2022.07.04.71
Files > Volume 7 > Vol 7 No 4 2022
Mohammed Abd Ali Jabber Al-Saady1,2, Nawfal H. Aldujaili3,4, Shiama Rabeea Banoon5* , Aswan Al-Abboodi6
, Aswan Al-Abboodi6
1:AL Sader Teaching Hospital, Maysan Health Directorate, Ministry of Health, Maysan, Iraq
2:Department of Mechanical and Aerospace Engineering, Monash University, VIC 3800, Australia
3:Department of Biology, Faculty of Science, University of Kufa, Najaf, Iraq.
4: Alameen Center for Advanced Research and Biotechnology, Imam Ali Holy Shrine, Najaf, Iraq
5:Department of Biology, College of Science, University of Misan, Maysan, Iraq.
6: Department of Biology, College of Science, University of Misan, Maysan, Iraq.
Corresponding author: [email protected]
Available from: http://dx.doi.org/10.21931/RB/2022.07.04.71
ABSTRACT
Biofilm is a structure in the shape of a surface adherent composed of a microbe’s community and plays a crucial role in stimulating the infection. Due to the Biofilm’s complex structure compared with the individual microbe, it occasionally develops recalcitrant to the host immune system, which may lead to antibiotic resistance. The National Institutes of Health has reported that more than 80% of bacterial infections are caused by biofilm formation. Removing biofilm-mediated infections is an immense challenge that should involve various strategies that may induce sensitive and effective antibiofilm therapy. In the last decade, nanoparticle NPs application has been employed as one of the strategies that have grown great stimulus to target antibiofilm treatment due to their unique properties. Nanobiotechnology holds promise for the future because it has various antimicrobial properties in biofilms and promising new drug delivery methods that stand out from conventional antibiotics. Studying the interaction between the Biofilm and the nanoparticles can deliver additional insights regarding the mechanism of biofilm regulation. This review article will define synthetic nanoparticle NPs, their medical applications, and their potential use against a broad range of microbial biofilms in the coming years. The motivation of the current review is to focus on NPs materials’ properties and applications and their use as antimicrobial agents to fight resistant infections, which can locally terminate bacteria without being toxic to the surrounding tissue and share its role in improving human health in the future.
Keywords: Biofilms, antimicrobial, nanoparticles, bio-nanotechnology, drug resistance.
INTRODUCTION
Nanobiotechnology syndicates biological applications with physical or chemical methods to produce nanoparticles with specific characterizations. Nanotechnology is a complicated science as it uses materials and compounds to create devices on near-atomic levels and is a new promising emerging field 1, 2.
Nanoparticles have unique properties to combat conditions and diseases and have established substantial consideration in various majors such as biomedicine. Technology is in the nano range 3. Nanoparticles come in multiple shapes, such as in a spherical form, such as a rod or plate, among other shapes. They can also be rigid or incompact and fabricated from diverse resources. Nanoparticles can be synthesized from Top-down and Bottom-up (Figure 1). In the top and bottom-up (chemical and biological) process 4. The primary use of nanotechnology in the biomedical field is to deliver medications directly to cells or to create chemical cascades that can alter one’s health and immune system to combat a range of diseases such as cancer, infectious diseases, or autoimmune diseases 5,6.
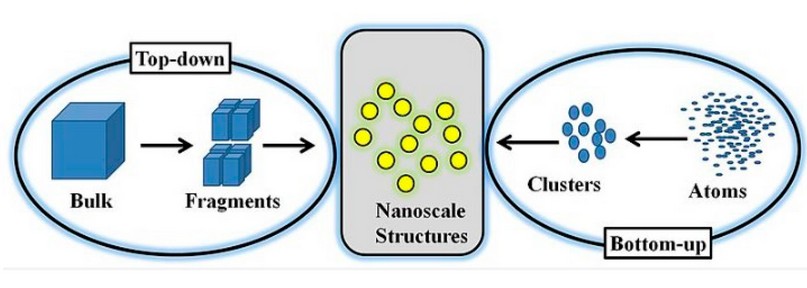
Figure 1. Illustrates the top-down and bottom-up approaches for making nanoparticles 7.
Physical-chemical properties of nanoparticles include particle size/size distribution, shape, solubility, agglomeration state/aggregation, purity and composition, surface area, surface chemistry, porosity, and other features that provide valuable information on nanoscale systems and could be melodramatically dissimilar from the particles in the range of micrometer size 8. The nanoparticle’s features, either the chemical or physical properties, should permit them to interrelate closely with bacterial biofilms and consequently provoke an antibacterial consequence that is not exclusively due to the release of metal ions which is extremely useful when used inside the body as it holds promising theories to how this technology can be used enhance one’s health and amplify what is possible 9.
Human infections can be caused by a wide variety of microorganisms plus bacteria, fungi, parasites, and viruses; microorganisms of all groups are related to infections. Bacteria are the predominant constituent of microflora, and the assortment of species reflects the extensive range of endogenously resulting nutrients and the wide-ranging types of habitats to build the colonization 10. The Biofilm could be categorized as a cumulative of microorganisms, including bacteria, in which microorganism cells adhere to each other and to a surface where they are accumulative. Though, the relationship between biofilms and the host can be disturbed in several pathways after the collapse of the microflora. Nanotechnology can interact with biofilms and microflora to improve drug delivery to these areas 11.
The polymicrobial phenomenon is dominant in most bacterial infections, and it is relatively infrequent to discover any that are obviously due to solitary types. The comparative influence of diverse bacterial species components in a specific infection is thus hard to control. The use of nanobiotechnology offers the possibility to control the formation of biofilms using nanoparticles as antimicrobial, antibiofilm, or with antibacterial, antiadhesive, and enhanced delivery competences (Figure 2) 12, 13.
