2022.07.01.15
Files > Volume 7 > Vol 7 No 1 2022
Antibacterial and cytotoxic evaluation of sequential extract of Moringa oleifera leaves
1 Department of Pharmacology and Toxicology, Faculty of Biosciences, University of Veterinary and Animal Sciences Lahore, Pakistan; [email protected];
2 Cell and Molecular Biology Lab, Institute of Zoology, University of the Punjab, Lahore, Pakistan; [email protected]
3 Department of Zoology, University of Narowal, Narowal-Pakistan.
* Correspondence: [email protected]; +92321-4575910
Available from: http://dx.doi.org/10.21931/RB/2022.07.01.15
ABSTRACT
Moringa Oleifera is an interesting plant used in Asian traditional medicine. In this study, in vitro antibacterial and cytotoxic evaluation of sequential extracts (aqueous, ethanol, and chloroform) of Moringa Oleifera was carried out. The antibacterial analysis was estimated with minimum inhibitory concentration (MIC) by micro dilution of Moringa Oleifera against common poultry pathogens Clostridium perfringens type A and Escherichia coli, while cytotoxic evaluation was estimated by the reduction in cell viability due to apoptosis or necrosis by metabolic events in the presence and absence of crude extracts or tested component. The aqueous extract shows the highest percentage yield (45/50gm) succeeded by ethanol extract (5.5gm/50gm) and chloroform extract (0.2gm/50gm). In our study, the zone of inhibition of sequential extracts of Moringa Oleifera against Haemophilus species are highest for chloroform (17mm), intermediate for ethanol (13mm), and lowest for aqueous extract (12.3mm). For chloroform extract the CSP was calculated at 10 different concentrations, 2000 µg/ml, 1000µg/ml, 500µg/ml, 250µg/ml, 125µg/ml, 62.5µg/ml, 31.25µg/ml, 15.63µg/ml, 7.81µg/ml and 3.91µg/ml .The results for cell survival percentage (CSP) in the present research are 26%, 46%, 58%, 55%, 60%, 63%, 62%, 59%, 68% and 82% respectively. The CSP results of chloroform extract indicated that it is toxic for cells at ≥1000µg/ml. At 1000µg/ml concentration CSP was 46% which is > 50% and therefore it is cytotoxic. At higher concentrations, chloroform is more cytotoxic than hexane because at > 1000µg/ml the cell survival percentage was recorded to be < 50%. For ethanol extract CSP was calculated at 10 concentrations, 6000µg/ml, 3000µg/ml, 1500µg/ml, 750µg/ml, 375µg/ml, 187.5µg/ml, 93.75µg/ml, 46.85µg/ml, 23.43µg/ml and 11.71µg/ml. The CSP values are 18%, 48%, 60%, 58%, 69%, 56%, 59%, 74%, 57% and 78% respectively which indicate that at concentrations ≥3000µg/ml the chloroform extract is toxic for cells. At a concentration less than 3000µg/ml, the CSP is more than 50%. So, as compared to hexane and chloroform, ethanol extract is less toxic at higher concentrations. Cytotoxicity of aqueous extract was calculated at 10 concentrations, 5000µg/ml, 2500µg/ml, 1250µg/ml, 625µg/ml, 312.5µg/ml, 156.25µg/ml, 78.125µg/ml, 39.06µg/ml, 19.53µg/ml and 9.76µg/ml. The CSP values are 8%, 18%, 42%, 56%, 54%, 59%, 55%, 62% 59% and 66% respectively. At a concentration ≥625µg/ml, the aqueous extract is toxic for cells. The CSP at 625µg/ml is 42%, hence toxic for cells. The cell survival percentage is more than 50% at a concentration > 625µg/ml, indicating that aqueous extract is more toxic to the cell than the rest of the three (Hexane, Chloroform, Ethanol extracts) at higher concentrations.
Keywords. Moringa oleifera; poultry pathogens; minimum inhibitory concentration; antibacterial; cytotoxicity; cell survival percentage.
