2023.08.02.78
Files > Volume 8 > Vol 8 No 2 2023
Antibacterial and anti-biofilm action of cobalt oxide nanoparticles beside persister Pseudomonas Aeruginosa isolates
Alaa M. Ahmed1,*, Mohammed F. Al Marjani2, and Ahmed M. Rheimah3
1Department of Biology, College of Science, Mustansiriyah University, Baghdad, Iraq
2Department of Biology, College of Science, Mustansiriyah University, Baghdad, Iraq
2Department of Chemistry, College of Science, Mustansiriyah University, Baghdad, Iraq
* Correspondence: [email protected] , [email protected] , [email protected]
Available from: http://dx.doi.org/10.21931/RB/2023.08.02.78
ABSTRACT
Persister cells of Pseudomonas aeruginosa have developed a wide-reaching public health problem. Although this is a medical concern, there is currently no effective means to remove P. aeruginosa persister cells. Nanoparticles containing cobalt oxide (Co3O4-NPs) were examined for their ability to impact the formation of biofilms and inhibit the growth of bacteria. Researchers found that Co3o4-NPs were effective against P. aeruginosa, with inhibition zones ranging from 11 to 19 mm and MIC (Minimum Inhibition Concentration) results of 156 to 312 g/ml. The Co3O4-NPs with a titration of 10 mg/mL (76.54 percent) had the maximum biofilm suppression activity, while a titration of 0.156 mg/ml had the lowest (11.50 percent). According to the findings, P. aeruginosa biofilms and persister cells can benefit from applying co3o4-NPs.
Keywords: Persister cell, SEM, co3o4-NPs, Anti-biofilm action
INTRODUCTION
Antibiotic resistance and biofilms have become a severe hazard to incurable infectious infections. Combating them should be a high priority, with the primary technique being to explore strategies to avoid their establishment 1. For further information on biofilms, see Biofilms: A community of adherent Bacteria with different metabolites from free-living bacteria 1. Bacterial infections are thought to be caused by biofilms in 80% of cases 2. These super constructs are inappropriately resistant to medications and the immunological defense system, creating a significant health concern. A good stress response and survival strategy for bacteria is the formation of biofilms3,4. P. aeruginosa is a life-threatening human opportunistic pathogen that flourishes in hosts that lack typical defenses, such as those suffering from severe illness or immunologically impaired. In addition to highly ill patients such as those in intensive care units (ICUs) 5, healthful persons can also take it. The most prevalent nosocomial infections that cause death and severe illness in hospitalized patients include 6. Bacterial persistence, known to create chronic intractable infections, is one of the primary reasons for antibiotic therapy failure. Sub-inhibitory levels of antibacterial medications cause persisters, which are antimicrobial-tolerant cells that do not grow and lack a conventional genetic resistance mechanism, to emerge in populations of bacteria. Persister cells are hereditarily equal to antibacterial sensitive cells in a resident that is not sure of MIC rate or persister cell. However, they take a diverse behavior in that they can survive antibacterial at titer that may be harmful to other cells7,8. Persister cells have been identified in bacterial populations before the antibiotic was applied inactive slow growing due to phenotypic variation 9. In general, nanomaterials have been demonstrated to have stronger microbicide activity than standard antibiotics and have even shown reactivity against numerous bacteria species 10. The existing work aims to examine the anti-biofilm and anti-persister cell development ability of Cobalt oxide nanoparticles against P. aeruginosa.
METHODS AND MATERIALS
Isolation, Collection, Identification and Growth Circumstances of Bacteria
From a variety of Baghdad-Iraqi hospitals. Fifty different clinical isolates of P. aeruginosa were studied. Bacterial isolates were detected by their characteristic on selective and differential culture media, biochemical assays, and the Vitek-2 system. All bacterial isolates were cultivated on Luria Bertani (LB, Difco Laboratories) agar or in Luria Bertani broth for 18-24 hr. at 37 °C.