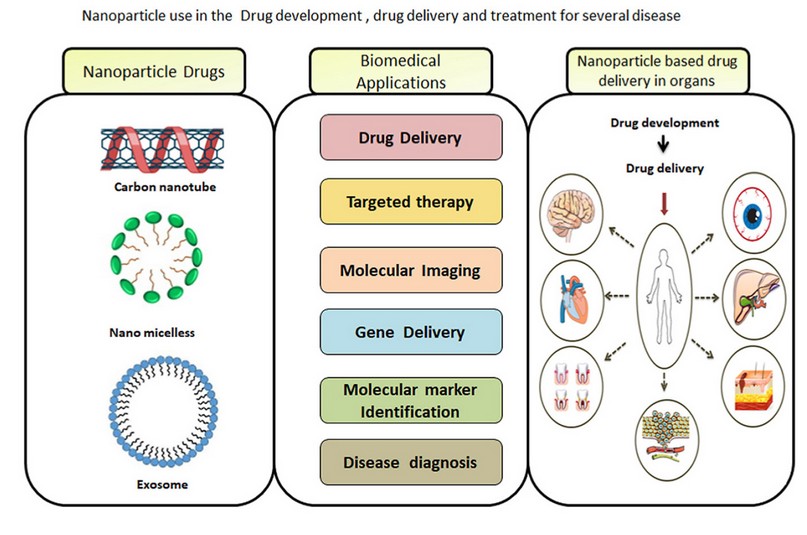
Figure 2. Nanobiotechnology offers the possibility to control many diseases 14.
Quorum Sensing (QS)
Quorum sensing is the cell-to-cell signaling procedure that determines multi-cellular performance in microorganisms involving bacteria. Gram-negative bacteria species habitually engage minor substances such as autoinducers, signaling substances, proteins, etc. These performances in recital with a protein receptor modulate alterations in gene expression to stimulate a response to vicissitudes in the population of the cells. At the same time, in Gram-positive bacteria, oligopeptides are preferred. Increasing concentrations will result in microorganisms producing and releasing chemical signal molecules as a function of cell density 15. Quorum Sensing has significant applications in nanotechnology because of its newfound antibacterial and antiviral properties. Due to Quorum Sensing essentially means the communication between multiple cells, nanotechnology can take advantage of this interaction and use it to help kill the Biofilm by doing things such as blocking communication, instead of taking the traditional course of broad-spectrum antibiotics 16.
Microbial biofilms and infection
Planktonic cells are isolated, free-living cells that can form the Biofilm that does not make part of the sessile cells. A biofilm is a cumulative of microorganisms with a diverse construction where cells adhere to a static surface, and biofilms are everywhere, such as on the surface of water or human teeth. Biofilms might be constructed on living and nonliving materials, which are of extensive alarm both from the environment and from a medical point of view. These cells are entrenched in the self-produced medium or environment 17.
Planktonic cells are much easier to kill with antibiotics than cells that are part of a biofilm. This is because, in a biofilm, all species in one can benefit from each other’s existence. After all, different microbes can collaboratively unlock newfound capabilities and amplify them, leading to rapid and uncontrolled growth of the Biofilm, and this can lead to severe illness or even death in humans due to the corresponding bacterial infection. This is where biomedical nanotechnology is useful, as it can interfere with the Biofilm using novel approaches to remove the infection at efficiency and speeds that antibiotics cannot achieve 18,19.
The biofilm milieu
The biofilm milieu has sponge-like makings that reflect organizational integrity to the Biofilm although still permitting the movement of small substances to penetrate the Biofilm and to spread on the out-layer surface. The milieu is highly hydrated and composed of up to approximately 97% H2O, often involving diverse types of polysaccharides along with other components such as proteins, lipids, DNA, and Ca+2 ions. The biofilm matrix is required for nanotechnology to pass through biofilms to enhance its drug delivery 20,21,22,23.
Factors of Microbial Biofilm
Generally, the Biofilm might be affected by many factors counting the cellular recognition for various attachment sites on a surface, contact with the planktonic cells with a sub-inhibitory concentration of antimicrobe, nutrition shortage, incidence of toxic metals, and other stress circumstances which can influence how the Biofilm grows and interacts with its environment 24. Conditions as to whether a biofilm even forms in the first place are numerous, including temperature and nutrients, among other external growth factors. Without the right conditions, no biofilm will form. For a biofilm to keep existing, there needs to be a continued nutrient supply and a good ecosystem, so the microflora remains stable 25,26.
Microbial Advantages of a Biofilm
Biofilm advantages include many aspects that may be briefed to increase the expression of valuable genes, phenotypic vicissitudes in colony morphology, the manufacture of copious quantities of extracellular polymers that improve the admission to nutrients, achievement of antibiotic resistance genes by plasmid transmission way, and closer proximity between cells easing mutualistic or synergistic links and protection 27,28. The biofilms usually assist bacteria in producing the virulence influences coordinately and disguising from the animal or plant’s immune system 29. The signal transduction could enhance bacterial mating among bacterial nearness available in a biofilm and, therefore, the achievement of original DNA by the transformation, which is improved and supplements the bacterial diversity 30.
The importance of Biofilm in the field of bacteriology
The reputation of biofilm development has been documented with microorganisms in one tend to vary decidedly from their planktonic counterparts in relation to behavior, construction, and physiology. These changes have consequences for the pathogenic possibility of microorganisms and their vulnerability to antimicrobials 31.
Biofilms make up a surprising amount of microbial activity, and planktonic bacteria are rarely a problem to human health. Adapting nanotechnology to biofilms to efficiently deliver drugs and disable bacteria means that the international scientific community must better understand biofilms and their relation to nanotechnology 32,33.
Biofilm stages
The development of biofilm formation is a highly complex process. Still, it is commonly recognized as containing five stages, starting with the development of a surface that the Biofilm can attach to, the crusade of microorganisms into the closeness with the surface, adhesion (in either way the reversible or irreversible of microbes adhesion to the habituated surface), development and reproduction of the organisms within the colonization of the biofilm surface, microcolony construction, and biofilm development; phenotype and genotype variations and biofilm cell detachment/dispersal 34. It is well known that identified antibiotics can attack single bacteria and Biofilm in their early formation stages. However, it is relatively tough to destroy the late formation stages of the biofilms using traditional antibiotics, and the nanoparticles may play a vital role in terminating the multiple layers of biofilms, as shown in Figure 3.
