2023.08.04.63
Files > Volume 8 > Vol 8 no 4 2023
First record of Fusarium brachygibbosum as a causal agent of seed decay and damping-off disease on cotton in Iraq and Control using some bioagents
Aymen Jasim Mahi 1, Yasir Naser Alhamiri 2, *
1 Plant Protection Department/Agriculture College/University of Kerbala / Karbala / Iraq; [email protected].
2 Plant Protection Department/Agriculture College/University of Kerbala / Karbala / Iraq; [email protected].
Available from. http://dx.doi.org/10.21931/RB/2023.08.04.63
ABSTRACT
The results of the isolation and identification of pathogens accompanying the symptomatic seeds and seedlings of cotton showed that the most common fungus was Fusarium brachygibbosum. Molecular identification of the studied fungus was performed using the universal primers: the results of the genetic analysis revealed the identities of the fungus as follows: a 100% identity for F. brachygibbosum that was deposited at the GenBank under accession number ON738702.1. This fungus has shown high pathogenicity against cotton seeds and seedlings by severely reducing their Germination and growth and treating cotton seeds with the biological factors of Trichoderma spp. It revealed a high efficiency in reducing disease incidence and increasing cotton germination percentage. Trichoderma viride showed the highest ability to increase seed germination to 94.44%.
In comparison, the lowest ability reached 77.77 % in Trichoderma pseudokoningii and Trichoderma reesei—the results of extracting toxins from the filters of Trichoderma spp. The study's use of trichodermin and Gliotoxin showed the presence of trichodermin and Gliotoxin in large quantities. The percentage of toxin inhibition was significant against the growth of pathogenic fungi. The highest percentage of inhibition was 86.1% for the isolate Trichoderma koningiopsis, and the lowest percentage was 66.65% for the isolate Trichoderma reesei. As for the effect of isolates of resistant fungus on the Pathogen in the field, the highest germination rate was 100%, and the inhibition rate was 0.00% when using the biological preparation prepared from the isolates (T. viride, T. pseudokoningii, T. koningiopsis and T. reesei).
In comparison, the lowest ability reached 77.77 % in Trichoderma pseudokoningii and Trichoderma reesei—the results of extracting toxins from the filters of Trichoderma spp. The study's use of trichodermin and Gliotoxin showed the presence of trichodermin and Gliotoxin in large quantities. The percentage of toxin inhibition was significant against the growth of pathogenic fungi. The highest percentage of inhibition was 86.1% for the isolate Trichoderma koningiopsis, and the lowest percentage was 66.65% for the isolate Trichoderma reesei. As for the effect of isolates of resistant fungus on the Pathogen in the field, the highest germination rate was 100%, and the inhibition rate was 0.00% when using the biological preparation prepared from the isolates (T. viride, T. pseudokoningii, T. koningiopsis and T. reesei).
Keywords: Fusarium brachygibbosum; Trichoderma spp.; Trichodermin; gliotoxin; Biological control.
INTRODUCTION
Cotton (Gossypium hirsutum L.) is one of the most important economic fiber crops, which is essential in agriculture and trade and which is grown to obtain fiber, which is used in the textile industry, and for obtaining oil from its seeds: 1. Cotton has a tremendous economic place in the world in general and Iraq in particular, as it is considered one of the cash crops that generate profits for farmers and its uses in many industries, 2. The cotton crop production in Iraq was estimated in the summer of 2020 to be 22 tons, with a cultivated area estimated at 60 dunums. The average yield per acre was 366.7. 3 Diseases caused by soil-borne fungi can significantly impact almost all crops 4, including cotton, and those that occur in the early stage of seedling growth have been a significant determinant of cotton production worldwide, 5. As indicated previously,6 eight isolates of Fusarium spp. were isolated from the roots of cotton seedlings and had a high pathogenicity on two cotton cultivars, causing seedling death. In addition, many Fusarium species caused various diseases such as seed rot, preemergence damping-off, and stem and root rot 7,8. In another study, the fungus Fusarium brachygibbosum was found to be a causative agent of cotton seedlings' death, 9. Some types of Trichoderma spp. have shown the ability to produce secondary metabolites such as Gliovirin, Gliotoxin, Trichodermin, Viridin, Viridol, koninginins, Pyrones, Peptaibols and others, which are characterized by their high biological control ability via inducing apoptosis in fungal pathogens. It is considered one of the critical antibiotics because it can antagonize and inhibit the growth of pathogens, 10, 11. Trichodermin is one of the compounds that inhibit the growth of many plant pathogens, 12. Recent research indicates that Gliotoxin is a determinant of pathogen virulence and is vital in protecting biological crops, 13. Therefore, the study aimed to isolate and diagnose the fungus that causes Seed decay and seedling damping in cotton and evaluate the efficacy of different types of Trichoderma spp against the Pathogen. This study aimed to identify the causal agent of the seed decay and damping-off disease on cotton and control it utilizing some biological control agents.
Isolation and identification of fungi
Fungi were isolated from rotting seeds and infected or dead cotton seedlings before and after emergence and were washed well, superficially sterilized by sodium hypochlorite at a concentration of 2% for two minutes, then washed well with sterile distilled water to remove the remnants of the sterile solution, then the excess water was removed using sterile filter paper. Then, the sterilized parts were transferred by sterile forceps to Petri dishes containing the nutrient medium P.D.A. With five seeds or parts of the infected seedling roots for each dish and five replicates, the dishes were incubated at 25 ± 2 ° C. After four days. The developing fungal colonies were examined under the microscope for their diagnosis and preservation. After isolating fungi from cotton seeds and seedlings, they were purified on several dishes by a single spore method using the Streak-plate method. The dishes were incubated in the incubator at a temperature of 25±2ºC for two days, after which the germinated colonies were taken. It was transferred to new dishes containing P.D.A medium was incubated for five days, 14. The fungal colonies that appeared were examined using a compound light microscope and then diagnosed phenotypically based on morphology and microscopic characteristics and by following the taxonomic keys mentioned previously, 15-17.
