Durkin D, Neild P, Rhodes G, Pickup R, Hermon-Taylor J. Detection and verification of Mycobacterium avium subsp. paratuberculosis in fresh ileocolonic mucosal biopsy specimens from individuals with and without Crohn's disease. Journal of clinical microbiology. 2003 ;41(7):2915-2923.
51- Shanahan F, O'Mahony J. The mycobacteria story in Crohn's disease. Am J Gastroenterol. 2005;100(7):1537–1538.
52- Cheng H, Xu M, Liu X, Zou X, Zhan N, Xia Y. TWEAK/Fn14 activation induces keratinocyte proliferation under psoriatic inflammation. Exp Dermatol. 2016; 25(1):32–37.
53- Liu Y, Peng L, Li L, Liu C, Hu X, Xiao S, et al. TWEAK/Fn14 activation contributes to the pathogenesis of bullous pemphigoid. J Invest Dermatol . 2017; 137(7):1512–1522.
54- Kawashima R, Kawamura YI, Oshio T, Son A, Yamazaki M, Hagiwara T, Okada T, Inagaki–Ohara K, Wu P, Szak S, Kawamura YJ. Interleukin-13 damages intestinal mucosa via TWEAK and Fn14 in mice—a pathway associated with ulcerative colitis. Gastroenterology. 2011; 141(6):2119-2129.
55- Di Martino L, Osme A, Kossak-Gupta S, Pizarro TT, Cominelli F. TWEAK/Fn14 is overexpressed in Crohn's disease and mediates experimental ileitis by regulating critical innate and adaptive immune pathways. Cellular and Molecular Gastroenterology and Hepatology. 2019;8(3):427-446.
56- Rieder F, Brenmoehl J, Leeb S, Schölmerich J, Rogler G. Wound healing and fibrosis in intestinal disease. Gut. 2007; 56(1):130–139.
57- Rogler G, Hausmann M. Factor's promoting development of fibrosis in Crohn's disease. Front Med (Lausanne) .2017; 4:96.
Received: 26 December 2022 / Accepted: 15 March 2023 / Published:15 June 2023
Citation: Sameer A F, Barraj A H, Mahmood H J. Investigation of Crohn's Disease by Immunohistochemistry Technique in Iraqi Patients. Revis Bionatura 2023;8 (2) 2. http://dx.doi.org/10.21931/RB/2023.08.02.2