Synthesis and Characterization of Cobalt Oxide Nanoparticles
The Sigma-Aldrich Company provided all of the components and the highest quality. UV irradiation was used to create cobalt oxide nanoparticles11,12,13,14,15. A 125-watt UV source (with a maximum wavelength of 365 nm) and an ice-bath-cooled Pyrex tube make up the photocell, eliminating an increase in temperature due to UV radiation. Our results were as follows: we added 0.06-mole Ethylene glycol (CH2OH)2 to 75 ml of 0.03-mole cobalt (II) acetate for 20 minutes with a magnetic stirring rod. For 30 minutes, the mixture was exposed to light from a photocell. This morning's precipitation was a dark brown. The material was repeatedly separated and washed using ion-free water in a centrifuge. The material has been dehydrated for 24 hours. After calcining it for three hours at 450 °C, a black powder of co3o4-NPs was produced.
Persister Cells Detection
The test for detecting persister cells was produced by applying rabidly killing techniques and freshly cultured cells with lytic solutions 16. For the McFarland standard of 0.5 McFarland, P. aeruginosa isolates were cultured for 18-24 hours at 37 °C in Luria Bertani broth. Then, 200μL of buffer lysis solution was poured into a sterile 10 mL sterile test tube, vortex spun for 10 seconds, and then for 10 minutes were incubated at room temperature. After that, 2 μL 00 of the enzymatic lysis solution was gently poured over the mixture. Finally, incubate for 15 minutes at 200 rpm at 37 °C in a shaking incubator. A 10 μL smear on Luria Bertani agar was used to determine the frequency of persister cells, and the plates were incubated for 18-24 hours at 37 °C.
Biofilm Assay
According to 17, the following was done to evaluate the biofilm of P. aeruginosa: First, in brain heart infusion (BHI) broth, they were cultivated for 18-24 hours. The growth was then diluted to 1:100 and pipetted onto the wells of a 96-well polystyrene flat bottom plate containing BHI broth medium with 2 percent (w/w) sucrose, where it was cultivated for 24 hours at 37 °C, after being rinsed 3 times in phosphate-buffered saline and leaving it to dry at room temperature earlier, being colored for fifteen minutes with 200μL of 1% crystal violet. The stain was eluted using 200μL of absolute ethanol after aeration at room temperature, and OD was measured using an ELISA reader at 630 nm. The experiment was done three times for each isolate. Separate groups of test isolates were tested for adherence, each with a different mean optical density value.
No biofilms if the OD ≤ ODc, weak biofilms, If ODc < OD ≤ 2 × ODc
Moderate biofilms, if 2 × ODc < OD ≤ 4 × ODc and if 4 × ODc < OD, strong biofilms
Antimicrobial Effect and MIC of Co3O4-Nps
To study the inhibitory impact of Co3O4-Nanoparticales against persistent P. aeruginosa Strains, according to 30, agar well diffusion methods were utilized. DMSO in the other served as a negative control, and an antibiotic disc in the other well was a positive control, the co3o4-Nanoparticales in the latter well. In addition, the MIC was determined using a 96-well polystyrene microtiter plate. 100μL of (MHB) Muller Hinton broth was put on a microtiter plate for each well, and then 100μL Co3O4-NPs (10 mg/mL) was added to the first vertical row from A1 to A10, followed by 1:2 serial dilutions. The remaining 100 mL of the last well had been abandoned. After 24 hours of incubation, 5 μL of bacterial suspension (108 CFU/mL or 105 CFU/well) was added to all wells except the negative control row from A12 to H12. After dyeing with 10μL of resazurin dye were put to each well and then incubated for around four hours at 37 °C to get prepared for reading, the effects of diverse titer of Co3O4-NPs for the microbiological growth were determined by utilizing UV visible spectrophotometer 18.