Antagonism testing of isolates of the fungus Trichoderma spp. against the fungus F. brachygibbosum in vitro:
The antagonistic ability of four isolates of Trichoderma spp. It was obtained from previous studies in the Pathology laboratory at the College of Agriculture, as it was tested against the fungus (F. brachygibbosum) that causes seed rot disease and the death of cotton seedlings. In the double culture method, 18, a petri dish with a diameter of 9 cm containing the P.D.A. culture medium was divided into two equal parts. The center of the first section of the dish was inoculated with the pathogenic fungus inoculation, where a 0.5 cm diameter piece was taken from the fungus culture at the age of seven days, while the center of the section was inoculated. The other is from the dish with a 0.5 cm diameter piece from the fungus culture Trichoderma spp. At the age of seven days, the experiment was carried out with four replicates, and the dishes were placed in an incubator at a temperature of 25 ± 2º C for one week. The antagonistic ability was estimated according to the scale of Bell, which consists of five degrees,19:
Degree Specifications
1-The biological control fungus covers the entire dish area without allowing the fungus to grow Pathogen.
2- The biological control fungus covers two-thirds of the plate area and covers the fungus Pathogen, the remaining third of the dish.
3- The biological control fungus covers half the plate area, and the fungus Pathogen covers the other half.
4- The biological control fungus covers one-third of the plate area, while the fungus covers one-third. Pathogen the remaining two-thirds of the plate.
5- Pathogenic fungus covers the dish.
The biological agent is considered antagonistically adequate when it shows a degree of antagonism equal to 1 to 2 with the isolates of the pathogenic fungus. The percentage of inhibition was calculated by measuring the radius of the colony of the biological fungus towards the Pathogen, compared to the control treatment. According to the following Abbot equation, 20, 21: 1

Quantitative and qualitative determination of Trichodermin and Gliotoxin using High-Performance Liquid Chromatography (HPLC)
The quantitative and qualitative estimation process was carried out in the Ministry of Science and Technology laboratories using an HPLC device model (SYKAMN) (German). Mobile phase: Isocoratic acetic acid: acetonitrile: D.W(2:68:30) (V\V) Flow rate: at 1ml/min column : C18-ODS(25cm*4.6mm) UV-Vis meter: nm254 The concentration is calculated using the following equation:
Sample Concentration = Standard Substance Concentration x Sample Area x Standard Substance Area x (Number of Dilutions / Sample Volume)
Effect of Trichodermin and Glyotoxin in inhibiting fungal growth F. brachygibbosum
To study the effect of Trichodermin and Gliotoxin in inhibiting the Diameter growth of the pathogenic fungus isolate, the fungal filtrate of the isolates of the biological fungus Trichoderma sp. The selected medium of liquid Potato Sucrose Broth (P.S.B.) was prepared in the laboratory. This medium was prepared by boiling 200 gm of potatoes per liter of water for 30 minutes. The filtrate was taken, and we added 10 gm of sucrose, mixed well, closed tightly and sterilized with an autoclave at a temperature of 121 °C and a pressure of 15 pounds for 20 minutes and after the end of the sterilization period. It was left until the temperature reached 45 ° C. It was placed in closed plastic test tubes of 50 ml capacity, each of which was inoculated with 3 pieces (0.5 cm) of all fungal isolates individually, were taken from seven-day-old colonies grown on P.D.A. medium with 3 tubes / fungal isolate, then placed in the incubator at a temperature of 25 ± 2º for fifteen days and then kept in the refrigerator until use. Then, the medium was filtered using filter paper (Whatman filter paper No.4), the centrifugation process was carried out, and the filtrate was centrifuged at a speed of 2000 rpm for five minutes. Then, the extract was filtered using a 0.22 mm micron mellipore filter 22.
The filtrate of each fungal isolate was added at the rate of 2 ml to the Petri dishes, and then 10 ml of the P.D.A. was poured over it, stirring the plates with a capillary movement to mix the filtrate with the medium. Three replicates were made for each treatment, Considering the existence of a control treatment that was P.D.A. + 2 ml sterile distilled water only. The treatments were placed in the incubator at 25 ± 2 °C. After the growth of the control treatment of each fungal isolate was completed, the diameter growth of the pathogenic fungus was measured. By taking the growth rate of two perpendicular diagonals passing from the center of the dish and the percentage of inhibition according to the following equation: 2

Testing the synergistic effect of the combination of isolates of the fungus Trichoderma spp. Producers of Trichodermin and Gliotoxin against isolates of field pathogenic fungi in greenhouse
This test was performed on four isolates of Trichoderma spp as well as testing its antagonistic ability by testing the antagonistic ability in the laboratory and the field against pathogenic fungi on M.E.A. culture media and their ability to produce the mycotoxin Trichodermin and Gliotoxin, which did not show any indications of antibiotics among them. For this experiment, a mixture of soils was sterilized by autoclave At a temperature of 121ºC and a pressure of 15 pounds for an hour and two consecutive days. Then, it was placed in plastic pots) size 2 kg) and moistened with water. The soil was treated with biological preparations for the fungus Trichoderma spp Single and combined, grown on corn grits and the integrated combination of the four isolates of the fungus Trichoderma spp represented by the biological preparation, and by placing 10 g (total) for three replicates for each sample 7 days before sowing the superficially sterilized cotton seeds 10 seeds/pot, and (10 g for sample) of the pathogenic fungus was placed for each pot grown on millet medium, two days before planting:
The percentage of disease was measured two weeks after the start of the experiment according to the following equation: 3

The percentage of disease severity was measured according to equation 23 as follows: 4

Molecular diagnosis of Fusarium brachygibbosum on cotton
Molecular diagnosis was made of the fungal isolate that showed significant pathogenicity and high virulence against seed germination and damping-off cotton seedlings diagnosed phenotypically and tentatively under study. Molecular diagnosis of these isolates was done by analyzing the D.N.A. base sequences of the ITS region and comparing them with previously diagnosed isolates. After, it was sent to the South Korean company Macrogen to determine the nucleotide sequence. After receiving the nucleotide sequences of the fungal isolate, the nucleotide sequences were analyzed using the Basic Local Alignment Search Tool (BLAST) to compare them with the data available at the National Center for Biotechnology Information (NCBI) within the Gen Bank, which belong to the identical fungal isolates, which has been diagnosed globally. Fungal isolates that did not match the nucleotide sequences 100% were registered at the National Center for Biotechnology Information (NCBI). Genetic kinship analyses were also carried out using the MEGA (Molecular Evolutionary Genetics Analysis) program to analyze the isolates and draw a kinship tree between each of these isolates and similar isolates registered at the (NCBI) Center (the phylogenetic tree of the type Neighbor-joining, which was built from the sequence The molecular nucleotide of the ITS region of each of the isolates (Table 5 and Figure 4).
