2023.08.03.26
Files > Volume 8 > Vol 8 No 3 2023
Preview / Index / Next
Interaction of Lactoferrin with Neutroplile Gelatinase Association Lipocalin in Treatment of Chronic Kidney Diseases Induced Experimentally by K. pneumonia

1Department of X-ray, Medical Technical College, the University of Al-Zahraa for Women, Karbala -Iraq
2Department of Physiology, Biochemistry, and Pharmacology, College of Veterinary Medicine, University of Baghdad, Iraq.
3Departement of X-ray and sonar, Medical Technical College, Al Farahidi University, Baghdad-Iraq
Corresponding author: [email protected],
Available from: http://dx.doi.org/10.21931/RB/2023.08.03.26
ABSTRACT
Lactoferrin (Lf), among the critical components of the innate immune system, has excellent iron-carrying properties that provide valuable protection against all types of bacterial infections, inflammatory diseases and other forms of cellular stress. This study hypothesized an essential role for Lf in the treatment of chronic kidney disease (CKD) and the mechanism of neutrophil gelatinase association with lipocalin action through its role in iron sequestration and its effectiveness in reducing oxidative stress caused by Klebsiella pneumoniae in infected rats. Thirty adult female rats were divided into three equal groups; 1st group was kept healthy as a control, and the second and third groups were infected with K. pneumoniae by the intraurethral route. Following 60 days of infection, the 3rd group was treated for 30 days with Lf (0.5 gm/kg BW). Results of kidney function tests (urea, creatinine, and Kidney injury molecule-1 KIM-1) indicated the change of K. pneumoniae infection from acute to chronic. Lactoferrin-treated rats had reduced urea, creatinine, KIM-1, neutrophil gelatinase-associated Lipocalin (NGAL), erythropoietin (EPO), malondialdehyde acid (MDA), and interleukin-6 (IL-6), and elevated total antioxidant capacity (TAOC) in comparison with untreated rats. It can be postulated that Lf activity in improving kidney function and histopathological changes following chronic K. pneumoniae infection belongs to its antioxidant, anti-inflammatory, and bacteriostatic role.
Keywords: Lactoferrin, CKD, K. pneumoniae 16SrRNA, NGAL, Antioxidant
INTRODUCTION
Chronic kidney disease (CKD) is a global epidemic recognized as impairment of renal functions shown by a glomerular filtration rate less than usual, signs of kidney damage, or both, for at least three months 1.2. Renal failure due to acute kidney injury can be fatal3 or might lead to progression to chronic kidney disease4. Direct infection of renal tissue by different bacteria induces urinary tract infection, which can progress to renal dysfunction in pyelonephritis by ascending infection or hematogenous dissemination5. Occasionally, renal injury may be due to the nephrotoxic effects of various antimicrobial agents used in management. Patients who are elderly, diabetic, pregnant, or immunocompromised are at an increased risk of acute injury, which has high mortality and morbidity6. As many as 20% of critically ill patients have irreversible renal damage due to acute cortical necrosis, and another 40% have incomplete renal recovery, leading to CKD7. It is not uncommon to observe that episodes of bacterial sepsis accelerate the rate of progression of pre-existing CKD by multiple mechanisms8. Because serum urea levels rise in acute and chronic renal disease, and serum creatinine is a late marker of acute kidney injury, estimating those biomarkers assesses renal function9. Kidney molecular injury-1 (KIM-1) and neutrophil gelatinase-associated Lipocalin (NGAL) are tubular injury biomarkers for acute kidney injury (AKI). Both may also add information on the persistence of renal injury in AKI and the risk of progression to CKD10.11.
Klebsiella pneumoniae is a facultative anaerobic bacterium that is Gram-negative, non-motile, rod-shaped, lactose-fermenting and has a conspicuous polysaccharide capsule. Within the genus Klebsiella, K. pneumoniae is the most common human pathogen, causing a variety of infections in hospitals, long-term care facilities, and communities around the world, including diseases of the lungs, urinary tract, abdominal cavity, surgical sites, soft tissue infections, and even bacteremia 12.13. This bacterium may be found in the natural flora of the mouth, skin, and gut and is the third most often isolated pathogen in blood culture patients with sepsis14. In addition, K. pneumoniae has been identified as a cause of severe and life-threatening infections, such as pyogenic liver abscesses, endophthalmitis, and meningitis, and is becoming a public health problem15.16.
Lactoferrin (lactotransferrin; Lf) is a globular glycoprotein with a strong affinity for metal ions, particularly iron, copper, zinc, and manganese17. Recent research shows that Lf treatment can protect against AKI and early renal fibrosis while restoring kidney function18. Lf shows protective effects against AKI and CKD; this reduces markers related to inflammation, oxidative stress, apoptosis, and kidney fibrosis and induces autophagy and mitochondrial biogenesis in the kidney19. LF has a variety of pharmacological effects that are mediated through a variety of receptors, including antibacterial, antifungal, antiviral, anti-inflammatory, and antioxidative activities, as well as the ability to regulate the amount of iron absorbed in the gut and can also chelate iron, either directly or indirectly, through its involvement in iron transport and modulates immunological responses20.
Neutrophil gelatinase-associated lipocalin (NGAL) is one of the proteins that are expressed in damaged kidneys and has endogenous antimicrobial and iron scavenging activity21; therefore, it can be considered as a tissue injury marker and a marker for the gradual loss of nephron function and CKD progression22.23. Lactoferrin (Lf) and neutrophil gelatinase-associated lipocalin (NGAL) are among the critical components of the innate immune system co-localized in specific (secondary) granules of human neutrophils, Lf and NGAL are released as acute phase proteins following neutrophil activation and degranulation24.25. Because their excellent iron-carrying properties provide valuable protection against all types of bacterial infection, inflammatory disease and other cellular stress, they have been linked to high affinity for Fe3+. By removing iron from the site of injury, Lf and NGAL may limit iron-mediated toxicity, although the mechanisms employed by each differ. Antibiotic resistance makes infections caused by K. pneumoniae increasingly challenging to treat and directly threaten health28. Thus, this study aimed to investigate the role of lactoferrin as a new therapeutic for CKD to limit K. pneumoniae infections via modulating the anti-inflammatory, antioxidant, and tissue repair responses.