Anti-Biofilm Activity of Co3o4-Nps
Co3O4-NPs' anti-biofilm impact on persistent P. aeruginosa strains was studied following 19. The isolates of P. aeruginosa were injected in BHI broth at 37 degrees Celsius. After that, 20 μL of bacterial suspension was put into each microtiter plate well, containing 80 μL of BHI broth with 2 % sucrose. 100 μL of co3o4-NPs was added and mixed well before incubating at 37°C for 24 hours. Then, the plate was allowed to dry at room temperature for two further washes in PBS. Cells of Bacteria that cannot be linked to one another were removed. Biofilms were stained with 200 μL of 1% crystal violet solution. The stain was eluted with 200μL of absolute ethanol, and the OD was calculated using an ELISA reader at 630nm. Only B.H.I broth was used as a negative control. The experiment was done three times for each bacterial isolate. The following equation was used to calculate the rate of inhibition rate of co3o4-NPs:
Rate of inhibition (%) =( OD of control – Od of treated ) / (OD of control) × 100
RESULTS
Persister Cells Detection
Rapidly killing approach, used to examine the formation of persister cells in P. aeruginosa isolates. According to the findings, only 5 out of 50 P. aeruginosa isolates (PA4, PA5, PA21, PA27, and PA47) had persister cells 20 seen in Figure 1.
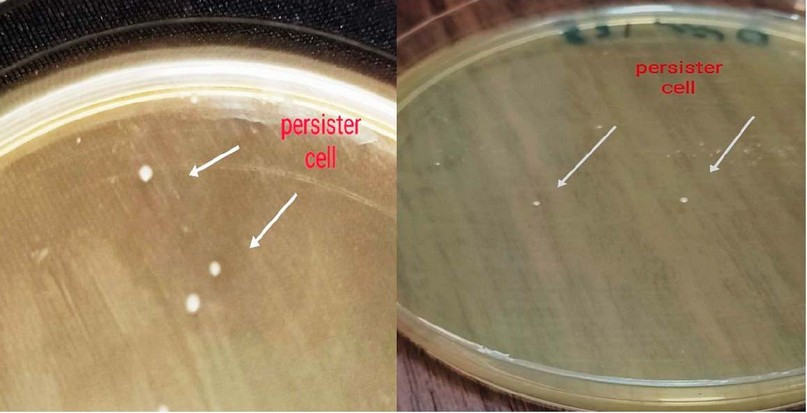
Figure 1. Formation of Persister cell in P. aeruginosa By Rapidly Killing Method.
As a result, it is an optimal procedure that operates without influencing the culture's bacterial population size, strain, or physiological state.
Cobalt Oxide Nanoparticles, Synthesis and Characterization
A distinctive XRD design of the precursor synthesized at 450 °C for three hours is shown in Figure 2. All of the reflection peaks in this pattern (JCPDS Card File No. 76–1802) might be easily attributed to crystalline cobalt with oxygen. In this pattern, there were no noticeable impurity peaks. The diffraction peaks of 2θ = 19.50°, 31.37°, 37.02°, 39.10°, 44.97°, 55.84°, 59.58°, 65.46°, and 77.62° appeared. The mean crystallite sizes were 25 nm.
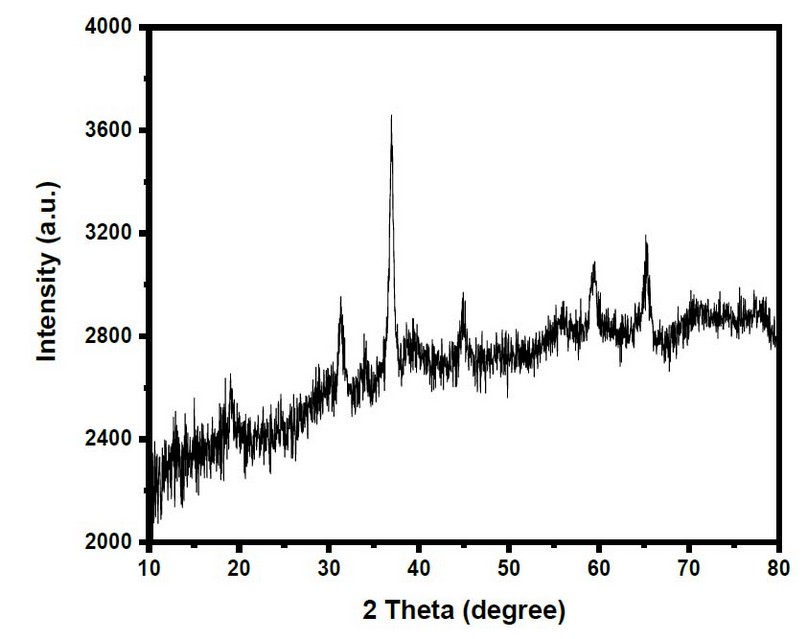
Figure 2. XRD Pattern Of Co3O4 -Nanoparticles
The ingredient of the created elements at 450°C was fixed by EDS analysis. Figure 3 shows that the result contained just cobalt (Co) and oxygen (O) elements, with a cobalt/oxygen atomic ratio of roughly 3:4, which is compatible with the predicted value of Co3O4. No additional elements are found, indicating that the Co3O4 NPs are highly pure.