Statistical designs for laboratory and field experiments
Complete randomized design (C.R.D.) was used for all experiments conducted under controlled conditions (laboratory experiments and greenhouse experiments), and the data were analyzed using the (S.A.S.) Statistical Analysis System program after converting percentages to the angular transformation and the significant differences between the averages were compared using the L.S.D. test Below the level of significance of 0.05
Antagonistic ability test of Trichoderma spp. in vitro against the fungus F. brachygibbosum
The antagonistic ability of (4) pre-selected isolates was tested from the interaction test among isolates of Trichoderma spp. Two isolates produce Gliotoxin, and two produce Trichodermin against the pathogenic fungus Fusarium. brachygibbosum, were isolates of Trichoderma spp. High antagonistic ability against pathogenic fungi under laboratory conditions, the highest antagonistic ability was 94.44% for isolate T. viride on pathogenic fungus Fusarium brachygibbosum, and the lowest antagonistic ability was 77.77 for T. pseudokoningii and T. reesei isolates. In contrast, isolate T. koningiopsis had 88.88% antagonistic ability. (Table 1 and Figure 1).
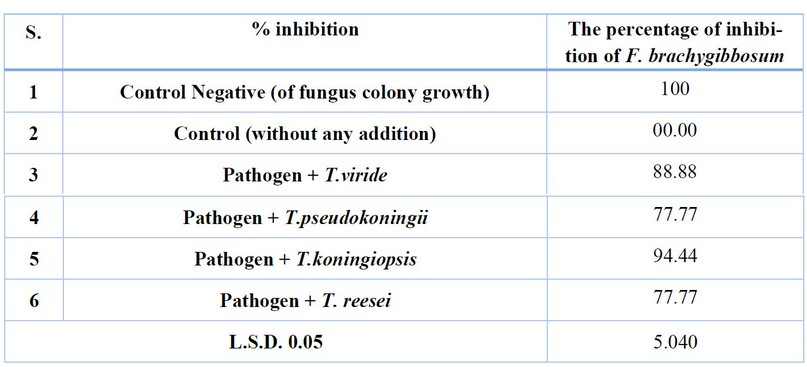
Table 1. Antagonistic ability test of Trichoderma spp. Isolates. In vitro against the fungus F.brachygibbosum.
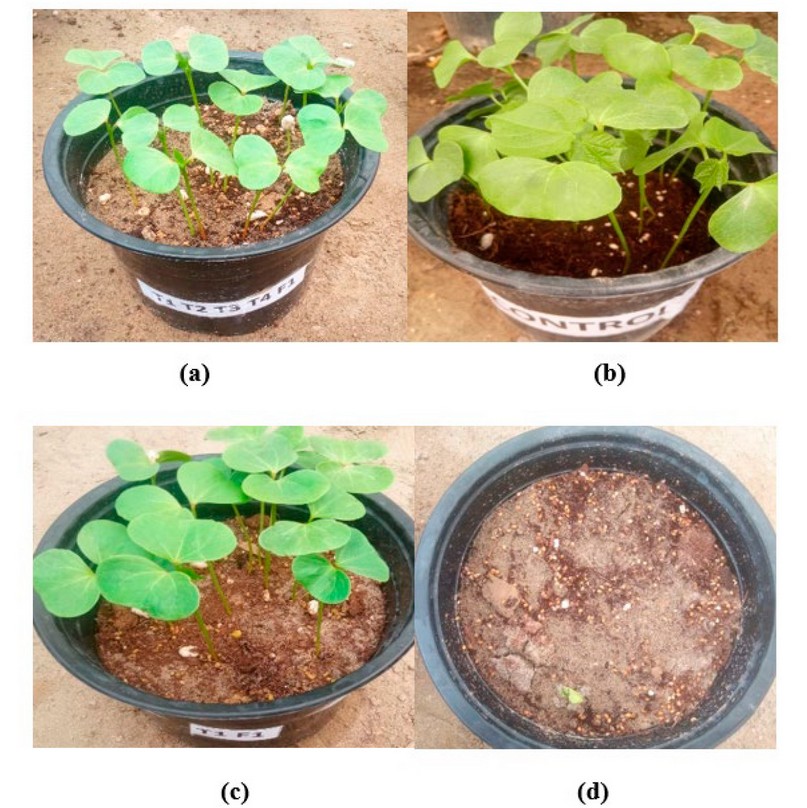
Figure 1. (a) T.viride + T.pseudokoningii + T.koningiopsis + T. reesei + F. .brachygibbosum ; (b) Control without adding ; (c ) T.viride + F. .brachygibbosum; (d) F. .brachygibbosum (only).