MATERIALS AND METHODS
Bacterial isolation and identification
Klebsiella pneumonia was obtained from Medical City Hospital, Baghdad, Iraq, isolated from a sputum swap from a Covid-19 patient. Firstly, the trade was transported into a tube containing 3 ml of brain_heart infusion broth and incubated at 37ºC for 48 hours. A slant of brain-heart infusion agar was used to maintain the bacterial isolate. To improve bacterial identification, selected colonies were cultured on blood, congo red, and MacConkey's agars. The VITEK®2 system did biochemical identification of the bacteria. Then, the isolate was confirmed at the molecular level using polymerase chain reaction (PCR) targeting the 16S rRNA gene. The bacterial DNA was extracted from the bacterial growth according to the protocol of ABIO pure. The extracted DNA was quantified by Quantus Fluorometer, briefly mixing 1 μl of DNA with 200 μl of diluted Quantifluor Dye. The DNA concentration values were detected after 5 min incubation at room temperature; for the molecular identification of K. pneumoniae, 27 F and 1492 R primers (supplied by the Macrogen Company) for the 16S rRNA gene were used to amplify the DNA sequence. After PCR amplification, agarose gel electrophoresis was adopted to confirm the presence of amplification. Finally, the PCR products were sent for Sanger sequencing using ABI3730XL, an automated DNA sequencer, by Macrogen Corporation – Korea. The results were received by email and then analyzed using Genius software.

Table 1. Primers and their sequences for K. pneumoniae molecular identification
Experimental Design
The thirty adult female Albino Wister Rats' body weight ranged between 150 and 200 gm and were randomly divided into two groups. The first group was considered the control. The second group was infected with K. pneumoniae using an experimental model of ascending pyelonephritis29, in which 0.5 ml of the bacteria suspension (105–107 bacteria/ ml) was introduced within 5 seconds via the urethra into the urinary bladder. The infection was repeated after 20 days to ensure condition. Following 60 days of disease, the second group was divided into two subgroups; the first sub-group continued with infection, and the second sub-group was administered lactoferrin orally at a dose of 0.5 g/kg BW for 30 days30. Blood samples were collected from all groups of anesthetized rats at 60 days of infection and after 30 days of treatment. This study was carried out according to the approval of the scientific committee in the Department of Physiology, Biochemistry and Pharmacology, College of Veterinary Medicine, University of Baghdad, following the ethical animal welfare standards.
Kidney function markers: Serum urea and creatinine were determined using a commercial kit (Linear Chemicals, SLU/Spain) by an enzymatic colorimetric method. Kidney injury molecule-1 (KIM-1) was measured using a commercial kit (Al-Shkairate / Jordan). Serum Neutrophil Gelatinase Associated Lipocalin (NGAL) was determined using the Rat Neutrophil Gelatinase Associated Lipocalin ELISA Kit (MyBioSource/USA).
Anti-inflammatory and antioxidant tests.
Serum malondialdehyde (MDA) and Total Antioxidant Capacity (TAOC) were determined by a colorimetric assay kit (MyBioSource/USA). Furthermore, serum Interleukin-6 (IL-6) was determined by the Rat Interleukin-6 ELISA Kit (MyBioSource/USA).
Statistical analysis
Data presented as a mean ±SE. Statistical analysis of data was performed by two-way analysis using SAS (Statistical Analysis System) and Microsoft Office Excel for Windows (2010). Least significant differences (LSD) were performed multiple (multiple comparisons) to evaluate significant differences at p ≤ 0.0531.
RESULTS
Bacterial identification and confirmation.
Klebsiella pneumoniae grew successfully on selective agar media, giving its characterized feature on MacConkey's agar, i.e., mucoid pink color, due to lactose fermentation; they also showed gray-white, large mucoid translucent and non-hemolytic colonies on blood agar, while on Congo red agar they were black due to biofilm formation. The bacterial identification was confirmed at 99% using the VITEK2 system. The PCR amplification fractionated on agarose gel electrophoresis showed a band of roughly 1500 bp representing 16 S rRNA of K. pneumoniae (Figure 1). The above bacterial gene sequence analysis using the BLAST program in NCBI confirmed that the 16S rRNA gene belonged to K. pneumoniae.
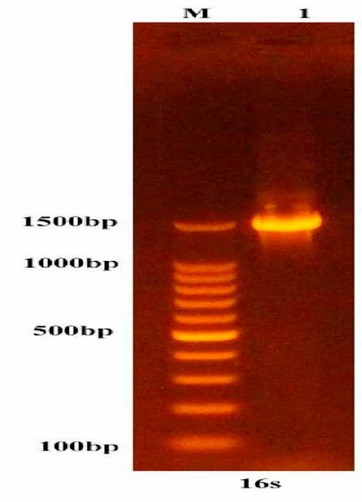
Figure 1: 1.5% Agarose gel electrophoresis shows a band of approximately 1500 bp of the 16S rRNA gene expected to belong to K. pneumoniae. M: 100bp DNA size marker, 1: PCR amplicon.
Kidney function markers
Treatment of rats with lactoferrin for 30 days reduced significantly (p< 0.05), the elevated serum urea, creatinine, KIM-1, and NGAL of rats with CKD caused by K. pneumoniae (Tables 2,3,4,5). Erythropoietin showed a significant reduction in K. pneumoniae-infected rats before treatment, but lactoferrin pushed it up to the normal range of the control group (Table 6).
Kidney histopathological changes
Histopathological analysis of the light microscopic image of the kidney section of control rats revealed normal tissue (Figure 2A,2B). K. pneumoniae infection with no treatment for about 90 days revealed severe destruction of the glomerular in the cortical region of the kidney with loss of parenchyma, multifocal mononuclear cells (MNCs), Tubular dilation, interstitial MNCs infiltration forming granuloma-like lesion with giant cell formation (figure 2C), all these changes indicated the chronic changes due to bacterial infection. The medullary section of the kidney from infected rats showed few hyaline casts with cystic tubular dilation (Figure 2D). The results of histopathological changes showed that treatment for 30 days with lactoferrin produced no apparent pathological changes in the cortical. Although, there was prevascular mononuclear cell aggregation, indicating a delayed hypersensitivity reaction (Figure 2E). The medullary region showed that lactoferrin treatment produced nodular granulomatous lesions composed mainly of lymphocytes and plasma (Figure 2F).