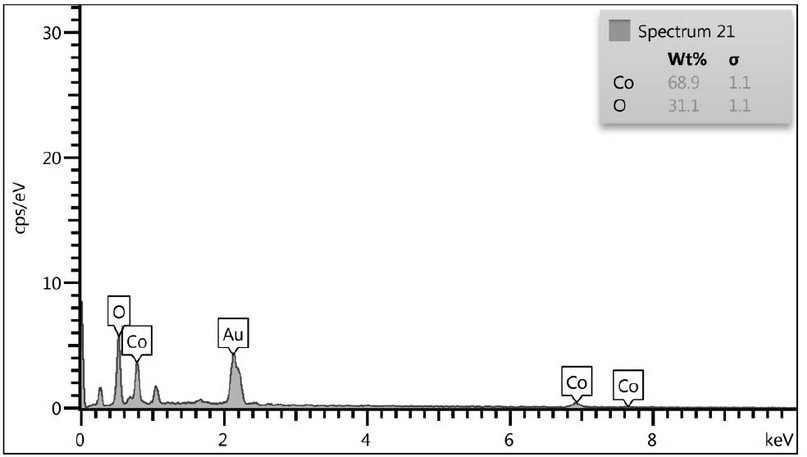
Figure 3. EDS Measurement of S the Co3O4 Nps
SEM was used to evaluate the morphology of the created units. The SEM image of produced Co3O4 nanoparticles is shown in Figure 4. The nanoparticles have a sphere-like morphology in the SEM picture, and they are created of agglomerated collected particles representing connectivity well among particles. The size of nanoparticles, as well as their homogeneity and size distribution, can be detected by using SEM. The SEM image shows that the particles are evenly scattered. The mean particle size was randomly estimated from the figures to be 37nm.

Figure 4. SEM Image Of Co3O4 Nps
Co3O4-Nps, Antibacterial Activity And MIC
On persistence, Co3O4-Nanoparticales were proven to have antibacterial efficacies against P. aeruginosa. Co3O4-NPs displayed antibacterial solid activity in the agar-well diffusion technique, with inhibition zones (11-19 mm) at 10.000g /ml. According to the MIC results, CO3O4-NPs had high antibacterial activity (156.25 -312.5 g/ml), as demonstrated in Table 1 and Figure 5 A, B. The ability of persistent P. aeruginosa to proliferate was often reduced during 16 hours of CO3O4-NPs exposure, as was also observed
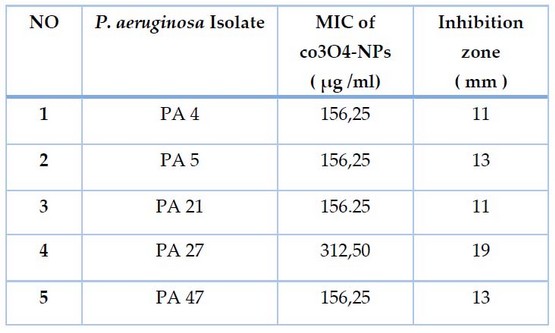
Table. 1 Antibacterial Effect of Co3o4-NPs Against Persister Cell of P. aeruginosa Isolates.