Effect of Trichodermin and Gliotoxin in Inhibiting Diameter Growth of Pathogenic Fungi Isolates The results showed that the effect of Trichodermin and Gliotoxin in the Leachate of the fungus Trichoderma spp has a clear and significant difference in inhibiting the growth of pathogenic fungi compared to the growth of pathogenic fungi without the addition of Trichoderma spp. The results on the fungus F.brachygibbosum had the highest inhibition rate of 86.1% for isolate T .koningiopsis, and the lowest inhibition rate was 66.65% for T. reesei (Table 2 and Figure 2).
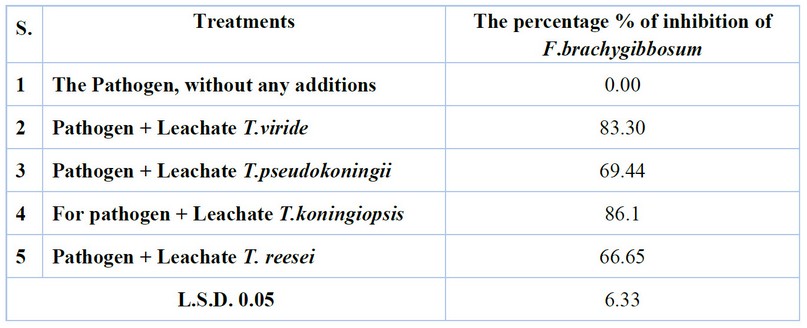
Table 2. The effect of Trichodermin and Gliotoxin in inhibiting the Diameter growth of pathogenic fungi isolates on P.D.A. culture media in vitro.
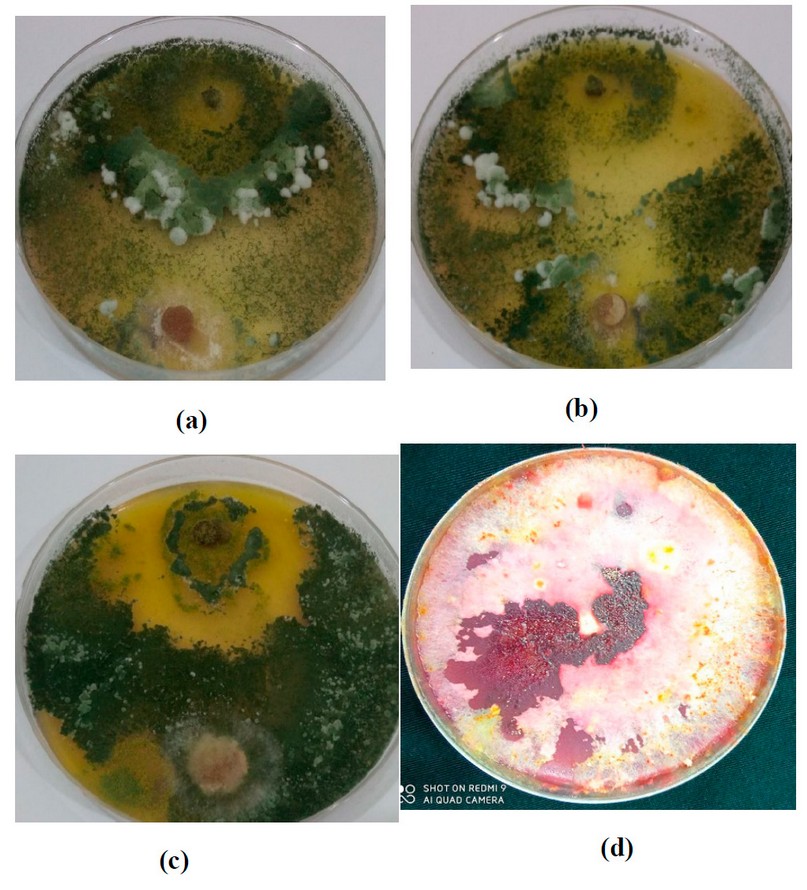
Figure 2. (a) T1+ F.brachygibbosum; (b) T2+F. brachygibbosum; (c ) (T3+ F. brachygibbosum); (d) Control F. brachygibbosum.