INTRODUCTION
"The tree of life" named Moringa oleifera, is regarded as one of the most valuable and useful trees because its all parts can be used for medication and food,1,2. The leaves of Moringa are eaten either cooked or fresh in salads. Leaves in the form of dried powder can be stored without losing nutritional value for months. However, it is essential to screen Moringa oleifera for antimicrobial properties and investigate its sanitizing /preservative potential,3. In developing countries, untreated water-born life-threatening infections are increasing,4. World Health Organization (WHO) has warned that microbial resistance to typical water treatment mechanisms is increasing, and medicinal plants offer a good source of choice. The assessment of all the drugs is based on phytochemical and pharmacological approaches, which lead to the drug discovery referred to as natural product screening,5. Antibacterial resistance is a global problem; strategies to improve the current situation include controlling infections and minimizing the incidence of bacterial resistance by making new findings and innovations in antibacterial antibiotics and chemotherapeutic agents,6. Almost 20% of the plants found in the world have been investigated through pharmacological or biological tests, and a considerable number of new antibiotics are synthesized using natural or semi-synthetic resources,7. Due to the high cost of antibacterial medicines in developing countries like Pakistan, a significant portion of the population uses medicinal plants as medicaments against infectious diseases.
MATERIALS AND METHODS
All experimental work was performed at the Department of Pharmacology and Toxicology, UVAS, Lahore. The fresh mature leaves of Moringa Oleifera were shadow dried at room temperature. Leaves were turned in powder form and stored in a dry place. Soxhlet apparatus as done by,8 with slight modification was used to collect the sequential extracts from dried leaves powder of Moringa Oleifera and dried through the rotary evaporator. For further analysis, sequential extracts were used to estimate the antibacterial activity. Cytotoxicity was conducted by colorimetric analysis, MTT (methyl thiazole tetrazolium) assay in vitro.
Antibacterial activity
identification based on Biochemical tests
All the selected microorganisms, Staphylococcus aureus, Escherichia coli, Salmonella enterica, Clostridium perfringens type A, Haemophilus spp., were biochemically characterized by a series of biochemical tests performed for Gram reaction, catalase test, carbohydrate (lactate) fermentation, IMViC tests (Indole test, Methyl Red test, Citrate utilization test, Voges–Proskauer test) and oxidase test as per the standard methods reported in The "Bergey's Manual of Determinative Bacteriology’’,9.
2.1.2. Antimicrobial susceptibility testing (AST) by Agar well diffusion method
Plant extract containing antimicrobial constituents is allowed to soak or diffuse properly in the agar medium on a plate with fresh growth of test microorganisms. A zone of inhibitions is produced circularly due to inhibition of the confluent lawn of growth. The zone of inhibition's diameter is measured in mm.
Minimum inhibitory concentration (MIC) calculation by Micro dilution method
96-well plates were used and labeled for every three isolates of a microorganism and their crude extracts. 100µl MHB media was introduced to each well up to 12th well by sterile micropipette. 100µl of each plant extract was added to the first well, mixed properly, and then serially two-fold diluted up to 10th well by taking 100µl from first well and shifted to second well then took a sample from the second well and shifted to third well and so on. The sample taken from the 10th well was discarded at the end. Now, 100µl of suspension inoculum was introduced up to the 11th well. Standardized inoculum and broth media presence in the 11th well was considered positive control while only broth media presence in the 12th well was considered a negative control.
The final volume in each well was 200µl, except in the 12th well, which has 100µl. The plates were wrapped, labeled, and incubated for 24 hours at 37°C. The plate's optical density (OD value) was measured using an ELISA reader at 595nm wavelength. Note the MIC endpoints as the lowest concentration of crude extract where no visible growth was shown,10. The experiment was performed in triplicate, and the results were noted in mean ± standard deviation format. The data was analyzed statistically by using one-way ANOVA, and the treatment means were compared by Duncan's Multiple Range (DMR) posthoc test at significance level P ≤0.05 (Dzomba and Muchanyereyi 2012),11
The final volume in each well was 200µl, except in the 12th well, which has 100µl. The plates were wrapped, labeled, and incubated for 24 hours at 37°C. The plate's optical density (OD value) was measured using an ELISA reader at 595nm wavelength. Note the MIC endpoints as the lowest concentration of crude extract where no visible growth was shown,10. The experiment was performed in triplicate, and the results were noted in mean ± standard deviation format. The data was analyzed statistically by using one-way ANOVA, and the treatment means were compared by Duncan's Multiple Range (DMR) posthoc test at significance level P ≤0.05 (Dzomba and Muchanyereyi 2012),11
Cytotoxic evaluation
Preparation of MTT dye
In a sterile test tube, 50 mg of MTT dye was taken. 0.22 µm syringe was used to filter water and then added to test tube containing MTT dye. The prepared MTT dye was stored at 4°C for further use
Vero cell line
The Vero cell line was taken from WTO-QOL (Quality Operation Laboratory) UVAS, Lahore. Lahore. For future use in research, it was cryopreserved by a method described by Day and Stacey, 2007,12
Cell line revival
The cryovial labeled Vero cells were removed from the liquid nitrogen container. Cells were decontaminated with 70% isopropyl alcohol and then thawed at 37°C in a water bath. The Vero cells suspension was shifted to a centrifuge tube containing cell culture media from cryovial. After centrifugation, the supernatant was discarded to remove DMSO, and pellets of Vero cells were resuspended by adding 5ml of cell culture media (M-199). The Vero cells were transferred to the Carrel cell flask for further use.
