2023.08.04.20
Files > Volume 8 > Vol 8 no 4 2023
Study of the protective effect of ginseng against testicular oxidative stress biomarkers and its gene expression induced by ciprofloxacin
Haitham M. Mokhimar1 , Hozaifa K. Elsawah2
, Hozaifa K. Elsawah2 , Mohamed M. Kandiel3
, Mohamed M. Kandiel3 , Faten E. Elsaid4, AbuBakr M. El-Mahmoudy5
, Faten E. Elsaid4, AbuBakr M. El-Mahmoudy5
1Department of Pharmacology, Benha College of Vet. Medicine, Benha, Qaliobia, Egypt.
2Department of Pharmacology, Benha College of Vet. Medicine, Benha, Qaliobia, Egypt. Email: [email protected]
3Department of Theriogenology, Benha College of Vet. Medicine, Benha, Qaliobia, Egypt. Email: [email protected]
4Department of Pharmacology, Benha College of Vet. Medicine, Benha, Qaliobia, Egypt. Email: [email protected]
5Department of Pharmacology, Benha College of Vet. Medicine, Benha, Qaliobia, Egypt. Email: [email protected]
Available from. http://dx.doi.org/10.21931/RB/2023.08.04.20
ABSTRACT
Ciprofloxacin is the first-choice member of the fluoroquinolone antibacterials for treating testicular infections, but it may harm testicular tissue because of oxidative stress. Many mechanisms are involved, like decreasing antioxidant enzymes and suppressing gene expression. This study intends to investigate the possible protective role of ginseng against ciprofloxacin-induced testicular oxidative stress and its mechanism, if any. For this purpose, 50 adult male albino rats were randomly divided into five groups, ten rats in each group. Rats in group 1 received only ciprofloxacin at a daily dose of 156.46 mg/kg. Rats in groups 2, 3 and 4 received ciprofloxacin in a daily dose of 156.46 mg/kg, ginseng in two doses of 100 and 200 mg/kg, and vitamin E as a standard in a daily dose of 100 mg/kg, respectively. Group 5 served as control and received carboxymethylcellulose in normal saline. All these treatments were applied orally during 14 14-day experimental courses. Half the animals in each group were euthanized on day 15 from the start of the treatment, while the second half was euthanized on day 60. Both testes were dissected, immediately frozen, and evaluated for oxidative stress biomarkers and gene expression antioxidant enzymes. We found that ciprofloxacin significantly (P ≤ 0.05) increased MDA and decreased total antioxidant capacity (TAC), superoxide dismutase (SOD) and catalase (CAT) compared to the control group. Also, the drug downregulated gene expression of SOD and CAT. Compared to all groups, the co-administration of ginseng or vitamin E with ciprofloxacin almost normalized antioxidant enzymes and upregulated the tested gene expressions. It could be concluded that ginseng ameliorates the testicular adverse effect of ciprofloxacin. So, it is highly recommended to be used as an adjunct remedy during ciprofloxacin administration for its antioxidant properties.
Keywords: Ciprofloxacin, Gene expression, Ginseng, Infertility, ROS, Testicular oxidative stress, Vitamin-E.
INTRODUCTION
Reactive oxygen species (ROS) potentially contribute to male infertility 1, leading to defective sperm function, metabolism and motility 2, 3.
Oxidative stress describes when a system has imbalanced oxidation and reduction reactions leading to oxidative stress 4, which can lead to nuclear and mitochondrial DNA damage and Y chromosomal changes 5.
Former studies reported that ciprofloxacin harmfully impacted male fertility via increasing testicular oxidative stress marked by depletion in SOD (Superoxide dismutase) and GPx (glutathione peroxidase) 6-8. Ciprofloxacin is effective in the treatment of a wide variety of infections, particularly those caused by Gram-negative pathogens and is considered to be the best choice for patients with complicated urinary tract infections 9, also frequently prescribed by fertility specialists in the therapy of many types of bacterial infections when leukocytospermia or before in vitro fertilization program 5.
Daily 400 mg/kg of ciprofloxacin for 7 days induced elevation in oxidative stress biomarkers 10. Also, high doses of ciprofloxacin and enrofloxacin increase blood oxidative stress 11.