Testing the synergistic effect of the combination between isolates of the fungus Trichoderma spp. Producing Trichodermin and Gliotoxin against pathogenic fungal isolates in the field in plastic pots
The results showed the antagonistic ability of isolates of Trichoderma spp. Against pathogenic fungal isolates in plastic pots, there was a significant difference between the resistant fungus and the pathogenic fungus, which gave the highest germination rate of cotton seeds when using Trichoderma spp. Isolates alone when the seeds were treated with the pathogenic fungus Fusarium brachygibbosum was followed by isolates of T.pseudokoningii, T.koningiopsis and T. reesei, with a seed germination rate of 86.66% and inhibition rate of 13.33% for each isolate. The fungus Trichoderma spp showed high resistance against the pathogenic fungus Fusarium. brachygibbosum when using synergistic combinations of isolates of Trichoderma spp. The results showed the highest germination rate of 100% and inhibition rate of 0.00% when using the biological preparation from isolates (T. viride and T. pseudokoningii, T.koningiopsis and T. reesei), followed by (T1+T2+T3) and (T1+T2+T4), (T1+T3+T4), (T2+T3+T4) and (T1+T2) combinations with a percentage of Germination was 96.66%. The inhibition rate was 3.33, while the germination percentage of (T1+T3), (T1+T4), (T2+T3) and (T3+T4) isolates was 93.33%, and the inhibition percentage was 6.66%. The lowest germination percentage in the combination was 90% for isolate (T2 + T4), and the inhibition rate was 10%. It was noticed that there was a significant difference compared to the germination rate when adding the Pathogen only, which amounted to 10%, and the inhibition rate was 90%. The infection rate and disease severity results significantly differed when adding Trichoderma spp isolates compared to the infection rate of the plant pathogenic fungus Fusarium brachygibbosum without adding resistant fungus. Where there was an effect of Trichoderma spp isolates on the growth of pathogenic fungi when added alone, the highest infection rate was 20%, and the highest disease severity was 15.55% for isolate T. reesei The infection rate and disease severity decreased when using combinations of Trichoderma spp isolates, reaching the highest infection rate of 16.66% and the highest severity 10% disease for the combination (T.2) + (T.3), the lowest infection rate is 3.33%, and the severity of the disease is 3.33% for the combination (T.1) + (T.2) + (T.3) + (T.4). Table (3),
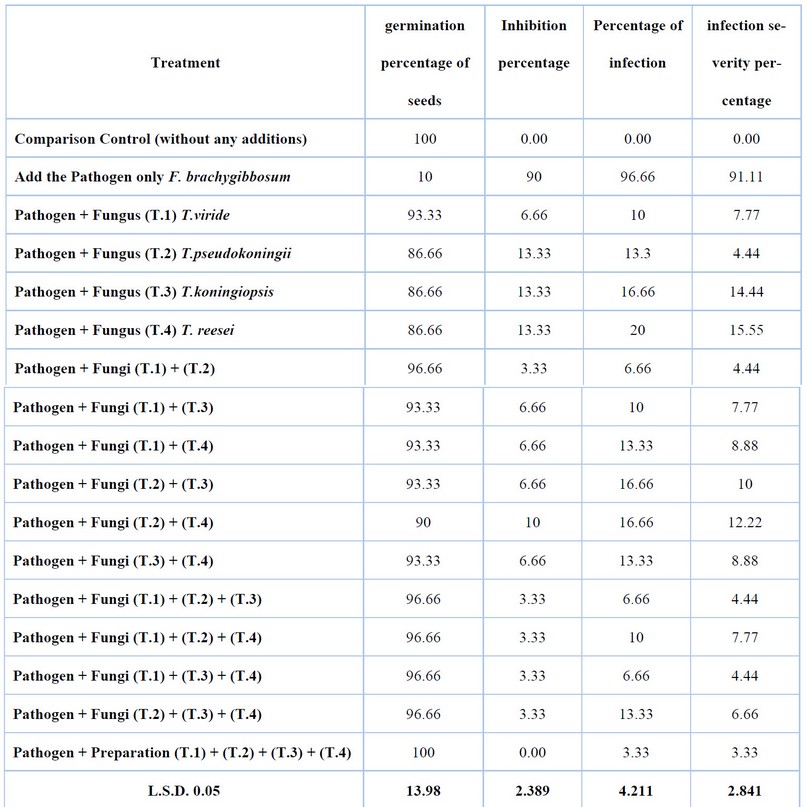
Table 3. Synergistic effect test between isolates of Trichoderma spp. The mycotoxins producing Gliotoxin and trichodermin reduce the incidence and severity of F. brachygibbosum infection on cotton seedlings in the field.
The results of Table (4) showed that the fungus Trichoderma spp. The mycotoxins producing Gliotoxin and Trichodermin significantly affected the growth Standards of cotton seedlings compared to plant growth when F. brachygibbosum was added only without the resistant fungus. In contrast, Trichoderma spp isolates affected the characteristics of fresh vegetative weight, dry weight and seedling length, where the highest fresh vegetative weight of cotton seedlings was 6 g, dry vegetative weight 3 g, seedling length 38 cm for the combination (T.1) + (T.2) + (T.3) + (T.4), and the lowest fresh vegetative weight 3 g and dry vegetative weight 1 g and weight Dry root 0.5 gm and 29 cm long for T. koningiopsis—the effect of isolates of the fungus Trichoderma spp.
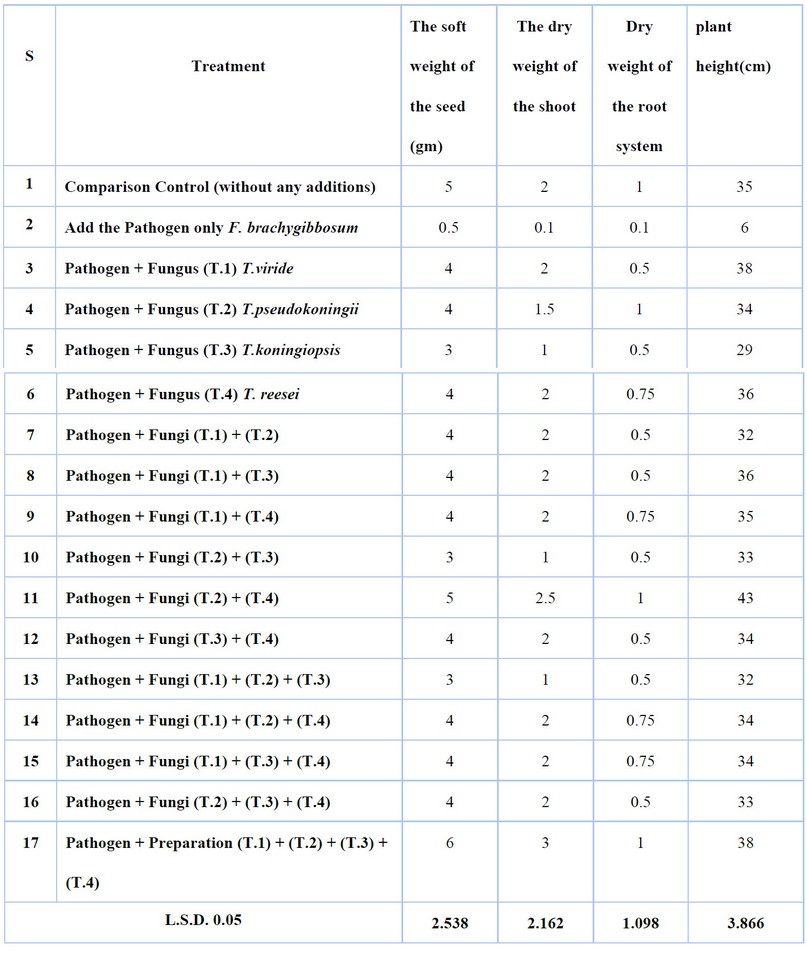
Table 4. Synergistic effect test between isolates of Trichoderma spp. Mycotoxin producing Gliotoxin and Trichodermin in some growth Standards of field cotton seedlings infected with F. brachygibbosum.