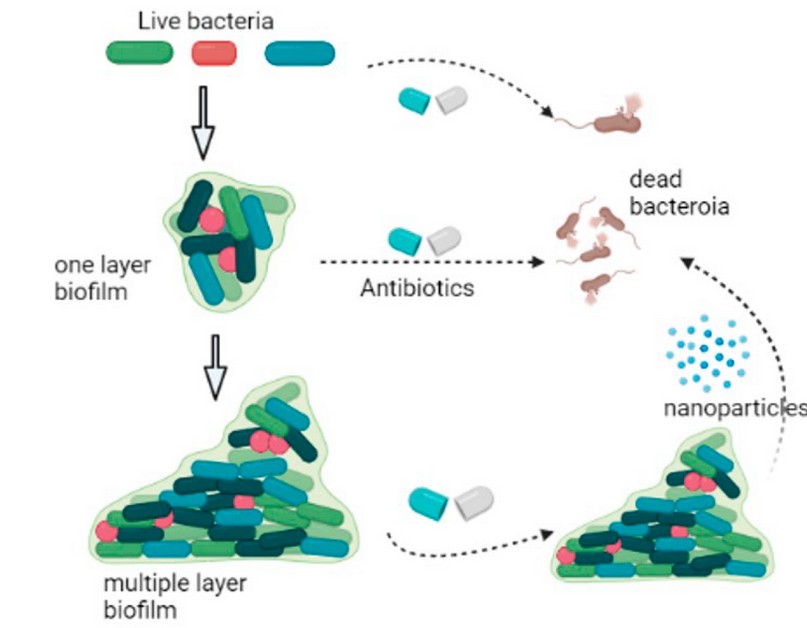
Figure 3. Biofilm growth stages, the inhibition of single bacteria cells and the one-layer Biofilm using traditional antibiotics, and the inhibition of the multiple-layer Biofilm using nanoparticles (Biorender Program).
Occurrence and examples of Biofilm
Dental plaque forms on our teeth when we neglect oral health and is an example of a biofilm; these have since been stated to be in diverse environments, for example, chronic wounds, in individuals with Cystic Fibrosis in their lungs, or plants and agricultural systems. It has been reported that 60–80% of microorganisms exist in the biofilm lifeform. Relevant biofilms are present in up to 80% of infections. Many bacterial species, such as Bacillus sp., Pseudomonas sp., E. coli, Lactobacillus sp., form biofilms under the correct environmental conditions 35.
Biofilms are everywhere on Earth and can rapidly grow to substantial sizes covering entire animals and rainforests and even growing to the size of small countries. There are many different types of biofilms, however, among the most common ones are algae, fungus (mold), dental plaque, and moss 36, 37.
Multispecies Biofilms
Multispecies biofilms often comprise algae, bacteria, fungi, and protozoa reliant on the colonization environment. Advantages include Interspecies interactions within biofilms resulting in increased antimicrobial tolerance, protection from foreign predators, degradation of pollutants, and indorse the spread of drug-resistance indicators and other virulence factors. Estimates of antimicrobial efficiency contrary to multispecies biofilms relying on monoculture assays may not be practicable 38.
The contaminating bacteria are adherent to some substratum or are surface-related; direct investigation of affected tissue displays bacteria living in cell clusters, or microcolonies, enclosed in an extracellular matrix. The matrix might often consist of bacterial and host contents. The infections are commonly limited to a specific site. Though dissemination might occur, it is a subordinate phenomenon. The infections are problematic or impossible to eliminate with antibiotics even though the accountable organisms are vulnerable to them in a planktonic state 39, 11.
Detection methods of microbial Biofilm
Biofilm can be detected with various methods such as a tissue culture plate, the tube method, the Congo Red Agar method, Confocal Laser Scanning Microscopy (CLSM), fluorescent microscopy, bioluminescence assays, electro voltammetric detection of biofilm markers, and the Biofilm Ring Test. Biofilms come in many shapes and forms, so frequently, a human can detect a biofilm with the naked eye, such as when algae form on stagnant water or dental plaque forms on teeth 3.
Fighting Microbial Biofilms
The omnipresence and difficulty of biofilms in industrial, environmental and clinical systems present challenges for therapeutic interference. Their aptitude to perform as a breeding base for multidrug resistance and horizontal gene transfer, enabling the emergence of pathogenic strains, additional highlights the need to report their control 40. The principal challenge is the broad-mindedness of these biofilms to most traditional or classical antibiotics. Most drugs currently used in the clinical setting have been developed and optimized to kill planktonic cells. Even when killing is accomplished, the concentration mandatory for biofilm control (minimum Biofilm inhibiting concentration, (MBIC) far exceeds that essential for control of planktonic cultures (MBIC). When the death of biofilm cells is accomplished, the capability of a subdivision of the population to survive this task, mentioned as persisted cells, means that the Biofilm can regenerate once conditions become favorable again. Therefore, new approaches are required to target the biofilm mode growth 41.
A diverse range of methods has been labeled in the literature. Antimicrobial peptides, exopolysaccharides, repurposed drugs, enzymes, chelating agents, bacteriophage therapy, quorum sensing inhibitors, and nanoparticles have all acknowledged significant considerations 42,43,44.
Antibiofilm activity of Nanoparticles
The administration of therapeutics into Biofilm is highly affected by the penetration of antimicrobial agents into the Biofilm. Nanotechnology deals with the design where the small size of the nanoparticles supports the procedure due to the relatively large surface area and potential group dynamic nature, these features play a crucial role in controlling biofilms through either their biocidal or antiadhesive activities 45. The research by Watson et al. used the “Leeds in situ models,” which considers a tool that assists dental plaque in growing in situ on a detachable human enamel layer, has aided in the valuation of innovative antimicrobial agents, and is considered the extremely complex microbial composition and architecture of plaque biofilms. Using such a tool model of intact Biofilm would help gain information on the penetration of the nanoparticles on natural tooth surfaces, which may indicate that there are channels and voids in the plaque. It may occasionally spread entirely through the biomass to the underlying enamel and considerably influence the transfer of nanoparticles through biofilms 46.