Ginseng is the most frequently used herbal medicine for immune system stimulation and as an adjuvant with prescribed drugs12. It is effective in the treatment of male infertility. It has been shown to induce testicular growth, increase the production of spermatozoa and testosterone levels, and sexual activity in animals 13. Ginseng inhibits oxidative stress in rats, which lowers lipid peroxidation and increases antioxidant capacity 14.
So, we hypothesize that ginseng as an antioxidant could protect from infertility caused by oxidative stress induced by ciprofloxacin.
The adverse effect of ciprofloxacin on testicular tissue via oxidative stress has been demonstrated to achieve this objective.
MATERIALS AND METHODS
Drug preparation
Ciprofloxacin was purchased as a generic pharmaceutical preparation Serviflox® with a concentration of 750 mg of ciprofloxacin in one tablet manufactured by Novartis Pharma-Cairo, under the license of Sandoz GmbH- Australia. The tablets were crushed and diluted with CMC (carboxymethylcellulose) in NS (normal saline) to a final volume of 1ml /rat dose. Ginseng was purchased with the generic name Ginsana® with a concentration of 100 mg of ginseng in one capsule manufactured by Egyptian International Pharmaceutical Industries Company (EIPICO). The capsules were opened and diluted with CMC in NS to a final 1ml /rat dose volume. Vitamin E was purchased with the generic name vitamin-E 1000® with a concentration of 1000 mg of vitamin E in each capsule manufactured by Pharco Pharmaceuticals. The tablets were opened and diluted with sunflower oil to a final 1ml /rat dose volume.
Animals
Fifty adult Wister albino male rats, 8 weeks old and weighing 200±20g, were used. They were obtained from Animal House, Faculty of Veterinary Medicine, Benha University. Male rats were housed at average room temperature (30°C), humidity (40-60%) and 12h/12h dark/ light cycle before the start of the experiment. The animals were fed laboratory formula 15 and tap water ad libitum.
Study design
After two weeks of adaptation to a standard diet, male rats were randomly divided into five groups, with ten in each group. Rats in group 1 received ciprofloxacin in daily doses of 156.46 mg/kg16. Rats in groups 2, 3 and 4 received ciprofloxacin in a daily doses of 156.46 mg/kg and ginseng in two doses of 100 and 200 mg/kg17 and vitamin E in a daily doses of 100 mg/kg, respectively. Group 5 served as control and received 1 ml carboxymethyl cellulose in normal saline. All treatments were orally administered for 14 days. Half the animals in each group were sacrificed by euthanasia using ether 18 on day 15 from the start of the treatment (1 euthanasia). In contrast, the second half was sacrificed by euthanasia 18 on day 60 from the beginning of the treatment (2nd euthanasia). Testis were immediately removed, frozen, and evaluated for oxidative stress biomarkers and gene expression antioxidant enzymes.
Sampling
Immediately after euthanasia, one testis from each animal was dissected, weighed and divided into two parts.
1- Testicular tissue samples for testicular oxidative stress biomarker concentrations were collected by centrifugation of the first part of the testis after homogenization with a phosphate buffer solution at pH 7.4 at 1500 xg for 5 minutes at 4º C 19. The supernatant was taken out and preserved at -20 C. till used analysis of testicular oxidative stress markers: MDA (Malondialdehyde) concentration, SOD activity, total antioxidant capacity and CAT (catalase) activity.
2- Testicular tissue samples for testicular gene expression were collected and immediately kept at – 80oC until the expression of testicular gene expression of antioxidant enzymes was quantified. Using a Qiagen RNeasy® Mini kit, total RNA was extracted from the frozen samples following the manufacturer's protocol. RNA was determined by using Spectro star Nanodrop (BMG LABTECH®) according to the high-capacity cDNA Reverse Transcription Kits (Applied Biosystems) protocol. Real-time polymerase chain reaction for each gene was carried out using (Quanti Tect SYBR Green PCR Kit, Qiagen), 1 μM of each forward and reverse primer for each gene (Table 1), and The real-time PCR equipment used the comparative CT method to calculate the changes in gene expression20.