Vero cell's seeding in 96-well cell culture plate
Vero cells suspension (100 µl) was seeded in 96 well plates in a safety cabinet aseptically. The plates seeded with Vero cells were incubated in 5% CO2 for 72 hours at 37ºC, and an inverted microscope for a complete monolayer was used to monitor cell plates.
Cell survival percentage
Calculation of cell survival percentage:
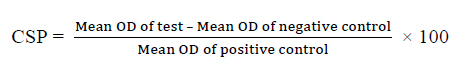
Statistical analysis
The parameters analyzed in all experiments were subjected to SPSS for windows version 16, SPSS inc, Chicago, IL, USA). One-way ANOVA analyzed the zone of inhibition and minimum inhibitory concentrations and then the means were compared with Duncan's Multiple Range (DMR) posthoc test at significance level P ≤0.05,13.
RESULTS
Selected bacteria identification
Staphylococcus aureus (S. aureus), Salmonella enterica (S. enterica), Escherichia coli (E. coli), Clostridium perfringens (C. perfringens) type A, and Haemophilus species (isolated from poultry) were collected from the Microbiology department of UVAS, Lahore. These bacteria were cultured. Microscopic evaluation and biochemical characterization were performed to confirm these bacteria. The summary of the biochemical test is shown in Table 1.
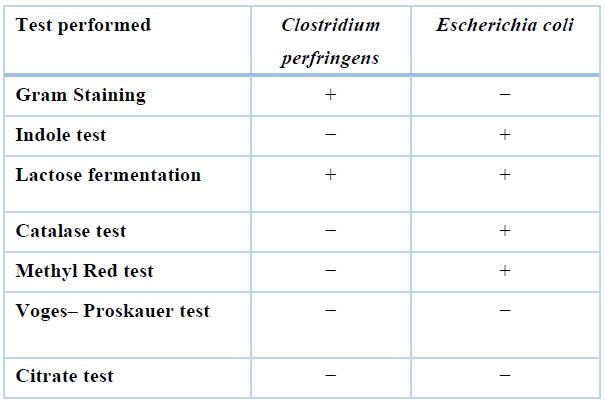
Table 1. Summary of biochemical test performed.
Minimum inhibitory concentration (MIC) values
Antibacterial activity of Moringa oleifera sequential leaves and root extracts against common poultry pathogens was evaluated using the MIC method. Minimum inhibitory concentrations of Clostridium perfringens and Escherichia coli were measured) and results are summarized in Tables 2 and 3, respectively. Means minimum inhibitory concentration of all extracts (chloroform, ethanol, and aqueous) differ non-significantly, as showed by ANOVA and Duncan's test. Minimum inhibitory concentration values were recorded highest for aqueous (5000µg/mL), while lowest for hexane (1250µg/mL), chloroform (1250µg/mL) and ethanol (1250µg/mL). So, hexane, chloroform, and ethanol were active at their respective lowest concentrations.
Cytotoxicity assay
Assay for cytotoxicity was performed by MTT assay using Vero cell lines for sequential extracts (Hexane, Chloroform, Ethanol, and Aqueous) of Moringa oleifera roots. Every extract was evaluated using different concentrations. Cell survival percentage and results were indicated as mean optical density ± standard deviation. CSP was calculated with the help of the optical density of each extract tested.