Metals such as copper, silver, zinc, and gold have been employed for the last period as antimicrobial agents; they have appealed to specific attention due to their particular chemical and mechanical properties that have affected their potential roles. Many products, including toothpaste, now incorporate powdered zinc citrate or acetate to control the formation of dental plaque. Metallic nanoparticles have also been considered to improve antimicrobial efficiency 47,48,49,50 .
The ability of the nanoparticles to be absorbed within the depth of the Biofilm is a key consideration in making these nanoparticles of potential effects. Therefore, the physical and chemical properties of the nanoparticles used, such as the biodegradability, biocompatibility, surface charge, and degree of hydrophobicity 51. Nanoparticles play a significant role in causing cell death or apoptosis through different mechanisms, as shown in Figure 4.
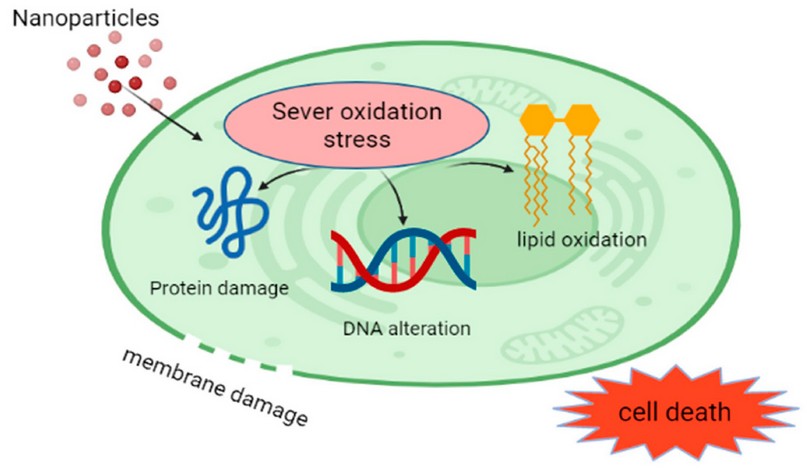
Figure 4. Nanoparticle effects on the living cells (Biorender Program).
The antimicrobial characterizations of silver and copper have established the most consideration. Both have been layered into various base materials, including PMMA and hydrogel. It has been shown that smaller silver nanoparticles are more toxic than larger particles, more so when oxidized. At the nanoscale, Ag1 ions are known to be released (leached) from the surface. Using silver (Ag) nanoparticles (100nm), the antimicrobial action was dominated by Ag1 ions.
In comparison, for larger particles (15 nm), the aids of Ag1 ions and particles to the antibacterial activity are comparable. The Ag1 ion release is proportional to the showing nano-silver surface area. Because of their small size, nanoparticles (Figure 5) may offer other advantages to the biomedical field by improved biocompatibility 52,53, 54,55.
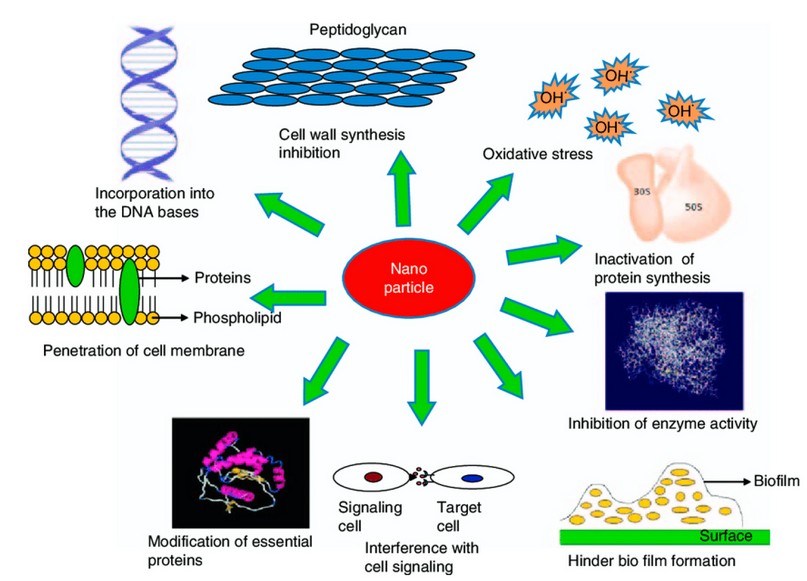
Figure 5. Mechanisms for antibacterial activity of nanoparticles 56.
Numerous theories and descriptions have been suggested for diverse nanoparticles for their microbicidal activity (Figure 2). It seems that bacteria are distant and less likely to obtain resistance to metal nanoparticles than they are to other traditional antibiotics with narrow-spectrum. This is supposed to happen because metals may act on a wide variety of bacterial boards, and frequent mutations would have to occur for the microorganisms to resist their antimicrobial activity 47. Shape may also affect the activity of nanoparticles. The form of silver nanoparticles has been studied. Table 1 explains the most updated information about the toxicity mechanisms of different nanoparticles against biofilms.