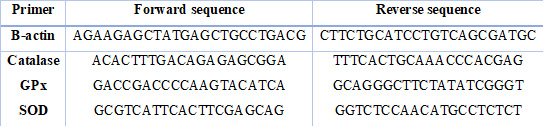
Table 1. Primer forward and reverse sequences for gene expression analysis
Statistical analysis
The multi-group comparisons were carried out using the one-way analysis of variance (ANOVA) technique, followed by post hoc Tukey's test for pairwise comparison at a 0.05 significance level. They were using GraphPad Prism program 21.
Ethics statement
The experiment was conducted in the Departments of Pharmacology and Theriogenology, Faculty of Veterinary Medicine, Benha University, with the ethical approval number BUFVTM 090422.
RESULTS
Effect of ciprofloxacin alone and with ginseng or vitamin E on testicular oxidative stress biomarkers:
As shown in Figure 1, on the 15th day from the start of treatment, group 1, which was treated with ciprofloxacin, only showed a significant increase in MDA. However, adding ginseng or vitamin E normalized MDA compared to control. However, group 3, which received a high dose of ginseng, showed a significant decrease in MDA compared to the control. CAT, TAC (total antioxidant capacity) and SOD enzyme activity were reduced in the group treated with ciprofloxacin compared to the control group (P < 0.05). However, adding ginseng or vitamin E with ciprofloxacin normalizes CAT, TAC and SOD enzyme activity. However, the group treated with a high dose of ginseng showed a higher increase in SOD enzyme activity than group 4, which received vitamin E, and the control group, Figure 2A.
On the 60th day of treatment, all groups have been approximately returned to normal figures 1-2.
Effect of ciprofloxacin alone and with ginseng or vitamin E on antioxidant gene expression
As shown in Figure 3, on the 15th day of treatment, group 1, which received ciprofloxacin, showed significant downregulation of relative gene expression of CAT, GPx and SOD genes compared with all groups. However, supplementation of ginseng or vitamin E with ciprofloxacin approximately normalized the relative gene expression of CAT, GPx and SOD compared to the control group (P < 0.05). Also, group 3, which received a high dose of ginseng with ciprofloxacin, showed a higher significant upregulation of relative gene expression of CAT, GPx and SOD compared with group 2 and group 4.
On the 60th day from the start of treatment, all groups returned approximately to normal compared with the control group Figure 3.
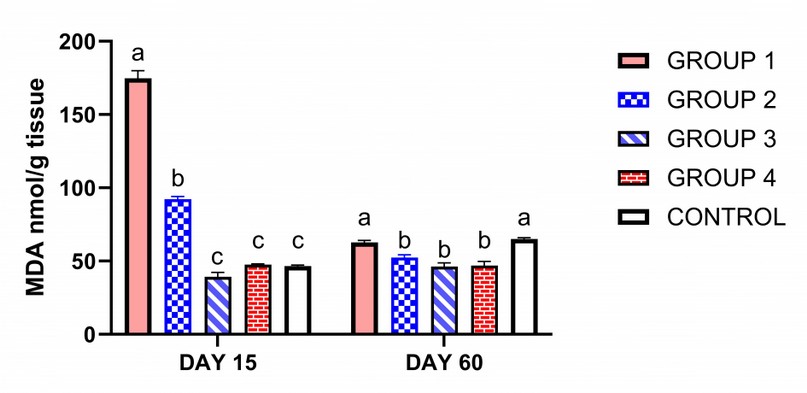
Figure 1. Influence of ciprofloxacin with or without ginseng or vitamin E administration on oxidative stress biomarker MDA obtained from rats' testis. Columns bearing different superscript letters on the same sacrifice day differ significantly at p < 0.05 in Tukey's multiple comparison post hoc test. (Group 1) treated with ciprofloxacin only. (Group 2). Moreover, (group 3) was treated with ciprofloxacin and ginseng at a low dose and ginseng at a high dose. (Group 4) treated with ciprofloxacin and vitamin E. (Group 5) serve as control. MDA: Malondialdehyde.