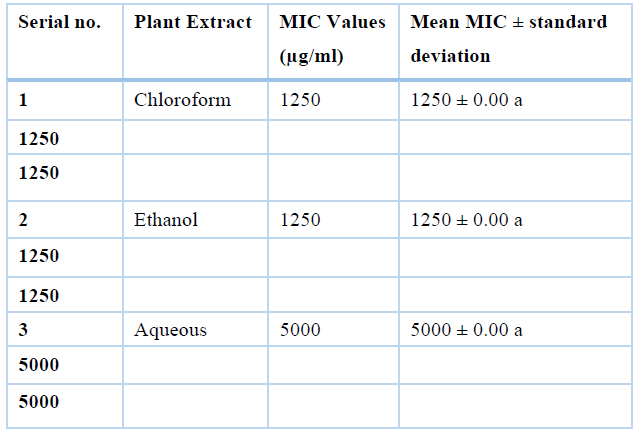
Table 2. Values of MIC for sequential extracts (Chloroform, Ethanol and Aqueous) of Moringa oleifera leaves against Clostridium perfringens isolates (n=3)
This means having the same superscripts differ non-significantly and with different superscripts differ significantly.
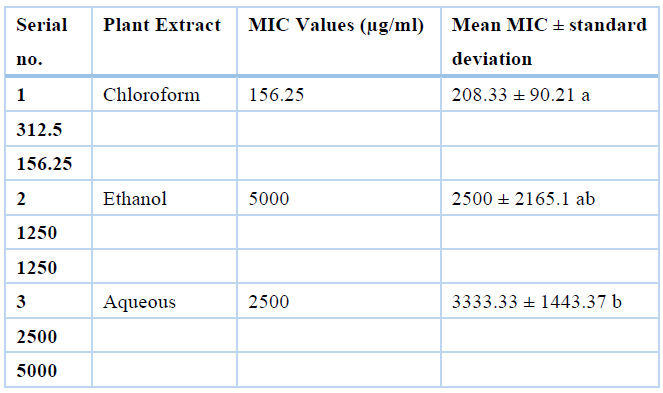
Table 3. Values of MIC for sequential extracts (Chloroform, Ethanol and Aqueous) of Moringa oleifera leaves against Escherichia coli isolates (n=3)
This means having the same superscripts differ non-significantly and with different superscripts differ significantly.
Chloroform extract was safe at concentrations of 500µg/ml, 250µg/ml, 125µg/ml, 62.5µg/ml, 31.25µg/ml, 15.63µg/ml, 7,.81µg/ml and 3.91µg/ml where cell survival percentage was more than 50% and the values are 58%, 55%, 60%, 63%, 62%, 59%, 68% and 82% respectively while it was toxic at the concentrations of 2000 µg/ml, 1000µg/ml where cell survival percentage was less than 50% i.e., 26% and 46% respectively, and results are summarized in Table 4.
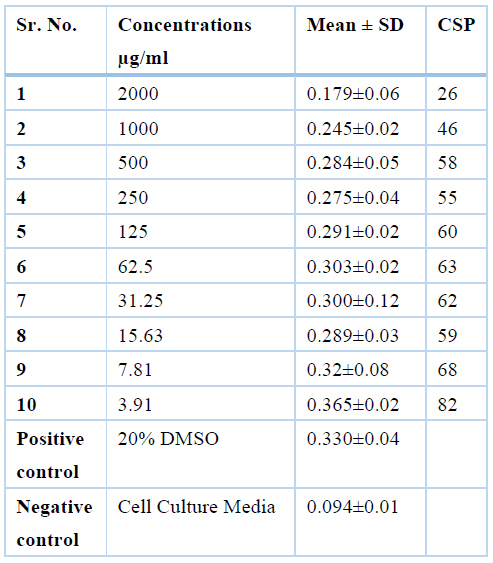
Table 4. CSP Values of Chloroform extract of Moringa Oleifera leaves at different concentrations by MTT assay
Ethanolic extract was safe at conc. of 1500µg/ml, 750µg/ml, 375µg/ml, 187.5µg/ml, 93.75µg/ml,46.85µg/ml, 23.43µg/ml and 11.71µg/ml where cell survival percentage was more than 50% and the values are 60%, 58%, 69%, 56%,59%, 74% 57% and 78% respectively while it was toxic at the concentrations of 6000µg/ml, 3000µg/ml where cell survival percentage was less than 50% i.e., 18% and 48% respectively and results are represented in Table 5.