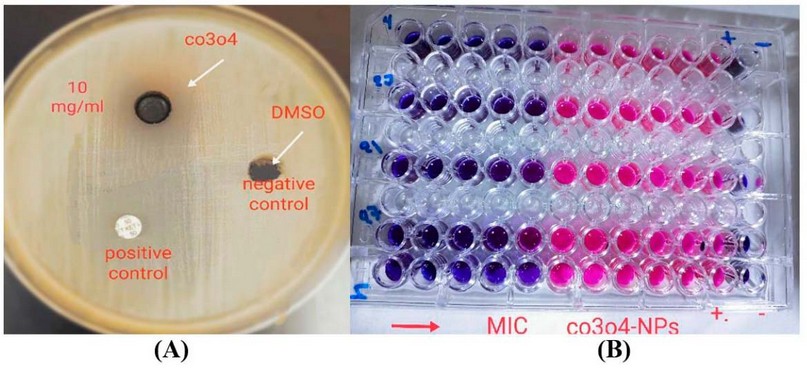
Figure 5 Antibacterial Activity Of Co3o4-Nps Against Persister Cell Of P. aeruginosa Isolates By A-Agar-Well Diffusion Method B-Micro Titer Plate Method.
Biofilm Inhibition Activity
The ability of co3o4-NPs to suppress biofilm formation by persister cells of P.aeruginosa isolates was examined in this work. Co3o4-NPs were examined for their average inhibition rate against P. aeruginosa. Co3o4 nanoparticles greatly assisted biofilm reduction. Biofilm was inhibited to a percentage of 76.54 percent at 10 mg/ml and 0.156 mg/ml. Because of this, co3o4-NPs had a considerable effect on P. aeruginosa persister cell biofilms.
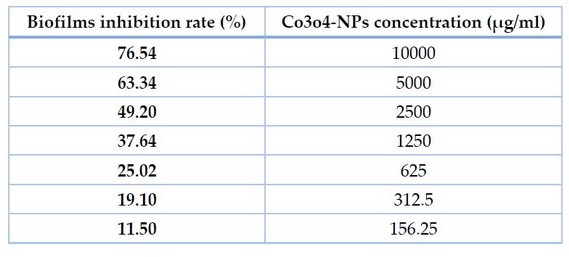
Table 2. AntiBiofilm Ability Of Co3o4-Nps Against Persister P. aeruginosa Isolates.
If we can diminish persister, we can reduce bacterial resistance to medicines and therapies, which will help treat chronic infections. We assessed the existence of the creature utilizing a quick-killing strategy. Following testing with lysis buffer solution and enzymatic lysis solutions, persister cells in P. aeruginosa isolate. Current work indicates the inhibitory zones of co3o4-NPs ranged from 11- 19 mm in a concentration of 10.000μg/ml to remove P. aeruginosa persister cells. For P. aeruginosa isolates, the MIC of Co3O4-Nanoparticales ranged from 156.25 to 312.5 μg/ml. Results showed that Co3O4-NPs demonstrated a significant anti-biofilm activity at 76.54 percent present in co3o4-Nanoparticales at a concentration of 10000 μg/mL correspondingly.
DISCUSSION
Methods that depend on antibacterial titer are substantially slower than the rapid killing method of persister cell isolation. Further studies can be conducted on the isolated persister cells because the fast killing approach is stable after isolation 16
The reflection peaks could be easily attributed to crystalline cubic phase Co3O4. The crystallite sizes of the as-produced were calculated by using the Scherer formula21,22,23.
P. aeruginosa is a hazardous microorganism that is a source of various infections in the community and hospitals. In recent years, the incidence of multidrug-resistant P. aeruginosa isolates has become a global public clinical issue 24. Bacterial persistence refers to the survival of a minor proportion of germs after being eliminated by bactericidal drugs at lethal levels. Co3o4-NPs were also tested against P. aeruginosa persister cells. It was evaluated as an antibacterial and suppression of biofilm formation agent concern to diverse reports 27. The antibacterial activity of Co3O4-NPs was improved against a wide range of microbial diseases 28. In general, the effects of Nanoparticles ca3o4 against bacteria were summarized as follows: electrostatic interaction causes mechanical harm to the cell wall, oxidative stress from the formation of reactive oxygen species (ROS), and damage to protein activities and cell structures from metal cation release 29.
CONCLUSIONS
To boost their bactericidal potency, co3o4-nps were synthesized utilizing a photolysis method. The current work was the first to indicate that co3o4-NPS may effectively suppress biofilm and persister cell production of P. aeruginosa.
Acknowledgement
To each person who aided us in accomplishing this project at Mustansiriyah University, Department of Biology and Department of Chemistry
Conflict of interest authors states that they do not have a conflict of interest.