Antioxidant and anti-inflammatory markers
Table 6 shows that MDA, an oxidative stress marker, was significantly (P ≤ 0.05) high before treatment in infected groups, but after 30 days of treatment with Lf, its serum levels significantly decreased (P ≤ 0.05). The serum TAOC was significantly increased in the K. pneumoniae infection treated with Lf for 30 days. The results also showed that the serum level of IL-6, a proinflammatory cytokine, was significantly (P ≤ 0.05) higher in the groups of K. pneumoniae-infected female rats before treatment compared with control female rats. After 30 days of treatment, with Lf, IL-6 levels reduced significantly (P ≤ 0.05) compared with the non-treated group (Table 7,8).
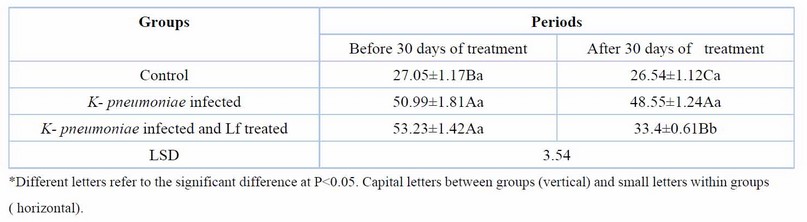
Table 2. Serum urea (mg/dl) at day 60 days of K. pneumoniae infection and after 30 days of lactoferrin treatment. Means ± SE, n= 6
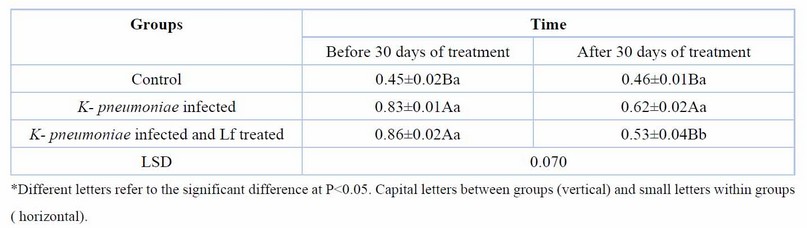
Table 3. Serum creatinine (mg/dl) at day 60 days of K. pneumoniae infection and after 30 days of lactoferrin treatment. Means ± SE, n= 6
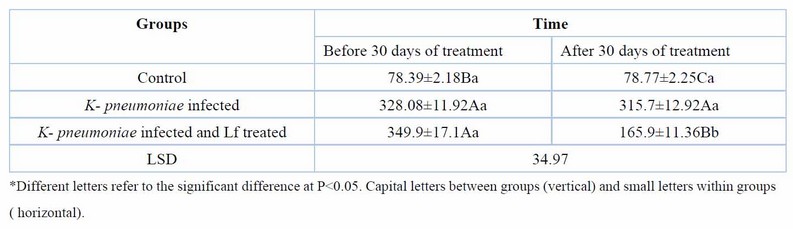
Table 4. Serum Kim1 (pg/ml) at day 60 days of K. pneumoniae infection and after 30 days of lactoferrin treatment. Means ± SE, n= 6
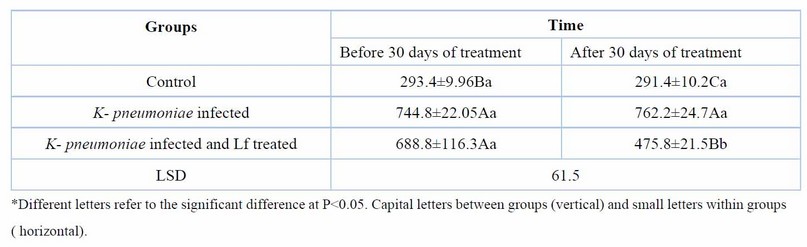
Table 5. Serum NGAL(pg/ml) at day 60 days of K. pneumoniae infection and after 30 days of lactoferrin treatment. Means ± SE, n= 6
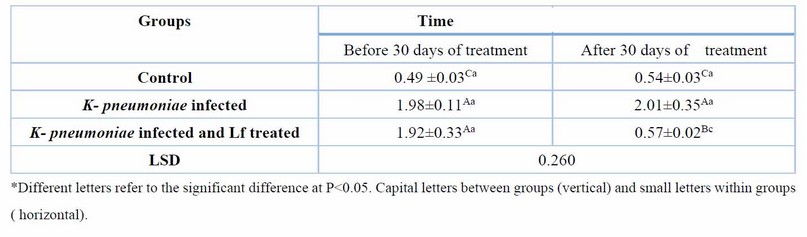
Table 6. Serum MDA (nmol/ml) at day 60 days of K. pneumoniae infection and after 30 days of lactoferrin treatment. Means ± SE, n= 6
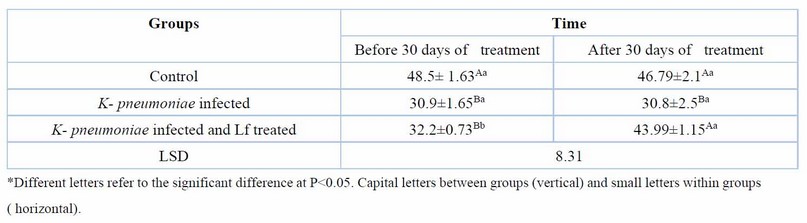
Table 7. Serum TAOC(U/ml) at day 60 days of K. pneumoniae infection and after 30 days of lactoferrin treatment. Means ± SE, n= 6
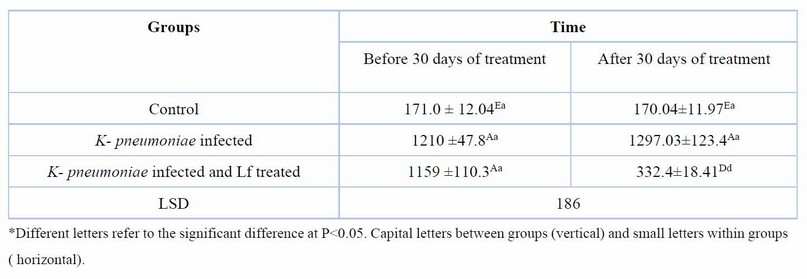
Table 8. Serum Il-6 (pg/ml) at day 60 days of K. pneumoniae infection and after 30 days of lactoferrin treatment. Means ± SE, n= 6
ular mononuclear cell
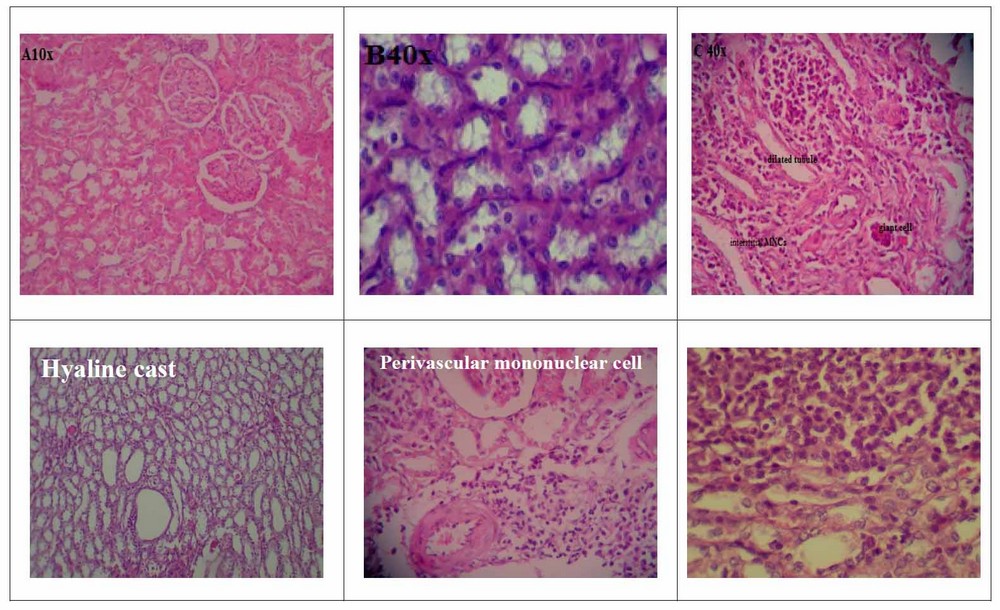
Figure 2. Light microscopic image for kidney tissues for A: cortical section of the control group (10x), B: kidney medulla section (40x), C: cortical area of K- pneumoniae infected (10x), D: medulla sections of K- pneumoniae infected (10x), E: cortical section of K- pneumoniae infected with Lf treatment (40x), E: medulla section of K- pneumoniae infected with Lf treatment (10x).
DISCUSSION
Animal models have been extensively used to clarify the pathophysiology and processes behind kidney failure. Rats are quickly produced and can be made to develop classic kidney damage in various ways, including surgery and the injection of medicines or poisons32. In the present study, the elevation of urea and creatinine to more than 50 %, according to KDIGO, indicates the transition of infection from acute to chronic, resulting in chronic kidney disease (CKD). Chronic kidney disease has been induced by an ascending method for kidney infection with K. pneumoniae29. K. pneumoniae is a highly virulent and antibiotic-resistant bacteria involved in CKD33. The pathogenicity of K. pneumoniae has been acquired through the activity of different virulence factors, such as capsular polysaccharide (CPS) and siderophores34. The mechanism by which rats infected with K. pneumoniae developed CKD could result in inflammation accompanied by oxidative stress and renal dysfunction35. As a primary histological hallmark of acute kidney