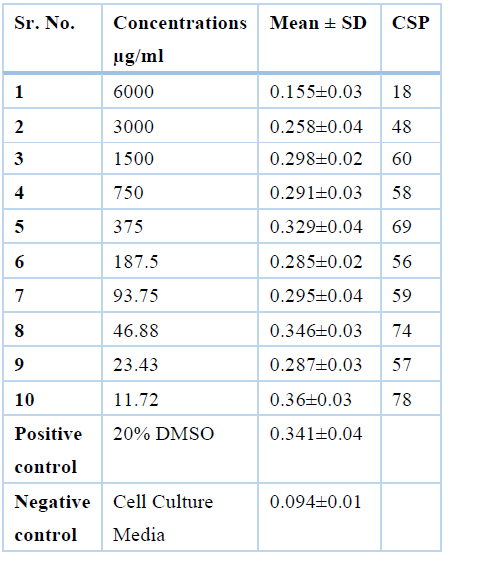
Table 5. Values for cell survival percentage of Moringa Oleifera leaves Ethanol extract of at various concentrations by MTT assay
Aqueous extract was safe at concentrations of 625µg/ml, 312.5µg/ml, 156.25µg/ml, 78.125µg/ml, 39.06µg/ml, 19.53µg/ml and 9.76µg/ml where cell survival percentage was more than 50% and the values are 56%, 54%, 59%, 55%, 62% 59% and 66% respectively while it was toxic at the concentrations of 5000µg/ml, 2500µg/ml, 1250µg/ml where cell survival percentage was less than 50% i.e., 8%, 18% and 42% respectively as summarized in Table 6.
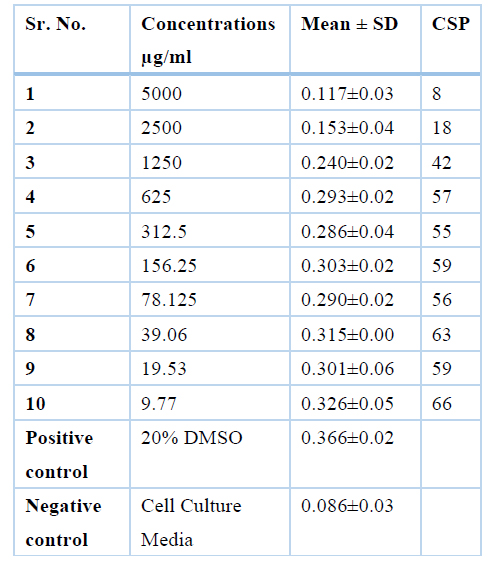
Table 6. Cell survival percentage of aqueous extract of Moringa oleifera Leaves.
DISCUSSION
The current study was designed to determine the antibacterial activity of Moringa oleifera against common poultry pathogens C. perfringens type A, E. coli, Haemophilus species, S. enterica, and S. aureus. Well-diffusion method for qualitative and minimum inhibitory concentration (micro-broth dilution method) for the quantitative study was performed to find out antibacterial activity of sequential extracts using hexane, chloroform, ethanol, and aqueous solvents. These minimum inhibitory concentrations were evaluated for cytotoxicity.
The aqueous extract shows the highest percentage yield (45/50gm) succeeded by ethanol extract (5.5gm/50gm), hexane extract (0.2gm/50gm), and chloroform extract (0.2gm/50gm). The percentage yield was 90%, 11%, 0.8%, 0.4% respectively. In a study the extraction of Astragalus Root was carried out with two-fold 95% ethanol, and the yield obtained was 17.1 %. The extraction was carried out for three days at room temperature. The filtrate then collected was concentrated under pressure and stored for further use. The yield obtained was closely related to our study's ethanol extract, which was reported 11%,13.
For Clostridium species, culture characteristics, gram staining, different biochemical tests such as Lipase test, Esculin Hydrolysis test, Catalase test, Indole test, Voges- Proskauer test Starch hydrolysis test Motility test, and Methyl Red test were performed,14.
Different biochemical tests were used for the identification of Escherichia coli. MacConkey's agar and Eosin methylene Blue agar were used to observe colony characters. Gram staining was also performed. To identify bacteria motility test by hanging drop method, mannitol, sucrose, lactose, maltose and dextrose fermentation, voges procure, indole, methyl red, and Catalase tests were used 15. E. coli isolates are catalase-positive, as tested in another study,16.