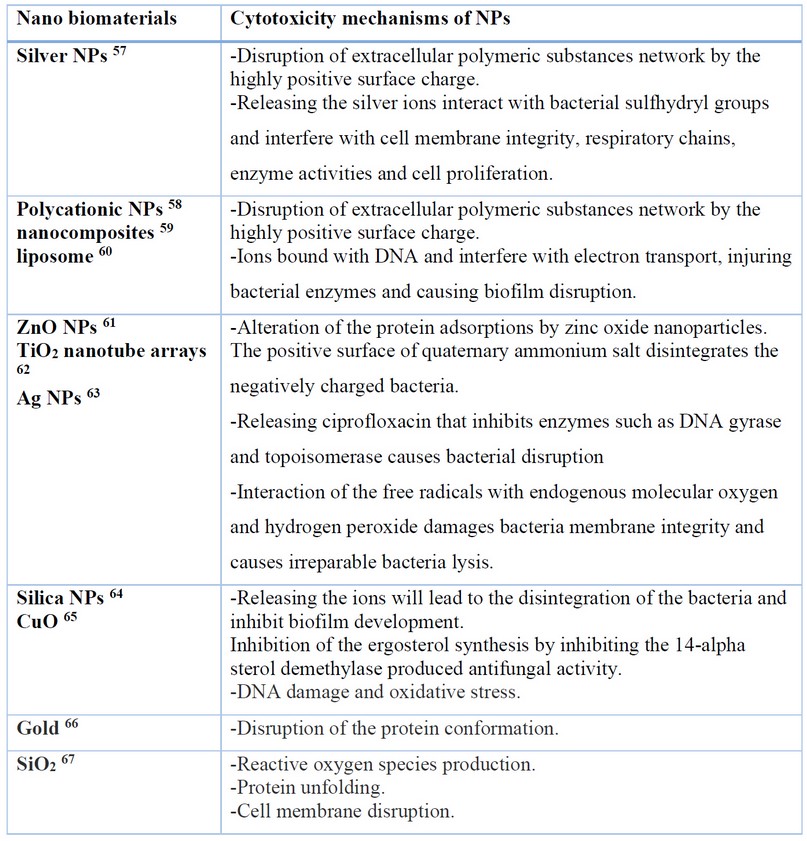
Table 1. An update on the Nanoparticle Cytotoxicity mechanisms to prevent and treat the biofilms
Since NPs were shown to affect antibacterial activity in Escherichia coli, this conclusion may be drawn. Exhibiting the exception of round and rod-shaped silver nanoparticles, those with a lattice structure on the basal plane demonstrated superior biocide activity. The discrepancies appear to be explained by the number of active facets in nanoparticles of various shapes 68.
This shows that mistreatment of the toxic properties of nano particulate metals and metal oxides is feasible; in particular, those that method reactive oxygen species under UV light, such as titanium dioxide and zinc oxide, are discovered to increased use in antimicrobial formulations, with silver metal nanoparticles (5240 nm) having been reported to inactivate most microorganisms, including HIV-1 69, 70.
The large responsiveness of nano titanium dioxide and nano silicon dioxide (SiO2) is exploited expansively for their bactericidal characterizations in filters and coatings on substrates for example, polymers, glasses, ceramics and alumina. In 2009, a novel strain of Influenza recombined in Mexico, leading to a pandemic of H1N1, a common strain of Influenza A. This led to renewed development in nanotechnology to combat such viruses and new interest in using them as antiviral medication. Substantial achievement using metal and metal oxide nanoparticles and their composite clusters against fungal and bacterial pathogens such as methicillin-resistant Staphylococcus aureus (MRSA) and E. coli has been confirmed. These have also shown the competence to inactivate viruses, including severe acute respiratory syndrome (SARS), H1N1 swine flu, and H5N1 bird flu. For example, new broad-spectrum materials (5260 nm) can reduce virus levels by anywhere from 80-2100% through direct or indirect exposure 71.
Nanoparticle arrangements, counting those based upon copper, titanium (TiO2), (ZnO), (Al2O3), nickel (Ni, NiO), zirconium (ZrO2), silicon (IV) nitride (Si3N4), silver (Ag), and tungsten carbide (WC) have been compared in regards to their antimicrobial potential 53, 72.
Substantial activity when using Ag, TiO2, and ZnO in the presence of UV light), SiO2, and CuO in contradiction of bacterial pathogens, counting MRSA and Pseudomonas aeruginosa, have been demonstrated. MBCs were found to be in the range of 0.1-5 mg/mL
In evaluation, the conventional antibiotics are potential at concentrations 1000-fold lower. NiO, Ni, Al2O3, Si3N4, TiO2 (in the absence of UV light), WC (tungsten carbide), and ZrO2 lead to a lack of antimicrobial ability at the concentrations experienced. The oral pathogens Streptococcus intermedius, P. gingivalis, F. nucleatum, P. intermedia, and A. actinomycete mcomitans were also found to be vulnerable to Ag and CuO nanoparticles under anaerobic conditions with MBC values in the range 0.025-2.5 mg/mL 73,74, 75.
CONCLUSIONS
Biofilms are structures of an accumulative of divided members of the microorganisms that may be of a single species or accumulative of a variety of microbial species communicating community-based drug resistance. Therefore, treating biofilm-mediated infections using the usual classic medicines is undoubtedly problematic. Treatment of biofilms using nanoparticle technology as antibiofilm agents is an up-and-coming method to eliminate conditions raised by microbes, including bacteria. Numerous types of NPs have been experimented with to check their activity as antibiofilm agents, and several of these NPs possess excellent anti-biofilm activity. Immobilization or impregnations of NPs are ways used to prepare biomedical surfaces. Fabrication of intelligent nanoparticles that can eradicate or treat biofilms is a step toward biofilm termination. However, many obstacles and limitations still require more research and trials. The toxic effects of some tested nanoparticles can be resolved by developing different eco-friendly methods. Despite numerous studies that have been conducted on experimenting with nanoparticles against biofilms, however, the mechanism of action is still a mystery. In the future, the potential nanoparticles that apply as antibiofilm agents will assist in improving human health by regulatory the Biofilm mediated infections.
REFERENCES
1- Beloin C, Renard S, Ghigo JM, Lebeaux D. Novel approaches to combat bacterial biofilms. Current opinion in pharmacology. 2014 October 1;18:61-8.
2- Albukhaty S, Naderi-Manesh H, Tiraihi T, Sakhi Jabir M. Poly-l-lysine-coated superparamagnetic nanoparticles: a novel method for the transfection of pro-BDNF into neural stem cells. Artificial cells, nanomedicine, and biotechnology. 2018 Nov 12;46(sup3):S125-32.
3- Williams DN, Ehrman SH, Pulliam Holoman TR. Evaluation of the microbial growth response to inorganic nanoparticles. Journal of nanobiotechnology. 2006 Dec;4(1):1-8.
4- Arbatan T, Al‐Abboodi A, Sarvi F, Chan PP, Shen W. Tumor inside a pearl drop. Advanced healthcare materials. 2012 Jul;1(4):467-9.