damage, the ascending manner of bacterial infection results in biological processes, such as tubular cell death, necrosis, inflammation, and apoptosis36. Fibrosis, vascular disruption, tubular loss, glomerulosclerosis, and persistent interstitial inflammation are all pathological changes of chronic kidney disease37. Siderophores secreted by K. pneumoniae induce stabilization of the transcription factor HIF-1α, increasing the expression of proinflammatory cytokines38. Various CKDs have been linked to multiple diseases, including chronic inflammation and renal fibrosis 39. The interaction of bacterial siderophores with host cells further promotes the pathogenicity of K. pneumoniae in different mechanisms40 and is likely relevant for other pathogens that also secrete siderophores in the host41. Because serum urea levels rise in acute and chronic renal diseases, estimating those biomarkers assesses renal function and indicates a defect in glomerular filtration42.43.9. Although many biomarkers in the blood can be measured to diagnose kidney diseases and are linked to kidney function, the kidney injury molecule-1 (KIM-1) is the most closely linked.
Kidney molecular injury and neutrophil gelatinase-associated lipocalin (NGAL) are tubular injury indicators for acute kidney injury (AKI), the persistence of renal injury, and the risk of progression to CKD45. The persistent serum NGAL and KIM above the control level for more than 90 days of infection approved the transition of acute to chronic disease; they improved as biomarkers of CKD progression of end-stage renal disease46. NGAL extensively impacts immune processes and has a direct bacteriostatic effect by blocking the siderophores on the Gram-negative bacterial wall47. Decrease in endogenous erythropoietin (EPO) production48. Erythropoietin, primarily generated by the kidney's fibroblast-like interstitial pericytes cells, surrounds pre-tubular capillaries and is considered a kidney biomarker. As shown by histopathological changes of kidney tissue in the present model of CKD, the kidney's interstitial showed young proliferated fibroblast cells, and differentiation of these pericytes or fibroblast to myofibroblasts cause interstitial fibrosis in CKD is indicative of long-standing kidney disease49 subsequently the ability of EPO production decreases50.51. Furthermore, EPO production inhibition or EPO resistance is affected by some inflammatory cytokines52. It is generally understood that CKD increases inflammation and immune activation molecules, which would impede hypoxia-induced EPO synthesis53.
During the host cell-pathogen interaction, a cytokine burst occurs to recruit the cells of the innate immune system and enhance the defense against pathogens. Mostly, interIeukin-654.55.56. Serum NGAL reflects renal impairment and presents a solid and independent risk marker for the progression of end-stage renal disease. Neutrophil gelatinase-associated lipocalin is a member of lipocalin proteins, which are extracellular proteins that bind small and hydrophobic molecules and play a role in cellular homeostasis NGAL has been used as a diagnostic marker in cases of glomerular and tubular dysfunction 57.58. Both NGAL and IL-6 are biomarkers for UTI infection59. Lactoferrin has recently become the subject of significant investigation. Lactoferrin has a considerable potential pharmacological role in treating kidney diseases.