REFERENCES
1 Stoodley, P., Sauer, K., Davies, D. G., & Costerton, J. W. Biofilms as complex differentiated communities. Annual Reviews in Microbiology, 2002; 56(1), 187-209.
2 Lamret, F., Colin, M., Mongaret, C., Gangloff, S. C., & Reffuveille, F. Antibiotic tolerance of Staphylococcus aureus biofilm in periprosthetic joint infections and antibiofilm strategies. Antibiotics, 2020; 9(9), 547.
3 Abidi, S. H., Sherwani, S. K., Siddiqui, T. R., Bashir, A., & Kazmi, S. U. Drug resistance profile and biofilm forming potential of Pseudomonas aeruginosa isolated from contact lenses in Karachi-Pakistan. BMC ophthalmology, 2013; 13(1), 1-6.
4 De la Fuente-Núñez, C., Reffuveille, F., Fernández, L., & Hancock, R. E. Bacterial biofilm development as a multicellular adaptation: antibiotic resistance and new therapeutic strategies. Current opinion in microbiology, 2013; 16(5), 580-589.
5 Wunderink , R. G., and Mendoza , D. L. Epidemiology of Pseudomonas aeruginosa in the Intensive Care Unit. infectious Diseases in Critical Care. Section III:218-225. 8. 2007.
6 Moradali, M. F., Ghods, S., & Rehm, B. H. Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Frontiers in cellular and infection microbiology, 7, 39. 2017.
7 Knudsen, G. M., Ng, Y., & Gram, L. Survival of bactericidal antibiotic treatment by a persister subpopulation of Listeria monocytogenes. Applied and environmental microbiology, 2013; 79(23), 7390-7397.-
8 Van den Bergh, B., Fauvart, M., & Michiels, J. Formation, physiology, ecology, evolution and clinical importance of bacterial persisters. FEMS Microbiology Reviews, 2017; 41(3), 219-251.
9 Balaban, N. Q., Merrin, J., Chait, R., Kowalik, L., & Leibler, S. Bacterial persistence as a phenotypic switch. Science, 2004; 305(5690), 1622-1625.
10 Akbar, A., Sadiq, M. B., Ali, I., Muhammad, N., Rehman, Z., Khan, M. N., ... & Anal, A. K. Synthesis and antimicrobial activity of zinc oxide nanoparticles against foodborne pathogens Salmonella typhimurium and Staphylococcus aureus. Biocatalysis and agricultural biotechnology, 2019; 17, 36-42.-.
11 Mohammed, M. A., Rheima, A. M., Jaber, S. H., & Hameed, S. A. The removal of zinc ions from their aqueous solutions by Cr2O3 nanoparticles synthesized via the UV-irradiation method. Egyptian Journal of Chemistry, 2020;63(2), 425-431
12 Kamil, A. F., Abdullah, H. I., Rheima, A. M., & Mohammed, S. H. Impact of Fe2NiO4 nanoparticles to increase the efficiency of dye-sensitized solar cells. Materials Today: Proceedings, 2022; 49, 2727-2732.
13 Kamil, A. F., Abdullah, H. I., & Mohammed, S. H. Cibacron red dye removal in aqueous solution using synthesized CuNiFe2O5 Nanocomposite: thermodynamic and kinetic studies. Egyptian Journal of Chemistry, 2021; 64(11), 5-6.
14 Rheima, A. M. Dye-sensitized Solar Cells Based on Silicon Dioxide Nanoparticles Photochemically Synthesized: A Comparative Study in the Concentration of the Dye-sensitized. Journal of Nanostructures, 2021; 11(3), 609-617.
15 Al Marjani, M., Aziz, S. N., Rheima, A. M., & Abbas, Z. S. Impact of Chromium Ooxide Nanoparticles on growth and biofilm formation of persistence Klebsiella pneumoniae isolates. Nano Biomed. Eng, 2021; 13(3), 321-327.
16 Cañas-Duarte, S. J., Restrepo, S., & Pedraza, J. M. Novel protocol for persister cells isolation. PLoS One, 2014; 9(2), e88660.