5- Monteiro DR, Gorup LF, Takamiya AS, Ruvollo-Filho AC, de Camargo ER, Barbosa DB. The growing importance of materials that prevent microbial adhesion: antimicrobial effect of medical devices containing silver. International journal of antimicrobial agents. 2009 Aug 1;34(2):103-10.
6- Banoon SR, Ghasemian A. The characters of graphene oxide nanoparticles and doxorubicin against HCT-116 colorectal cancer cells in vitro. Journal of Gastrointestinal Cancer. 2022 Jun;53(2):410-4.
7- Arole VM, Munde SV. Fabrication of nanomaterials by top-down and bottom-up approaches-an overview. J. Mater. Sci. 2014;1:89-93.
8- Al‐Abboodi A, Tjeung R, Doran PM, Yeo LY, Friend J, Yik Chan PP. In Situ Generation of Tunable Porosity Gradients in Hydrogel‐Based Scaffolds for Microfluidic Cell Culture. Advanced healthcare materials. 2014 Oct;3(10):1655-70.
9- Allaker RP, Ren G. Potential impact of nanotechnology on the control of infectious diseases. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2008 Jan 1;102(1):1-2.
10- Das S, Dash HR. Microbial biotechnology-A laboratory manual for bacterial systems. Springer; 2014 Nov 24.
11- Bjarnsholt T, Alhede M, Alhede M, Eickhardt-Sørensen SR, Moser C, Kühl M, Jensen PØ, Høiby N. The in vivo biofilm. Trends in microbiology. 2013 Sep 1;21(9):466-74.
12- Flemming HC, Wingender J. The biofilm matrix. Nature reviews microbiology. 2010 Sep;8(9):623-33.
13- Jihad MA, Noori F, Jabir MS, Albukhaty S, AlMalki FA, Alyamani AA. Polyethylene Glycol Functionalized Graphene Oxide Nanoparticles Loaded with Nigella sativa Extract: A Smart Antibacterial Therapeutic Drug Delivery System. Molecules. 2021 Jan;26(11):3067.
14- Sahu T, Ratre YK, Chauhan S, Bhaskar LV, Nair MP, Verma HK. Nanotechnology based drug delivery system: Current strategies and emerging therapeutic potential for medical science. Journal of Drug Delivery Science and Technology. 2021 June 1;63:102487.
15- Seil JT, Webster TJ. Antimicrobial applications of nanotechnology: methods and literature. International journal of nanomedicine. 2012;7:2767.
16- Abdalla SS, Katas H, Azmi F, Busra MF. Antibacterial and anti-biofilm biosynthesised silver and gold nanoparticles for medical applications: Mechanism of action, toxicity and current status. Current drug delivery. 2020 Feb 1;17(2):88-100.
17- Arita-Morioka KI, Yamanaka K, Mizunoe Y, Ogura T, Sugimoto S. Novel strategy for biofilm inhibition by using small molecules targeting molecular chaperone DnaK. Antimicrobial agents and chemotherapy. 2015 Jan 1;59(1):633-41.
18- Azam A, Ahmed AS, Oves M, Khan MS, Memic A. Size-dependent antimicrobial properties of CuO nanoparticles against Gram-positive and-negative bacterial strains. International journal of nanomedicine. 2012;7:3527.
19- Azam A, Ahmed AS, Oves M, Khan MS, Habib SS, Memic A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: a comparative study. International journal of nanomedicine. 2012;7:6003.
20- Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clinical microbiology reviews. 2002 Apr;15(2):167-93.
21- Ramos MA, Da Silva PB, Spósito L, De Toledo LG, Bonifácio BV, Rodero CF, Dos Santos KC, Chorilli M, Bauab TM. Nanotechnology-based drug delivery systems for control of microbial biofilms: a review. International journal of nanomedicine. 2018;13:1179.
22- Sahli C, Moya SE, Lomas JS, Gravier-Pelletier C, Briandet R, Hémadi M. Recent advances in nanotechnology for eradicating bacterial Biofilm. Theranostics. 2022;12(5):2383.
23- Bahadar H, Maqbool F, Niaz K, Abdollahi M. Toxicity of nanoparticles and an overview of current experimental models. Iranian biomedical journal. 2016 Jan;20(1):1.
24- Aldujaili NH, Abdullah NY, Khaqani RL, Al-Tfaly SA, Al-Shammary AH. Biosynthesis and antibacterial activity of titanium nanoparticles using Lactobacillus. Int J Recent Sci Res. 2015;6(12):7741-51.
25- Guzmán-Soto I, McTiernan C, Gonzalez-Gomez M, Ross A, Gupta K, Suuronen EJ, Mah TF, Griffith M, Alarcon EI. Mimicking biofilm formation and development: Recent progress in in vitro and in vivo biofilm models. Iscience. 2021 May 21;24(5):102443.
26- Krsmanovic M, Biswas D, Ali H, Kumar A, Ghosh R, Dickerson AK. Hydrodynamics and surface properties influence biofilm proliferation. Advances in Colloid and Interface Science. 2021 February 1;288:102336.
27- Wilson M. Susceptibility of oral bacterial biofilms to antimicrobial agents. Journal of medical microbiology. 1996 Feb 1;44(2):79-87.
28- Balcázar JL, Subirats J, Borrego CM. The role of biofilms as environmental reservoirs of antibiotic resistance. Frontiers in microbiology. 2015 October 31;6:1216.
29- Al-Kaabi WJ, Albukhaty S, Al-Fartosy AJ, Al-Karagoly HK, Al-Musawi S, Sulaiman GM, Dewir YH, Alwahibi MS, Soliman DA. Development of Inula graveolens (L.) plant extract electrospun/polycaprolactone nanofibers: a novel material for biomedical application. Applied Sciences. 2021 Jan 17;11(2):828.
30- Wang R, Zhang W, Ding W, Liang Z, Long L, Wong WC, Qian PY. Profiling signal transduction in global marine biofilms. Frontiers in microbiology. 2021;12.
31- Hagens WI, Oomen AG, de Jong WH, Cassee FR, Sips AJ. What do we (need to) know about the kinetic properties of nanoparticles in the body?. Regulatory toxicology and pharmacology. 2007 Dec 1;49(3):217-29.