Upregulated innate lymphocyte activation and cytokine production from injured epithelial cells leads to kidney disease60. Innate lymphoid cells (ILCs) have emerged as essential mediators of mucosal surface protection and repair during infection61. The innate immune system is capable of guarding against K. pneumoniae infection62.63. Both inflammation and oxidative stress can contribute to the development of CKD and its various complications. Permanent oxidative stress occurs in patients with chronic kidney disease. Increased ROS production and/or insufficient antioxidant system performance (TAOC) can lead to oxidative stress, which is associated with the damage or oxidative modification of vital molecules such as nucleic acid, proteins (enzymes), and lipids, producing malondialdehyde (MDA). In CKD patients, oxidative stress causes lipid and protein peroxidation 64.65. Most kidney failure indices, oxidative stress and inflammation measured in plasma were improved in rats given lactoferrin, suggesting different mechanisms of K. pneumoniae-induced CKD. Lactoferrin acts as an antioxidant, the principal component of immune homeostasis. It may decrease intracellular ROS levels and oxidative stress-induced apoptosis. Transitional metals such as iron are the leading oxidative stress cause, which controls ROS production. Lactoferrin can inhibit lipid peroxidation due to iron sequestration66.
The results indicate that Lf ingestion effectively treated kidney tubular oxidative damage by protecting kidney tissue from ferric nitrilotriacetate-induced kidney tubular oxidative injury in rat models. This antioxidant activity is mediated by diminishing H2O2-induced apoptosis by inducing autophagy69. Treatment with Lf restored kidney function and prevented fibrosis by inhibiting apoptosis and augmenting autophagy. The antifibrotic activity of lactoferrin in the treated group results from the role of lactoferrin in down-regulation of tissue growth factor β1 gene expression18. As an anti-inflammatory, lactoferrin has been used successfully against kidney inflammation70. The anti-inflammatory mechanism of lactoferrin could be through iron metabolism. Lf can reduce the damage of excessive inflammatory responses by sequestering free iron and directing reactive oxygen intermediates71 or by reducing hyperoxia-mediated kidney inflammation and kidney injury in mice models. The PCR technique in the medical field was applied in diagnosing pathogenic microorganisms 72-83 and genetic-related diseases 84-88.
CONCLUSION
The results of lowering inflammatory biomarkers indicated lactoferrin's multifactorial activity in CKD caused by K. pneumoniae. In the current work, lactoferrin improved kidney tissue by acting as an antioxidant and antifibrotic; when Lf is administered as an iron sequestration molecule, which sequesters and conjugates bacterial siderophores and inhibits bacterial absorption, this conjugation of lactoferrin to bacterial siderophores are improving the therapy of CKD and renal repair, which lowers bacterial resistance and has anti-inflammatory properties.
Funding: Self-Funding.
Acknowledgments: The authors thank everyone who helped with this work.
Conflicts of Interest: No conflict of interest.
REFERENCES
1- Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. The Lancet. 2017 Mar 25;389(10075):1238-52.
2- Benjamin O, Lappin SL. End-Stage Renal Disease. 2021 Sep 16. StatPearls. Treasure Island (FL): StatPearls Publishing. 2022.
3- Zarjou A, Agarwal A. Sepsis and acute kidney injury. Journal of the American Society of Nephrology. 2011 Jun 1;22(6):999-1006.
4- Ferenbach DA, Bonventre JV. Acute kidney injury and chronic kidney disease: From the laboratory to the clinic. Nephrologie & therapeutique. 2016 Apr 1;12:S41-8.
5- Mandracchia VJ, Hayes DW, Yoho RM, Hayes MF. Diagnosis, differential and treatment options. Nat Rev Microbiol [Internet]. 2000 Mar;13:269-84.
6- Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Disease-a-month. 2003 Feb 1;49(2):53-70.
7- Schiffl H. Renal recovery from acute tubular necrosis requiring renal replacement therapy: a prospective study in critically ill patients. Nephrology Dialysis Transplantation. 2006 May 1;21(5):1248-52.
8- Huang T, Gu W, Wang B, Zhang Y, Cui L, Yao Z, Zhao C, Xu G. Identification and expression of the hepcidin gene from brown trout (Salmo trutta) and functional analysis of its synthetic peptide. Fish & shellfish immunology. 2019 Apr 1;87:243-53.
9- Gounden V, Bhatt H, Jialal I. Renal function tests.
10- Castillo-Rodriguez E, Fernandez-Prado R, Martin-Cleary C, Pizarro-Sánchez MS, Sanchez-Niño MD, Sanz AB, Fernandez-Fernandez B, Ortiz A. Kidney injury marker 1 and neutrophil gelatinase-associated Lipocalin in chronic kidney disease. Nephron. 2017;136(4):263-7.
11- Al-ghareebaw AM, Al-Okaily BN, Ibrahim OM, Mohammed AD. Role of Olive leaves Zinc Oxide Nanoparticles in Alleviating The Molecular and Histological Changes of Kidney in Female Goats-Induced by Gentamicin (Part III). The Iraqi Journal of Veterinary Medicine. 2020 Dec 28;44(E0)):14-20.
12- Alyssa SS, Rajinder PS, Thomas AR. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae. A new and dangerous breed. Virulence. 2013;4:2.
13- Mody L, Juthani-Mehta M. Urinary tract infections in older women: a clinical review. Jama. 2014 Feb 26;311(8):844-54.
14- Li X, Zhou X, Ye Y, Li Y, Li J, Privratsky B, Wu E, Gao H, Huang C, Wu M. Lyn regulates inflammatory responses in Klebsiella pneumoniae infection via the p38/NF‐κB pathway. European journal of immunology. 2014 Mar;44(3):763-73.
15- Struve C, Roe CC, Stegger M, Stahlhut SG, Hansen DS, Engelthaler DM, Andersen PS, Driebe EM, Keim P, Krogfelt KA. Mapping the evolution of hypervirulent Klebsiella pneumoniae. MBio. 2015; 6: e00630. DOI.;10:00630-15.
16- Zhan L, Wang S, Guo Y, Jin Y, Duan J, Hao Z, Lv J, Qi X, Hu L, Chen L, Kreiswirth BN. Outbreak by hypermucoviscous Klebsiella pneumoniae ST11 isolates with carbapenem resistance in a tertiary hospital in China. Frontiers in cellular and infection microbiology. 2017 May 16;7:182.