The minimum inhibitory concentration of sequential extracts of Moringa Oleifera leaves against Clostridium perfringens are highest for aqueous (5000µg/mL), while lowest for chloroform (1250µg/mL) and ethanol (1250µg/mL). So, chloroform and ethanol are active at their lowest concentration, the MIC values against Escherichia coli are highest for aqueous (3333.33µg/mL), medium for ethanol (2500µg/mL) while lowest for chloroform (208µg/mL).
For chloroform extract the CSP was calculated at 10 concentrations, 2000 µg/ml, 1000µg/ml, 500µg/ml, 250µg/ml, 125µg/ml, 62.5µg/ml, 31.25µg/ml, 15.63µg/ml, 7.81µg/ml and 3.91µg/ml. The results for cell survival percentage (CSP) in the present research are 26%, 46%, 58%, 55%, 60%, 63%, 62%, 59%, 68% and 82% respectively. The CSP results indicate that chloroform extract is toxic to cells at concentration ≥1000µg/ml. CSP at 1000µg/ml is 46% means toxic to cell because value is less than 50%. At a concentration less than 1000µg/ml the CSP is more than 50%. So, at higher concentrations chloroform is more cytotoxic to cell than hexane.
The CSP for Ethanol extract was calculated at 10 concentrations, 6000µg/ml, 3000µg/ml, 1500µg/ml, 750µg/ml, 375µg/ml, 187.5µg/ml, 93.75µg/ml, 46.85µg/ml, 23.43µg/ml and 11.71µg/ml. The CSP values are 18%, 48%, 60%, 58%, 69%, 56%, 59%, 74%, 57% and 78% respectively which indicates that chloroform extract at concentration ≥3000µg/ml is 48% so cytotoxic. At a concentration less than 3000µg/ml the CSP is more than 50%. Ethanol as compared to hexane and chloroform is less toxic to cell at higher concentration. Cytotoxicity of aqueous extract was calculated at 10 concentrations, 5000µg/ml, 2500µg/ml, 1250µg/ml, 625µg/ml, 312.5µg/ml, 156.25µg/ml, 78.125µg/ml, 39.06µg/ml, 19.53µg/ml and 9.76µg/ml. The CSP values are 8%, 18%, 42%, 56%, 54%, 59%, 55%, 62% 59% and 66% respectively. The resulted values indicate that aqueous extract is cytotoxic at concentration ≥625µg/ml. The CSP at 625µg/ml is 42% which means it is toxic to the cells. At a concentration ≥625µg/ml the CSP is more than 50%. So, aqueous extract is more toxic to cell as compared to the rest of two (chloroform, ethanol extracts) at higher concentration.
CONCLUSIONS
The current study aimed to evaluate cytotoxic and antibacterial activities of different extracts of Moringa Oleifera against common poultry pathogens. Sequential extraction with ethanol, chloroform and aqueous solvents was prepared, and antibacterial activity was evaluated by using agar well diffusion. A micro broth dilution test was used to evaluate the MIC of plant extracts. The extracts exhibiting antimicrobial activity were further evaluated for cytotoxicity by using an MTT assay on the Vero cell line. Cell culture media was prepared, cell lines were propagated, the monolayer was formed. This monolayer was exposed to plant extract dilutions. After 24-48 hours, MTT dye was introduced, and cell survival percentage was calculated. SPSS software was used to analyze data. Results MTT assay and antibacterial activity were compared using DMR posthoc test. All extracts inhibited the growth of Clostridium perfringens and Escherichia coli except aqueous extract, which shows no zones of inhibition against C. perfringes. MIC values were higher for aqueous extract against all selected bacteria and lowest for chloroform against E. coli (208.3ug/ml); Moringa Oleifera leaves showed antibacterial activity against all selected pathogens. Chloroform extract showed more significant antibacterial activity than ethanol and aqueous. Cytotoxicity values for chloroform extract are safer than the other two extracts. Moringa Oleifera may be used to design traditional medicines for the development of therapeutic agents which will be more safe, effective, and economical.
Acknowledgments: Author Contributions: U. B. N.; W. I.; M.R.; and M. B. K. proposed the concept of this study. U. B. N. and W. I. did the experimental research. U. B. N.; W. I. and M.R. analyzed the results and wrote the initial draft. M.B.K. reviewed, finalized the manuscript, and supervised the study.