32- Ramasamy M, Lee J. Recent nanotechnology approaches for prevention and treatment of biofilm-associated infections on medical devices. BioMed Research International. 2016 October 31;2016.
33- Yeh YC, Huang TH, Yang SC, Chen CC, Fang JY. Nano-based drug delivery or targeting to eradicate bacteria for infection mitigation: a review of recent advances. Frontiers in chemistry. 2020 April 24;8:286.
34- Muhammad MH, Idris AL, Fan X, Guo Y, Yu Y, Jin X, Qiu J, Guan X, Huang T. Beyond risk: bacterial biofilms and their regulating approaches. Frontiers in microbiology. 2020 May 21;11:928.
35- Li P, Li J, Wu C, Wu Q, Li J. Synergistic antibacterial effects of β-lactam antibiotic combined with silver nanoparticles. Nanotechnology. 2005 Jul 28;16(9):1912.
36- Falsetta ML, Klein MI, Colonne PM, Scott-Anne K, Gregoire S, Pai CH, Gonzalez-Begne M, Watson G, Krysan DJ, Bowen WH, Koo H. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infection and immunity. 2014 May;82(5):1968-81.
37- Horev B, Klein MI, Hwang G, Li Y, Kim D, Koo H, Benoit DS. pH-activated nanoparticles for controlled topical delivery of farnesol to disrupt oral biofilm virulence. ACS nano. 2015 Mar 24;9(3):2390-404.
38- Furiga A, Lajoie B, El Hage S, Baziard G, Roques C. Impairment of Pseudomonas aeruginosa biofilm resistance to antibiotics by combining the drugs with a new quorum-sensing inhibitor. Antimicrobial agents and chemotherapy. 2015 Dec 28;60(3):1676-86.
39- Dalton T, Dowd SE, Wolcott RD, Sun Y, Watters C, Griswold JA, Rumbaugh KP. An in vivo polymicrobial Biofilm wound infection model to study interspecies interactions. PloS one. 2011 Nov 4;6(11):e27317.
40- Vargas-Reus MA, Memarzadeh K, Huang J, Ren GG, Allaker RP. Antimicrobial activity of nanoparticulate metal oxides against peri-implantitis pathogens. International journal of antimicrobial agents. 2012 Aug 1;40(2):135-9.
41- Hetrick EM, Shin JH, Paul HS, Schoenfisch MH. Anti-biofilm efficacy of nitric oxide-releasing silica nanoparticles. Biomaterials. 2009 May 1;30(14):2782-9.
42- Banoon S, Ali Z, Salih T. Antibiotic resistance profile of local thermophilic Bacillus licheniformis isolated from Maysan province soil. Comunicata Scientiae. 2020 Jul 13;11:e3291-.
43- Sedarat Z, Taylor-Robinson AW. Biofilm Formation by Pathogenic Bacteria: Applying a Staphylococcus aureus Model to Appraise Potential Targets for Therapeutic Intervention. Pathogens. 2022 Mar 23;11(4):388.
44- Topka-Bielecka G, Dydecka A, Necel A, Bloch S, Nejman-Faleńczyk B, Węgrzyn G, Węgrzyn A. Bacteriophage-derived depolymerases against bacterial Biofilm. Antibiotics. 2021 Feb 10;10(2):175.
45- Al‐Abboodi A, Fu J, Doran PM, Chan PP. Three‐dimensional nanocharacterization of porous hydrogel with ion and electron beams. Biotechnology and bioengineering. 2013 Jan;110(1):318-26.
46- Watson PS, Sissons CH, Devine DA, Shore RC, Kirkham J, Nattress BR, Marsh PD, Robinson C. Minimizing prion risk without compromising the microbial composition of biofilms grown in vivo in a human plaque model. Letters in applied microbiology. 2004 Mar;38(3):211-6.
47- Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free radical biology and medicine. 1995 Feb 1;18(2):321-36.
48- Aldujaili NH, Banoon SR. Antibacterial characterization of titanium nanoparticles nano synthesized by Streptococcus thermophilus. Periodico Tche Quimica (Online). 2020;17(34):311-20.
49- Albukhaty S, Al-Bayati L, Al-Karagoly H, Al-Musawi S. Preparation and characterization of titanium dioxide nanoparticles and in vitro investigation of their cytotoxicity and antibacterial activity against Staphylococcus aureus and Escherichia coli. Animal Biotechnology. 2020 Dec 1:1-7.
50- Hassan BA, Lawi ZKK, Banoon SR. Detecting the activity of silver nanoparticles, pseudomonas fluorescens and bacillus circulans on inhibition of aspergillus. Periodico Tche Quimica (Online). 2020;17 (35):678-690.
51- Niazi JH, Gu MB. Toxicity of metallic nanoparticles in microorganisms-a review. Atmospheric and biological environmental monitoring. 2009:193-206.
52- Sahib FH, Aldujaili NH, Alrufae MM. Biosynthesis of silver nanoparticles using Saccharomyces boulardii and study their biological activities. European journal of pharmaceutical and medical research. 2017;4(9):65-74.
53- Aldujaili NH, Alrufa MM, Sahib FH. Antibiofilm antibacterial and antioxidant activity of biosynthesized silver nanoparticles using Pantoea agglomerans. Journal of Pharmaceutical Sciences and Research. 2017 Jul 1;9(7):1220.
54- Albukhaty S, Al-Karagoly H, Allafchian AR, Jalali SA, Al-Kelabi T, Muhannad M. Production and characterization of biocompatible nanofibrous scaffolds made of β-sitosterol loaded polyvinyl alcohol/tragacanth gum composites. Nanotechnology. 2021 Nov 30;33(8):085102.
55- Al-Karagoly H, Rhyaf A, Naji H, Albukhaty S, AlMalki FA, Alyamani AA, Albaqami J, Aloufi S. Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract. Green Processing and Synthesis. 2022 Jan 1;11(1):254-65.
56- Singh R, Smitha MS, Singh SP. The role of nanotechnology in combating multidrug resistant bacteria. Journal of nanoscience and nanotechnology. 2014 Jul 1;14(7):4745-56.