17- Redwan EM, Uversky VN, El-Fakharany EM, Al-Mehdar H. Potential lactoferrin activity against pathogenic viruses. Comptes rendus biologies. 2014 Oct 1;337(10):581-95
18- Hsu YH, Chiu IJ, Lin YF, Chen YJ, Lee YH, Chiu HW. Lactoferrin contributes a renoprotective effect in acute kidney injury and early renal fibrosis. Pharmaceutics. 2020 May 8;12(5):434.
19- Zahan MS, Ahmed KA, Moni A, Sinopoli A, Ha H, Uddin MJ. Kidney protective potential of lactoferrin: pharmacological insights and therapeutic advances. The Korean Journal of Physiology & Pharmacology: Official Journal of the Korean Physiological Society and the Korean Society of Pharmacology. 2022 Jan 1;26(1):1-3.
20- Hao L, Shan Q, Wei J, Ma F, Sun P. Lactoferrin: major physiological functions and applications. Current Protein and Peptide Science. 2019 Feb 1;20(2):139-44.
21- Albert C, Zapf A, Haase M, Röver C, Pickering JW, Albert A, Bellomo R, Breidthardt T, Camou F, Chen Z, Chocron S. Neutrophil gelatinase-associated lipocalin measured on clinical laboratory platforms for the prediction of acute kidney injury and the associated need for dialysis therapy: a systematic review and meta-analysis. American Journal of Kidney Diseases. 2020 Dec 1;76(6):826-41.
22- Alderson HV, Ritchie JP, Pagano S, Middleton RJ, Pruijm M, Vuilleumier N, Kalra PA. The associations of blood kidney injury Molecule-1 and neutrophil Gelatinase–associated Lipocalin with progression from CKD to ESRD. Clinical Journal of the American Society of Nephrology. 2016 Dec 7;11(12):2141-9.
23- Anderson AH, Xie D, Wang X, Baudier RL, Orlandi P, Appel LJ, Dember LM, He J, Kusek JW, Lash JP, Navaneethan SD. Novel risk factors for progression of diabetic and nondiabetic CKD: findings from the chronic renal insufficiency cohort (CRIC) study. American Journal of Kidney Diseases. 2021 Jan 1;77(1):56-73.
24- OE BN, Theilgaard-Monch K. Neutrophil granules: a library of innate immunity proteins. Trends Immunol. 2007;28(8):340-5.
25- Parrow NL, Fleming RE, Minnick MF. Sequestration and scavenging of iron in infection. Infection and immunity. 2013 Oct;81(10):3503-14.
26- Yang J, Goetz D, Li JY, Wang W, Mori K, Setlik D, Du T, Erdjument-Bromage H, Tempst P, Strong R, Barasch J. An iron delivery pathway mediated by a lipocalin. Molecular cell. 2002 Nov 1;10(5):1045-56.
27- Pandita A, Sharma D, Kumar C. Lactoferrin and its role in neonatology: a review article. J Pediatr Neonatal Care. 2015;2(2):00062.
28- Yan X, Su X, Ren Z, Fan X, Li Y, Yue C, Yang M, Deng H, Deng Y, Xu Z, Zhang D. High Prevalence of Antimicrobial Resistance and Integron Gene Cassettes in Multi-Drug-Resistant Klebsiella pneumoniae Isolates From Captive Giant Pandas (Ailuropoda melanoleuca). Frontiers in Microbiology. 2022 Feb 3;12:801292.
29- Ibrahim ZI. Experimental infection of Klebsiella pneumoniae in the urinary tract of rats and guinea pigs: Ibrahim ZI and Alwaan MJ. The Iraqi Journal of Veterinary Medicine. 2008 Dec 31;32(2):68-79.
30- Wu J, Chen J, Wu W, Shi J, Zhong Y, van Tol EA, Tang Q, Cai W. Enteral supplementation of bovine lactoferrin improves gut barrier function in rats after massive bowel resection. British Journal of Nutrition. 2014 Aug;112(4):486-92.
31- Lee S, Lee DK. What is the proper way to apply the multiple comparison test? Korean journal of anesthesiology. 2018 Oct 1;71(5):353-60.
32- Libetta C, Sepe V, Esposito P, Galli F, Dal Canton A. Oxidative stress and inflammation: implications in uremia and hemodialysis. Clinical biochemistry. 2011 Oct 1;44(14-15):1189-98.
33- Majeed HT, Aljanaby AAJ. Antibiotic Susceptibility Patterns and Prevalence of Some Extended Spectrum Beta-Lactamases Genes in Gram-Negative Bacteria Isolated from Patients Infected with Urinary Tract Infections in Al-Najaf City, Avicenna Journal of Medical Biotechnology. 2019 Apr-Jun;11(2):192-201.
34- Vuotto C, Longo F, Balice MP, Donelli G, Varaldo PE. Antibiotic resistance related to biofilm formation in Klebsiella pneumoniae. Pathogens. 2014 Sep 5;3(3):743-58.
35- Uddin MJ, Kim EH, Hannan MA, Ha H. Pharmacotherapy against oxidative stress in chronic kidney disease: Promising small molecule natural products targeting nrf2-ho-1 signaling. Antioxidants. 2021 Feb 7;10(2):258.
36- Gupta J, Mitra N, Kanetsky PA, Devaney J, Wing MR, Reilly M, Shah VO, Balakrishnan VS, Guzman NJ, Girndt M, Periera BG. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clinical Journal of the American Society of Nephrology. 2012 Dec 1;7(12):1938-46.
37- Wagner M, Alam A, Zimmermann J, Rauh K, Koljaja-Batzner A, Raff U, Wanner C, Schramm L. Endogenous erythropoietin and the association with inflammation and mortality in diabetic chronic kidney disease. Clinical Journal of the American Society of Nephrology. 2011 Jul 1;6(7):1573-9.
38- Russo TA, Olson R, Fang CT, Stoesser N, Miller M, MacDonald U, Hutson A, Barker JH, La Hoz RM, Johnson JR. Identification of biomarkers for differentiation of hypervirulent Klebsiella pneumoniae from classical K. pneumoniae. Journal of clinical microbiology. 2018 Sep;56(9):e00776-18.
39- Goldstein SL, Leung JC, Silverstein DM. Pro-and anti-inflammatory cytokines in chronic pediatric dialysis patients: effect of aspirin. Clinical Journal of the American Society of Nephrology. 2006 Sep 1;1(5):979-86.
40- Holden VI, Bachman MA. Diverging roles of bacterial siderophores during infection. Metallomics. 2015 Jun;7(6):986-95.