Description of the fungus F. brachygibbosum
The fungus F. brachygibbosum appeared equally red on the dish, and from below, the dish is dark red, as described by Padwick 0, and a slightly sharp apex and basal cells of a foot-like shape, 24.
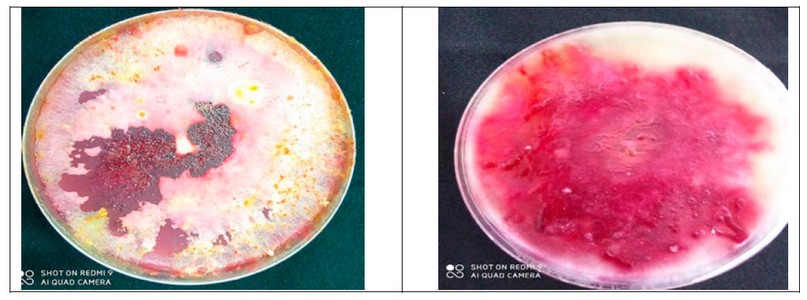
Figure 3. Fusarium brachygibbosum.
Molecular diagnosis of F. brachygibbosum on cotton
It was noticed by comparing the nucleotide sequence of the D.N.A. bundle of the fungus Fusarium brachygibbosum Y.N.147Aymen) isolated from cotton seeds and seedlings with the data available in the Center for Biotechnology Information (NCBI) that the percentage of genetic similarity reached (100%) with all isolates of F. brachygibbosum (Table 5).
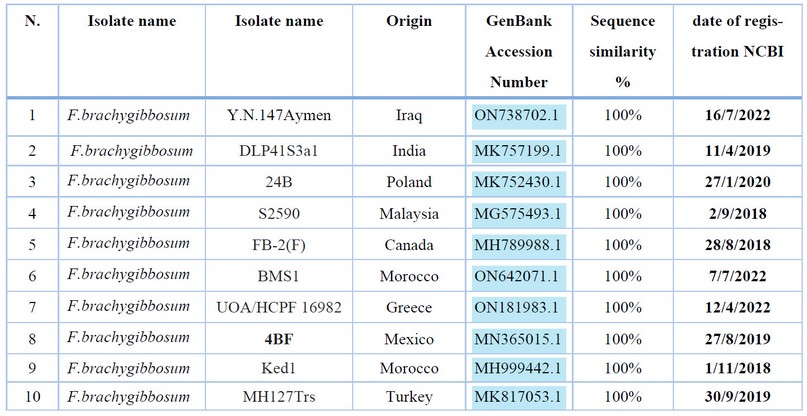
Table 5. Comparison of nucleotide base sequence similarity ratios for the ITS gene region of the fungus isolates F. brachygibbosum (Y.N.147Aymen).
Figure (4), represented by the genetic tree, showed that the isolate (Y.N.147Aymen) appeared in the same clade in which the Indian isolate (MK757199.1) appeared and with separate branches (clades) from the Moroccan and Turkish isolates (MH999442.1 and MK817053.1) respectively due to genetic divergence between them.
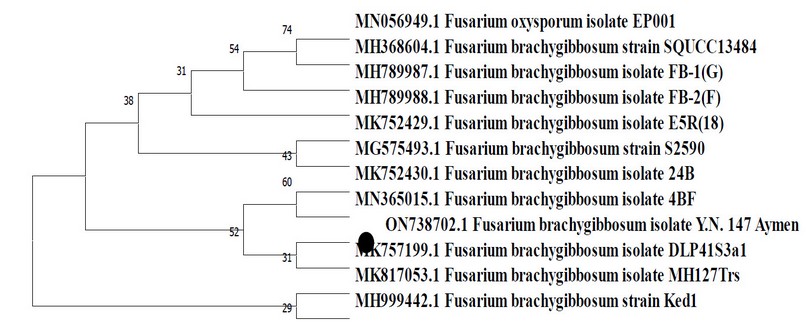
Figure 4. Phylogenetic tree of F. brachygibbosum fungus Y.N.147Aymen.
Antagonistic ability test of Trichoderma spp. in vitro against the fungus F. brachygibbosum
The high antagonistic efficiency of Trichoderma spp. Species of pathogens are attributed to the formation of many secondary metabolites, including antibiotics such as gilotoxin, virindin and Trichoderma, which have been shown to have antifungal activity to control various soil-borne pathogens. Conclude that Trichoderma spp. It has an antagonistic property in controlling pathogens, 25-27.
Effect of Trichodermin and Gliotoxin in inhibiting Diameter growth of pathogenic fungi isolates
These results are attributed to the effect of toxins produced by synergistic fungal isolates. These results are consistent with the previous study, 28, In which the effect of toxins produced by the synergistic isolates of Trichoderma spp in inhibiting pathogenic fungi on okra was demonstrated.