Funding: This research received no external funding.
Acknowledgments: The authors are thankful to the Vice-chancellor of the University of Veterinary and Animal Sciences Lahore, Pakistan, for supporting this study's accomplishment.
Conflicts of Interest: The authors declare no conflict of interest.
REFERENCES
1. Moyo, B.; Masika, P.J.; Hugo, A.; Muchenje, V. Nutritional characterization of Moringa (Moringa oleifera Lam.) leaves. Afr. J. Biotechnol. 2011, 10, 12925–12933.
2. Ma, Z. F.; Ahmad, J.; Zhang, H.; Khan, I.; Muhammad, S. Evaluation of phytochemical and medicinal properties of Moringa (Moringa oleifera) as a potential functional food. S. Afr. J. Botany. 2020, 129, 40-46.
3. Bukar, A.; Uba, A; Oyeyi, T.I. Phytochemical analysis and antimicrobial activity of parkia biglobosa (Jacq.) Benth. Extracts against some food – borne microorganisms. Adv. Environ. Biol. 2010, 74-80
4. Al-Bari, M.; Sayeed, M.; Rahman, M.; Mossadik, M. Characterization and antimicrobial activities of a phenolic acid derivative produced by Streptomyces bangladeshiensis, a novel species collected in Bangladesh. Res. J. Med. Med. Sci. 2006, 1, 77- 81.
5. Foye, W.O.; Lemke, T.L.; Williams, D.A. Foye's Principles of Medicinal Chemistry, 6th Ed.; Lippincott Williams and Wilkins: Philadelphiap, 2008; pp 44-45.
6. Danso, A.A. Interpretation of low praziquantel cure rates of Schistosoma mansoni. Trends Parasitol. 2002, 18, 95- 143.
7. Mothana, R.A.; Lindequist, U. Antimicrobial activity of some medicinal plants of the island Soqotra. J. Ethnopharmacol. 2005, 96(1-2), 177-181.
8. Balachandar, S.; Jagadeeswari, M.; Dhanabalan, R.; Meenachi, M. 2012. Antimicrobial Activity of Astragalus membranaceus against Diarrheal Bacterial Pathogens. Int. J. Pharm. 2012, 2(2), 2416-418.
9. Lederberg, J. Isolation and characterization of biochemical mutants of bacteria. Methods Med. Res. 1950, 3(195), 5-22.
10. Andrews, J. M. (2001). Determination of minimum inhibitory concentrations. J. antimicrob. Chemother. 2001, 48(suppl_1), 5-16.
11. Dzomba, P.; Muchanyereyi, N. Potential antimicrobial plant extract based therapeutics from temnocalyx obovatus roots. Eur. J. Med. Chem. 2012, 209-215
12. Day, J. G.; Stacey, G. Cryopreservation and freeze-drying protocols. Methods in molecular biology. Humana Press: New York, 2007
13. Wang, Y.; Auyeung, K.K.; Zhang, X.; Ko, J.K. Astragalus saponins modulates colon cancer development by regulating calpain-mediated glucose-regulated protein expression. BMC Complement. Altern. Med. 2014, 1-12.
14. Chaturvedi, A.; Shukla, S. Occurance of Clostridium species in different dairy products and its associated health risk. Int. J. Recent Sci. Res. 2015, 2, 2827-2829.
15. Zinnah, M.; Bari, M.; Islam, M.; Hossain, M.; Rahman, M.; Haque, M.; Babu, S.; Ruma, R.; Islam, M. Characterization of Escherichia coli isolated from samples of different biological and environmental sources. Bangladesh J. Vet. Med. 2007, 5 (1), 25-32.
16. Soomro, A.; Arain, M.; Khaskheli, M.; Bhutto, B. Isolation of Escherichia coli from raw milk and milk products in relation to public health sold under market conditions at Tandojam. Pak. J. Nutr. 2002, 1 (3), 151-152.
Received: 25 August / Accepted: 20 November 2021 / Published: date. 15 February 2022
Citation: Bin Naeem U, Iftikhar W, Rafiq M, Babar Khawar M. Antibacterial and cytotoxic evaluation of sequential extract of Moringa oleifera leaves. Revis Bionatura 2022;7(1). 15. http://dx.doi.org/10.21931/RB/2022.07.01.15