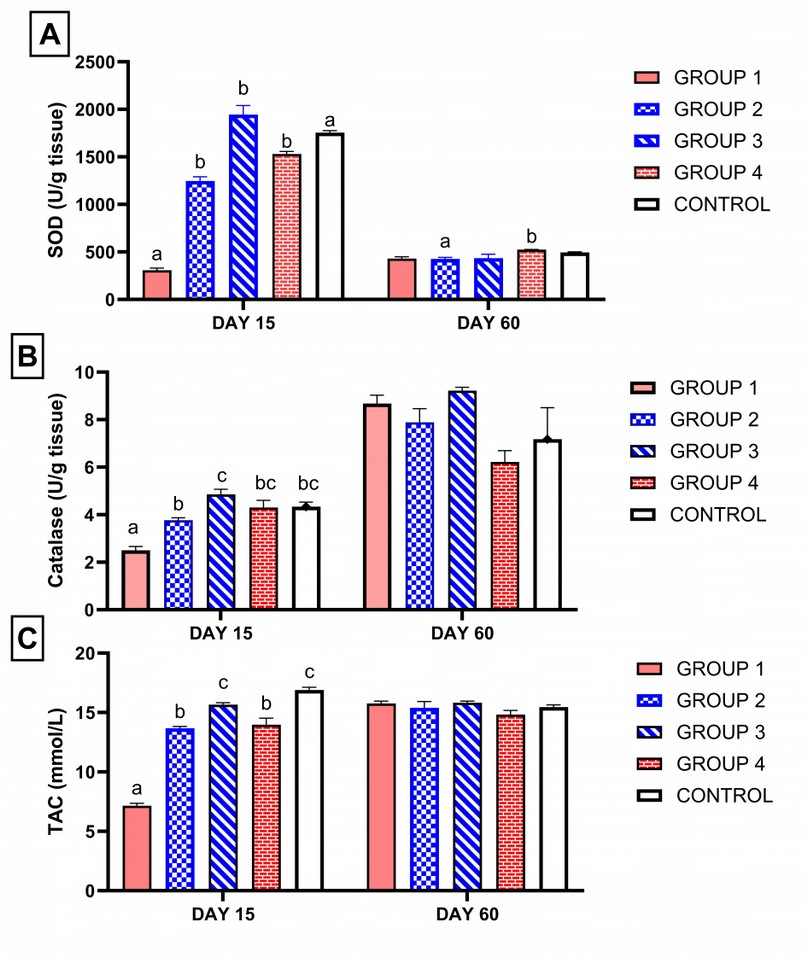
Figure 2. Influence of ciprofloxacin with or without ginseng or vitamin E administration on antioxidant enzymes SOD, Catalase, TAC obtained from rats' testis. Columns bearing different superscript letters on the same sacrifice day differ significantly at p < 0.05 in Tukey's multiple comparison post hoc test. (Group 1) treated with ciprofloxacin only. (Group 2) Moreover, (group 3) was treated with ciprofloxacin and ginseng at a low dose and ginseng at a high dose relatively. (Group 4) treated with ciprofloxacin and vitamin E. (Group 5) serve as control. SOD: superoxide dismutase. TAC: total antioxidant capacity.