Testing the synergistic effect of the combination between isolates of the fungus Trichoderma spp. Producing Trichodermin and Gliotoxin against pathogenic fungal isolates in the field in plastic pots
The decrease in infection rate and disease severity in combinations of resistant fungus isolates is due to the increase in the amount of Gliotoxin and Trichodermin produced from compatible isolates with each other, which significantly affects the inhibition of the growth of plant pathogenic fungi and protection of plants from infection. The growth Standards of cotton seedlings were improved considerably due to the plant-induced growth by the fungus Trichoderma spp. Previous studies supported these results conducted 29,30 on the effect of Trichoderma isolates and other bioagents in inhibiting pathogens, inducing pest control and increasing Germination and growth in cucumber plants.
This study showed isolation and identification of the fungus F. brachygibbosum was first recorded as a cause of seeds decay and seedlings damping off on cotton, where the fungus isolated from seeds and seedlings showed high virulence in attacking cotton seedlings and significantly reduced both germination and growth rates, but the treatment of seeds with isolates of Trichoderma spp. It has proven highly efficient in reducing infection percentage and increasing germination and growth rates in cotton seedlings. F. brachygibbosum isolate was diagnosed morphologically and molecularly by analyzing the D.N.A. base sequences of the ITS region after it was sent to the South Korean Macrogen Company to determine the nucleotide sequence. The deposit number of this isolate was in the GenBank (ON738702.1) and compared with previously diagnosed isolates. The results of the synthesis of isolates of the bio-resistant fungus Trichoderma spp. (T.viride, T.pseudokoningii, T.koningiopsis, T. reesei) High efficiency against the fungus F. brachygibbosum.The results of extracting toxins from the Leachates of Trichoderma spp used in the study showed the presence of Trichodermin and Gliotoxin in large quantities, and the percentage of toxin inhibition in the filtrates had laboratory inhibition. Trichoderma spp isolates also affected fresh vegetative weight, dry weight and seedling length characteristics. The study also showed the effectiveness of all treatments used to prevent the growth of pathogenic fungi.
Author Contributions: Conceptualization, A.M. and Y.A.; methodology, A.M.; validation, Y.A.; formal analysis, A.M.; writing—original draft preparation, A.M.; supervision, Y.A. All authors have read and agreed to the published version of the manuscript.
Funding: This research received no external funding.
Data Availability Statement: Data supporting reported results can be found at https://www.ncbi.nlm.nih.gov/nuccore/ON738702.1
Acknowledgments: The authors would like to thank the administrative of the Plant Protection Department, Agriculture College, University of Kerbala.
Conflicts of Interest: The authors declare no conflict of interest.
1. Javied, M. A.; N. Ashfaq, M. A. Haider, F. Fatima, Q. Ali, A. Ali, and A. alik. Agrobacterium-mediated transformation of cotton (Gossypium hirsutum L.) using dmo gene for enhanced tolerance against dicamba pesticide. Biol. Clin. Sci. Res. J. 2021, 1,2708-2728.
2. Gneit, M. K. A geographical analysis of climate change in the change of cotton yield in Wasit Governorate. Lark Magazine. 2021, 2(41), 1093–1059.
3. Lahuff, A.A. First report of Fusarium proliferatum causing stem and root rot on lucky bamboo (Dracaena braunii) in Iraq, Hell. Plant Prot. .2019, 12, 1–5.
4. Goulart, A.C.P. Reação de cultivares de algodoeiro a Rhizoctonia solani na fase de plântula e benefícios do tratamento de sementes com fungicidas. Summa Phytopathologica, Botucatu, 2016, 42, 4, 308-312.
5. Dawoud, T.M.; Yassin, M.A.; El-Samawaty, A.R.M.; Elgorban, A.M. Silver nanoparticles synthesized by Nigrospora oryzae showed antifungal activity. Saudi J. Biol. Sci .2021. 28 (3), 1847-1852.
6. Lahuff, A. A.; Jaafar, O. H.; Al-mosoy, M.; Hameed, Z. L..First record of the crown rot fungus Fusarium equiseti affecting Triticum aestivum L. and Aptenia cordifolia in Iraq. Asian J. Agric. Biol. 2018, 6 (4): 543-548.
7. Abdulmoohsin, R. G.; Lahuf, A. A.; Husain, Y. N. and Hameed, Z. L. Bioefficiency of some indigenous biocontrol agents against Rhizoctonia solani causing cowpea seed rot and preemergence damping-off. I.O.P. Conf. Ser.: Earth Environ. Sci. 2019. 388, 012011.doi:10.1088/1755-1315/388/1/012011.
8. Le, D. P.; Tran, T. T.; Gregson, A.; Jackson, R. TEF1 sequence-based diversity of Fusarium species recovered from collar rot diseased cotton seedlings in New South Wales, Australia. Australas. Plant Pathol. 2020. 49(3), 277-284.
9. A. Al-Badawi, S., T. Al-Wasity, R. AN .Economic Analysis Of The Most Important Variables Affecting Agricultural Employment In Iraq For The Period (1998 - 2019). Anbar Journal Of Agricultural Sciences, 2023; 21(1): 224-249. doi: 10.32649/ajas.2023.179764
10. Lahuff, A. A.; Kareem, A. A.; AL-Sweedi, T. M. and Alfarttoosi, H. A. Evaluation the potential of indigenous biocontrol agent Trichoderma harzianum and its interactive effect with nanosized ZnO particles against the sunflower damping-off pathogen, Rhizoctonia solani. I.O.P. Conf. Ser.: Earth Environ. Sci. 2019, 365, 012033. doi:10.1088/1755-1315/365/1/012033.