17 Badmasti, F., Siadat, S. D., Bouzari, S., Ajdary, S., & Shahcheraghi, F. Molecular detection of genes related to biofilm formation in multidrug-resistant Acinetobacter baumannii isolated from clinical settings. Journal of Medical Microbiology, 2015; 64(5), 559-564.
18 Teh, C. H., Nazni, W. A., Nurulhusna, A. H., Norazah, A., & Lee, H. L. Determination of antibacterial activity and minimum inhibitory concentration of larval extract of fly via resazurin-based turbidometric assay. BMC microbiology, 2017; 17(1), 1-8
19 Theodora, N. A., Dominika,V., & Waturangi, D. E. Screening and quantification of anti-quorum sensing and antibiofilm activities of phyllosphere bacteria against biofilm-forming bacteria. BMC Research Notes, 2019; 12(1), 1-5.
20 Aziz, S. N., Al Marjani, M. F., Rheima, A. M., & Al Kadmy, I. M. Antibacterial, antibiofilm, and antipersister cells formation of green synthesis silver nanoparticles and graphene nanosheets against Klebsiella pneumoniae. Reviews in Medical Microbiology, 2022; 33(1), 56-63.
21 Rheima, A. M., Al Marjani, M. F., Aboksour, M. F., & Mohammed, S. H. Evaluation of Anti-Biofilm Formation Effect of Nickel Oxide Nanoparticles (NiO-NPs) Against Methicillin-Resistant Staphylococcus Aureus (MRSA). International Journal of Nanoscience and Nanotechnology, 2021; 17(4), 221-230.
22 Rheima, A., Anber, A. A., Shakir, A., Salah Hammed, A., & Hameed, S. Novel method to synthesis nickel oxide nanoparticles for antibacterial activity. Iranian Journal of Physics Research, 2020; 20(3), 51-55.
23 Kamil, A. F., Abdullah, H. I., Rheima, A. M., & Mohammed, S. H. UV-Irradiation synthesized α-Fe2O3 nanoparticles based dye-sensitized solar cells. Materials Today: Proceedings. 2021.
24 Ratkai, C. Characterization of medically important Pseudomonas aeruginosa isolates. Institute of Clinical Microbiology, Faculty of Medicine University of Szeged, Szeged, Hungary. 2011.
25 Singh, R., Ray, P., Das, A., & Sharma, M. Role of persisters and small-colony variants in antibiotic resistance of planktonic and biofilm-associated Staphylococcus aureus: an in vitro study. Journal of Medical Microbiology, 2009; 58(8), 1067-1073.
26 Leung, V., & Lévesque, C. M. A stress-inducible quorum-sensing peptide mediates the formation of persister cells with noninherited multidrug tolerance. Journal of Bacteriology, 2012; 194(9), 2265-2274.
27 Parada, J., ATRIA, A., Wiese, G., Rivas, E., & Corsini, G. Synthesis, characterization and antibacterial activity of cobalt (Iii) complex with phenanthroline and maltose. Journal of the Chilean Chemical Society, 2014; 59(4), 2636-2639.
28 Alahmadi, N. S., Betts, J. W., Cheng, F., Francesconi, M. G., Kelly, S. M., Kornherr, A., ... & Wadhawan, J. D. Synthesis and antibacterial effects of cobalt–cellulose magnetic nanocomposites. RSC advances, 2017; 7(32), 20020-20026.
29 Wang, L., Hu, C., & Shao, L. The antimicrobial activity of nanoparticles: present situation and prospects for the future. International journal of nanomedicine, 2017; 12, 1227.
30 Yaseen, S. M., Abid, H. A., Kadhim , A. A., & Aboglida, E. E. Antibacterial activity of palm heart extracts collected from Iraqi Phoenix dactylifera L. Journal of Techniques, 2019; 1(1), 52-59.
Received: May 15, 2023/ Accepted: June 10, 2023 / Published: June 15, 2023
Citation: Ahmed, A.M.; Al Marjani, MF; Rheimah, A.M. Antibacterial And Anti-Biofilm Action Of Cobalt Oxide Nanoparticles Beside Persister Pseudomonas Aeruginosa Isolates. Revis Bionatura 2023;8 (2) 78. http://dx.doi.org/10.21931/RB/2023.08.02.78