41- Holden VI, Breen P, Houle S. Klebsiella pneumoniae Siderophores. Induce Inflammation, Bacterial Dissemination, and HIF-1α Stabilization during Pneumonia. mBio. 2016;7(5)..
42- Mihai S, Codrici E, Popescu ID, Enciu AM, Albulescu L, Necula LG, Mambet C, Anton G, Tanase C. Inflammation-related mechanisms in chronic kidney disease prediction, progression, and outcome. Journal of immunology research. 2018 Oct; 2018.
43- Okusa MD, Rosner MH. Overview of the management of acute kidney injury (AKI) in adults. UpToDate, topic last updated Oct. 2019;22:2019.
44- Navon-Venezia S, Kondratyeva K, Carattoli A. Klebsiella pneumoniae: a major worldwide source and shuttle for antibiotic resistance. FEMS microbiology reviews. 2017 May 1;41(3):252-75
45- Castillo-Rodriguez E, Fernandez-Prado R, Martin-Cleary C, Pizarro-Sánchez MS, Sanchez-Niño MD, Sanz AB, Fernandez-Fernandez B, Ortiz A. Kidney injury marker 1 and neutrophil gelatinase-associated Lipocalin in chronic kidney disease. Nephron. 2017;136(4):263-7.
46- Guo L, Zhu B, Yuan H, Zhao W. Evaluation of serum neutrophil gelatinase‐associated Lipocalin in older patients with chronic kidney disease. Aging Medicine. 2020 Mar;3(1):35-42.
47- Nasioudis D, Witkin SS. Neutrophil gelatinase-associated Lipocalin and innate immune responses to bacterial infections. Medical microbiology and immunology. 2015 Aug;204:471-9.
48- Estrela GR, Freitas-Lima LC, Budu A, Arruda AC, Perilhão MS, Fock RA, Barrera-Chimal J, Araújo RC. Chronic kidney disease induced by cisplatin, folic acid and renal ischemia-reperfusion induces anemia and promotes GATA-2 activation in mice. Biomedicines. 2021 Jul 2;9(7):769.
49- Souma T, Nezu M, Nakano D, Yamazaki S, Hirano I, Sekine H, Dan T, Takeda K, Fong GH, Nishiyama A, Ito S. Erythropoietin synthesis in renal myofibroblasts is restored by activation of hypoxia signaling. Journal of the American Society of Nephrology. 2016 Feb 1;27(2):428-38.
50- Shih HM, Wu CJ, Lin SL. Physiology and pathophysiology of renal erythropoietin-producing cells. Journal of the Formosan Medical Association. 2018 Nov 1;117(11):955-63
51- Bandach I, Segev Y, Landau D. Experimental modulation of Interleukin 1 shows its key role in chronic kidney disease progression and anemia. Scientific Reports. 2021 Mar 18;11(1):1-4.
52- Portolés J, Martín L, Broseta JJ, Cases A. Anemia in chronic kidney disease: from pathophysiology and current treatments, to future agents. Frontiers in Medicine. 2021 Mar 26;8:642296.
53- Nanda N, Juthani-Mehta M. Novel Biomarkers for the Diagnosis of Urinary Tract Infection–-A systematic Review. Biomarker insights. 2009 Jan;4:BMI-S3155.
54- Kuan PF, Powers S, He S, Li K, Zhao X, Huang B. A systematic evaluation of nucleotide properties for CRISPR sgRNA design. Bmc Bioinformatics. 2017 Dec;18(1):1-9.
55- Martino FK, Novara G. Asymptomatic Bacteriuria or Urinary Tract Infection? New and Old Biomarkers. International Journal of Translational Medicine. 2022 Feb 1;2(1):52-65.
56- Schmidt-Ott KM. Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, Barasch J. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2007;18:407-13.
57- Oksay SC, Dursun H, Neijmann ST, Hatipoglu S. Using urinary neutrophile gelatinase-associated Lipocalin for prognosticate renal dysfunction in children with familial Mediterranean fever the study design: a pilot study. Advances in Rheumatology. 2021 Apr 16;61.
58- Horváth J, Wullt B, Naber KG, Köves B. Biomarkers in urinary tract infections–which ones are suitable for diagnostics and follow-up?. GMS infectious diseases. 2020;8.
59- Bradburn MJ, Clark TG, Love SB, Altman DG. Survival analysis Part III: multivariate data analysis–choosing a model and assessing its adequacy and fit. British journal of cancer. 2003 Aug;89(4):605-11.
60- Liuzzo G. Biasucci LM, Gallimore JR, Grillo RL, Rebuzzi AG, Pepys MB, Maseri A. The prognostic value of C-reactive protein and serum amyloid, a protein in severe unstable angina. N Engl J Med. 1994;331:417-24.
61- Bazeley J, Bieber B, Li Y, Morgenstern H, de Sequera P, Combe C, Yamamoto H, Gallagher M, Port FK, Robinson BM. C-reactive protein and prediction of 1-year mortality in prevalent hemodialysis patients. Clinical Journal of the American Society of Nephrology. 2011 Oct 1;6(10):2452-61.
62- Roubicek T, Bartlova M, Krajickova J, Haluzikova D, Mraz M, Lacinova Z, Kudla M, Teplan V, Haluzik M. Increased production of proinflammatory cytokines in adipose tissue of patients with end-stage renal disease. Nutrition. 2009 Jul 1;25(7-8):762-8.
63- Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, Squadrito F, Altavilla D, Bitto A. Oxidative stress: harms and benefits for human health. Oxidative medicine and cellular longevity. 2017 Oct;2017.
64- Gwozdzinski K, Pieniazek A, Gwozdzinski L. Reactive oxygen species and their involvement in red blood cell damage in chronic kidney disease. Oxidative medicine and cellular longevity. 2021 Feb 25;2021:1-9.
65- Actor JK, Hwang SA, Kruzel ML. Lactoferrin is a natural immune modulator. Current pharmaceutical design. 2009 Jun 1;15(17):1956-73.
66- Okazaki Y, Kono I, Kuriki T, Funahashi S, Fushimi S, Iqbal M, Okada S, Toyokuni S. Bovine lactoferrin ameliorates ferric nitrilotriacetate-induced renal oxidative damage in rats. Journal of clinical biochemistry and nutrition. 2012;51(2):84-90.