11. Tijerino, A. et al. Overexpression of the trichodiene synthase gene tri5 increases trichodermin production and antimicrobial activity in Trichoderma brevicompactum. Fungal Genet. Biol..2011.48,3, 285-296.
12. Sulaiman, E. D.; Youns, A. N. Study the Mechanisms of Parasitism and Antagonism of Different Biocontrol Agents Against Sclerotinia sclerotiorum, the Causal Organism of White Rot Disease on Eggplant in the Laboratory. Tikrit J. Agric. Sci. 2019, 18(1), 47-56
13. Tomah, A. A.; Abd Alamer, I. S.; Li, B.; Zhang, J. Z. A new species of Trichoderma and gliotoxin role: A new observation in enhancing biocontrol potential of T. virens against Phytophthora capsici on chili pepper. Biol. Control, 2020, 145, 104261.
14. Odeh A.; Abdalmoohsin R.G.; AL-Abedy A.N. Molecular identification of Fusarium brachygibbosum and some isolates of Trichoderma spp. Int. J. Pharmaceut. Res. 2021, 13(1):1390-6.
15. Leslie, J. F.; Summerell, B. A. Fusarium laboratory workshops--A recent history. Mycotoxin Res. .2006, 22(2), 73.
16. Pandian, J. D.; Singh, G.; Kaur, P.; Bansal, R.; Paul, B. S.; Singla, M.; Sharma, M. Incidence, short-term outcome, and spatial distribution of stroke patients in Ludhiana, India. Neur. J. 2016. 86(5), 425-433.
17. Diaz-Najera, J. F.; Serna, S. A.; Bahena, A. M.; Cruz, E.B.; Hernandez, M.V.; Gomez, O.G.; Aragón, D.F. First Report of Fusarium falciforme (FSSC 3+4) Causing Wilt Disease of Phaseolus vulgaris in Mexico. Plant Dis. 2021, 105,710.
18. Baker, K. F.; Cook, R. J. Biological control of plant pathogens; WH Freeman and Company: U.S.A., 1974; pp.34-49.
19. Bell, D. E. Regret in decision making under uncertainty. Oper. Res. 1982, 30(5), 961-981.
20. Abbot, D. S. Analytical investigation of the decrease in the size of the habitable zone due to a limited CO2 outgassing rate. Astrophys. J. 2016. 827(2), 117.
21. Altindag, M.; Sahin, M.; Esitken, A.; Ercisli, S.; Guleryuz, M.; Donmez, M. F.; Sahin, F. Biological control of brown rot (Moniliana laxa Ehr.) on apricot (Prunus armeniaca L. cv. Hacıhaliloglu) by Bacillus, Burkholdria, and Pseudomonas application under in vitro and in vivo conditions. Biol. Control. 2006, 38(3), 369-372.
22. Konda, P. V. Magellan: Toward building entity matching management systems; The University of Wisconsin-Madison: Madison, U.S.A., 2018; pp. 122-133.
23. McKinney, H. H. Influence of soil temperature and moisture on infection of wheat seedlings by Helminthosporium sativum. J. Agric. Sci. 1925, XXXI, 827-840.
24. Ibraheem M W, Muhaimeed A R, Mohammed Th. T. Leg cuts from Awaasi lambs fed a diet with varying levels of Rhus coriaria L., Physical dissection and chemical composition. Revis Bionatura 2022;7(4) 4. http://dx.doi.org/10.21931/RB/2022.07.04.4.
25. Kareem, H. J.; Al-Araji, A. M. Evaluation of Trichoderma harzianum biological Control against Fusarium oxysporum f. sp. melongenae. Iraqi J. Sci. 2017, 58(4B), 2051-2060.
26. Inovejas, R. C.; Divina, C. C. Methanol extract and nanocomposite of Trichoderma sp. as a potential bio-control against Fusarium moniliforme in tomato (Lycopersicon esculentum). Int. J. Agric Technol. 2018, 14, 99-108.
27. Thabit S S, Awad M M, Abdulateef S M. Effects of In-Ovo Injection of sialic acid on Chick's embryonic development and physiological traits. Revis Bionatura 2023;8 (2) 49. http://dx.doi.org/10.21931/RB/2023.08.02.49.
28. Ali, A. F. .; Mohammed, T. T. .; Al-Bandar, L. K. . The Role Of Optifeed®, Vêo® Premium, And Oleobiotec® In Diets For Improvement Of The Production Performance Of Male Broilers In Heat Stress. JLSAR 2022, 3, 32-36.
29. Al-Bahrani, Sh. A. M. Molecular diagnosis of some isolates of Trichoderma spp. isolated from the governorates of Iraq, producing the mycotoxin Gliotoxin and evaluating its effectiveness against some pathogens of cucumber plant diseases. MSc. Thesis, College of Agriculture, University of Karbala, Karbala, 2021.
30. Abdalmoohsin, R.G.; Alhumairy, Y.N.; Abood, N.T.; Lahuf, A.A. Bioefficacy of Pseudomonas fluorescens and Saccharomyces cerevisiae against Pythium aphanidermatum under laboratory and greenhouse aquaculture conditions in cucumber plants, Cucumis sativus. Biopestic. Int. 2019, 15, 15–22.
Received: 26 September 2023 / Accepted: 15 April 2023 / Published:15 December 2023
Citation: Mahi, A.; Alhamiri, Y. First record of Fusarium brachygibbosum as causal agent of seeds decay and damping-off disease on cotton in Iraq and control it using some bioagents. Revis Bionatura 2023;8 (4) 63. http://dx.doi.org/10.21931/RB/2023.08.04.63
Publisher's Note: Bionatura stays neutral concerning jurisdictional claims in published maps and institutional affiliations.