57- Tran HA, Tran PA. In Situ Coatings of Silver Nanoparticles for Biofilm Treatment in Implant-Retention Surgeries: Antimicrobial Activities in Monoculture and Coculture. ACS Applied Materials & Interfaces. 2021 Aug 27;13(35):41435-44.
58- Thorn CR, Kopecki Z, Wignall A, Kral A, Prestidge CA, Thomas N. Liquid crystal nanoparticle platform for increased efficacy of cationic antimicrobials against biofilm infections. Nanomedicine: Nanotechnology, Biology and Medicine. 2022 June 1;42:102536.
59- Kleszcz K, Hebda M, Kyzioł A, Krawiec H, Kyzioł K. Towards prevention of biofilm formation: Ti6Al7Nb modified with nanocomposite layers of chitosan and Ag/Au nanoparticles. Applied Surface Science. 2021 August 15;557:149795.
60- Kannan S, Solomon A, Krishnamoorthy G, Marudhamuthu M. Liposome encapsulated surfactant abetted copper nanoparticles alleviates Biofilm mediated virulence in pathogenic Pseudomonas aeruginosa and MRSA. Scientific Reports. 2021 Jan 13;11(1):1-9.
61- Pugazhendhi A, Mathimani T, Alharbi SA, Chinnathambi A, Karuppusamy I, Brindhadevi K, Kheawfu K, Pikulkaew S. In vitro efficacy of green synthesized ZnO nanoparticles against Biofilm and virulence of Serratia marcescens. Progress in Organic Coatings. 2022 May 1;166:106781.
62- Perumal A, Kannan S, Nallaiyan R. Silver nanoparticles incorporated polyaniline on TiO2 nanotube arrays: A nanocomposite platform to enhance the biocompatibility and antibiofilm. Surfaces and Interfaces. 2021 February 1;22:100892.
63- Wang B, Wu Z, Lan J, Li Y, Xie L, Huang X, Zhang A, Qiao H, Chang X, Lin H, Zhang H. Surface modification of titanium implants by silk fibroin/Ag co-functionalized strontium titanate nanotubes for inhibition of bacterial-associated infection and enhancement of in vivo osseointegration. Surface and Coatings Technology. 2021 Jan 15;405:126700.
64- Ibrahim F. Synthesis of novel virus-like mesoporous silica-ZnO-Ag nanoparticles and quercetin synergize with NIR Laser for COVID-19 infectious diseases treatment. Brain Tumor Research and Treatment. 2022 Mar 15;10(Suppl).
65- Singh P, Singh KR, Verma R, Singh J, Singh RP. Efficient electro-optical characteristics of bioinspired iron oxide nanoparticles synthesized by Terminalia chebula dried seed extract. Materials Letters. 2022 Jan 15;307:131053.
66- Perveen K, Husain FM, Qais FA, Khan A, Razak S, Afsar T, Alam P, Almajwal AM, Abulmeaty MM. Microwave-assisted rapid green Synthesis of gold nanoparticles using seed extract of Trachyspermum ammi: ROS mediated biofilm inhibition and anticancer activity. Biomolecules. 2021 Jan 30;11(2):197.
67- García A, González B, Harvey C, Izquierdo-Barba I, Vallet-Regí M. Effective reduction of Biofilm through photothermal therapy by gold core@ shell based mesoporous silica nanoparticles. Microporous and Mesoporous Materials. 2021 December 1;328:111489.
68- Jaffat HS, Aldujaili NH, Hassan AJ. Antimicrobial activity of silver nano particles biosynthesized by Lactobacillus mixtures. RESEARCH JOURNAL OF PHARMACEUTICAL BIOLOGICAL AND CHEMICAL SCIENCES. 2017 Jan 1;8(1):1911-+.
69- Albukhaty S, Al-Bayati L, Al-Karagoly H, Al-Musawi S. Preparation and characterization of titanium dioxide nanoparticles and in vitro investigation of their cytotoxicity and antibacterial activity against Staphylococcus aureus and Escherichia coli. Animal Biotechnology. 2020 Dec 1:1-7.
70- Alyamani AA, Albukhaty S, Aloufi S, AlMalki FA, Al-Karagoly H, Sulaiman GM. Green fabrication of zinc oxide nanoparticles using phlomis leaf extract: characterization and in vitro evaluation of cytotoxicity and antibacterial properties. Molecules. 2021 Oct 11;26(20):6140.
71- Gallaher WR. Towards a sane and rational approach to management of Influenza H1N1 2009. Virology journal. 2009 Dec;6(1):1-7.
72- Khashan KS, Sulaiman GM, Abdulameer FA, Albukhaty S, Ibrahem MA, Al-Muhimeed T, AlObaid AA. Antibacterial activity of TiO2 nanoparticles prepared by one-step laser ablation in liquid. Applied Sciences. 2021 May 19;11(10):4623.
73- Allaker RP. The use of antimicrobial nanoparticles to control oral infections. InNano-Antimicrobials 2012 (pp. 395-425). Springer, Berlin, Heidelberg.
74- Reffuveille F, De La Fuente-Nú̃nez C, Mansour S, Hancock RE. A broad-spectrum antibiofilm peptide enhances antibiotic action against bacterial biofilms. Antimicrobial agents and chemotherapy. 2014 Sep;58(9):5363-71.
75- Saucedo-Vázquez, J.P.; Gushque, F.; Vispo, N.S.; Rodriguez, J.; Gudiño-Gomezjurado, M.E.; Albericio, F.; Tellkamp, M.P.; Alexis, F. Marine Arthropods as a Source of Antimicrobial Peptides. Mar. Drugs 2022, 20, 501. https://doi.org/10.3390/md20080501
Received: September 22, 2022 / Accepted: October 18, 2022 / Published:15 November 2022
Citation: Ali Jabber Al-Saady M A, Aldujaili N H, Banoon S R, Al-Abboodi A. Antimicrobial properties of nanoparticles in biofilms. Revis Bionatura 2022;7(4) 71. http://dx.doi.org/10.21931/RB/2022.07.04.71.