67- Kruzel ML, Zimecki M. acteur JK lactoferrine dans un contexte de pathologie induite par l'inflammation. Devant. Immunol. 2017;8:1438.
68- Gyurászová M, Kovalčíková AG, Renczés E, Kmeťová K, Celec P, Bábíčková J, Tóthová Ľ. Oxidative stress in animal models of acute and chronic renal failure. Disease markers. 2019 Oct;2019
69- Duffy S, Gokce N, Holbrook M, Huang A, Frei B, Keaney JF, Vita JA. Treatment of hypertension with ascorbic acid. The Lancet. 1999 Dec 11;354(9195):2048-9.
70- Åbrink M, Larsson E, Gobl A, Hellman L. Expression of lactoferrin in the kidney: implications for innate immunity and iron metabolism. Kidney international. 2000 May 1;57(5):2004-10.
71- Yen CC, Chang WH, Tung MC, Chen HL, Liu HC, Liao CH, Lan YW, Chong KY, Yang SH, Chen CM. Lactoferrin protects hyperoxia-induced lung and kidney systemic inflammation in an in vivo imaging model of NF-κB/luciferase transgenic mice. Molecular imaging and biology. 2020 Jun;22:526-38.
72- Fakhry SS, Hammed ZN, Bakir WA-E, ALRubaii BAL. Identification of methicillin-resistant strains of Staphylococcus aureus isolated from humans and food sources by use of mecA 1 and mecA 2 genes in Pulsed-field gel electrophoresis technique. Bionatura. 2022;7(2):44. doi: 10.21931/RB/2022.07.02.44.
73- Ali M, Al-Rubaii B. Study of the Effects of Audible Sounds and Magnetic Fields on Staphylococcus aureus Methicillin Resistance and mecA Gene Expression. Trop J Nat Prod Res. 2021;5(5):825-830.
74- Hashim ST, Fakhry SS, Rasoul LM, Saleh TH, Alrubaii BA. Genotyping toxins of Clostridium perfringens strains of rabbit and other animal origins. Trop J Nat Prod Res. 2021;5(4):613-616.
75- Abdulkaliq Awadh H, Hammed ZN, Hamzah SS, Saleh TH, AL-Rubaii BA. Molecular identification of intracellular survival related Brucella melitensis virulence factors. Biomedicine. 2022;42(4):761-765.
76- Abdul-Gani MN, Laftaah BA. Purification and characterization of chondroitinase ABC from Proteus vulgaris, an Iraqi clinically isolate. Curr Sci. 2017:2134-2140.
77- Kadhim AL-Imam MJ, AL-Rubaii BA. The influence of some amino acids, vitamins and anti-inflammatory drugs on the activity of chondroitinase produced by Proteus vulgaris caused urinary tract infection. Iraqi J Sci., 2016; 57 (4A):2412-2421.
78- Saleh, T.; Hashim, S.; Malik, S.N.; Al-Rubaii, B.A.L. The impact of some the nutrients on the swarming phenomenon and detection of the responsible gene RsbA in clinical isolates of Proteus mirabilis. International Journal of Research in Pharmaceutical Sciences.2020;1(6):437-444.
79- Shehab ZH, Laftah BA. Correlation of nan1 (Neuraminidase) and production of some type III secretion system in clinical isolates of Pseudomonas aeruginosa. BIOSCIENCE RESEARCH. 2018;15(3):1729-1738.
80- Al-Rubii, B.A.L. Cloning LasB gene of pseudomonas aeruginosa elastase 10104-2aI in E. coli BL21 and e. coli DH5? and investigated their effect on the stripping of Vero cells. Pakistan Journal of Biotechnology.2017;14(4):697-705.
81- Abdulla L, Ismael MK, Salih TA, Malik SN, Al-Rubaii BA. Genotyping and evaluation of interleukin-10 and soluble HLA-G in abortion due to toxoplasmosis and HSV-2 infections. Ann Parasitol., 68(2):385–390.
82- Jiad AL, Ismael MK, Muhsin SS, Al-Rubaii BA. ND2 Gene Sequencing of Subfertile Patients Recovered from COVID-19 in Association with Toxoplasmosis. Bionatura. 2022; 7(3):45. http://dx.doi.org/10.21931/RB/2022.07.03.45.
83- Rasoul LM, Nsaif MM, Al-Tameemi MT, Al-Rubaii BA. Estimation of primer efficiency in multiplex PCR for detecting SARS-Cov-2 variants. Bionatura, 2022, 7(3), 48. http://dx.doi.org/10.21931/RB/2022.07.03.49.
84- Rasoul, LM; Marhoon, A.A.; Albaayit, S.F.A.; Ali, R.W.; Saleh, T.H.; Al-Rubaii, B.A.L. Cytotoxic effect of cloned EGFP gene on NCI-H727 cell line via genetically engineered gene transfer system. Biomedicine (India). 2022,42(5): 938-942.
85- Ahmed, A.A., Khaleel, K.J., Fadhel, A.A., Al-Rubaii, B.A.L. Chronic Myeloid Leukemia: A retrospective study of clinical and pathological features. Bionatura, 2022, 7(3), 41. DOI. 10.21931/RB/2022.07.03.41.
86- Ali, S.M., Lafta, B.A., Al-Shammary, A.M., Salih, H.S. In vivo oncolytic activity of non-virulent Newcastle disease virus Iraqi strain against mouse mammary adenocarcinoma. AIP Conference Proceedings, 2021, 2372, 030010.
87- Ali SM, Laftah BA, Al-Shammary AM, Salih HS. Study the role of bacterial neuraminidase against adenocarcinoma cells in vivo. InAIP Conference Proceedings 2021, 2372, 030009.
88- Hamoode, R.H.; Alkubaisy, S.A.; Sattar, D.A.; Hamzah, SS; Saleh, T.H.; Al-Rubaii, B.A.L. Detection of anti-testicular antibodies among infertile males using indirect immunofluorescent technique. Biomedicine (India).2022; 42 (5):978-982.
Received: 28 May 2023/ Accepted: 15 July 2023 / Published:15 September 2023
Citation: Alajeli S H., Al-Qaiym M A., Altaie L H. Interaction of Lactoferrin with Neutroplile Gelatinase Association Lipocalin in Treatment of Chronic Kidney Diseases Induced Experimentally by K. pneumonia. Revis Bionatura 2023;8 (3) 26. http://dx.doi.org/10.21931/RB/2023.08.03.26