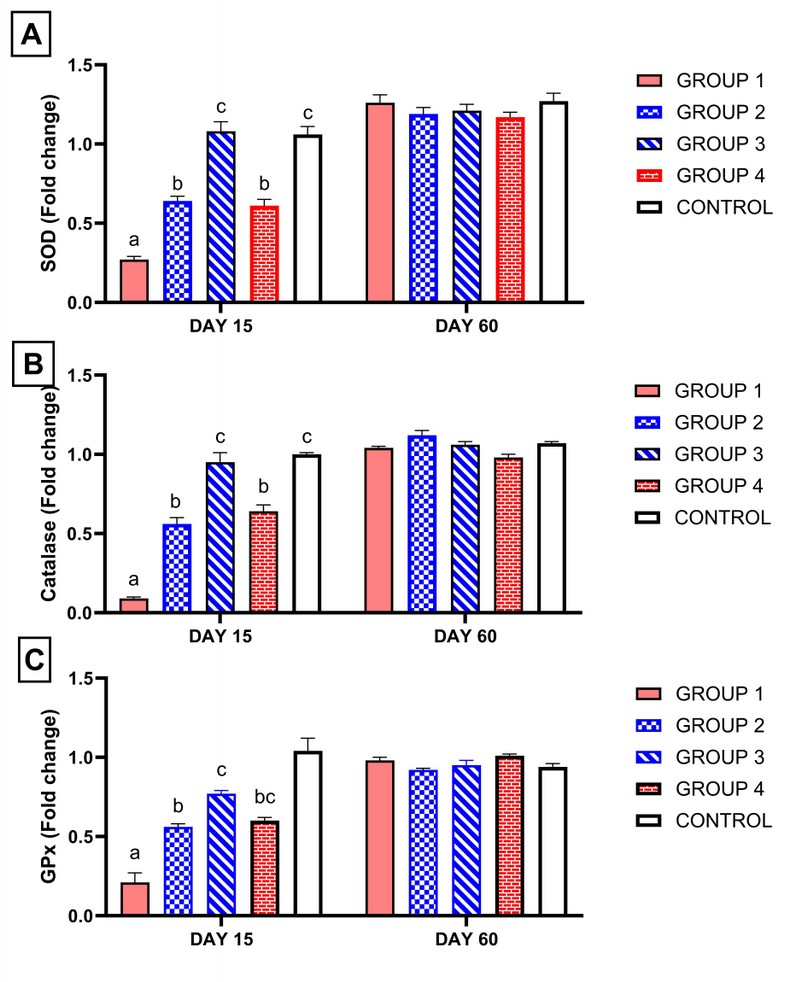
Figure 3. Influence of ciprofloxacin with or without ginseng or vitamin E administration on relative gene expression analysis of antioxidant enzymes obtained from rats' testis. Columns bearing different superscript letters on the same sacrifice day differ significantly at p < 0.05 in Tukey's multiple comparison post hoc test. (Group 1) treated with ciprofloxacin only. (Group 2)
Moreover, (group 3) was treated with ciprofloxacin and ginseng at a low dose ginseng high dose relatively. (Group 4) treated with ciprofloxacin and vitamin E. (Group 5) serve as control. GPx: glutathione peroxidase. SOD: superoxide dismutase.
DISCUSSION
The presented study is to evaluate the protective effect of ginseng against ciprofloxacin-induced oxidative stress causing male infertility. Many previous studies have demonstrated that ciprofloxacin-induced oxidative stress affects sperm parameters and function, leading to male infertility. In the present study, ciprofloxacin significantly increased MDA and decreased antioxidant enzyme activities (SOD, GPX and TAC). These results are consistent with former reports 6-8, 22. Ciprofloxacin is mainly related to reactive oxygen species (ROS) generation, besides metabolism-related toxicity 23. A rise in MDA indicates testicular cell injury and a reduction in sperm motility and sperm-oocyte fusion. 24, 25. There are many possible mechanisms for oxidative stress causing infertility related to damage of spermatozoa because its membrane consists of polyunsaturated fatty acids, which are susceptible to lipid peroxidation 26. Another hypothesis is that H2O2 can cross the membranes into the cells and inhibit the activity of some enzymes, such as G6PD, which leads to the accumulation of oxidized and reduced glutathione, which leads to lowering the antioxidant defenses of the spermatozoa 27. Also, oxidative stress is associated with reduced fertilization, miscarriage and congenital disabilities in the offspring due to several modifications leading to sperm DNA damage 28-30. Ciprofloxacin also induced a significant down-regulation of antioxidant gene expression after administration of 800 mg/kg/day for 15 days 31.
We found that groups treated with ginseng in two doses showed significant improvement in testicular function via increasing antioxidant enzyme activity (SOD, TAC and GPX) but decreasing in MDA with high dose only. These findings concern previous studies 13, 14, 32, 33. Furthermore, another study found a reduction in MDA besides an increase in SOD, CAT and GPX in rats supplemented with ginseng 34. The ginseng root contains many amino acids, vitamins A, B2, B12, C, and E and metals like sodium, potassium, calcium, phosphorus, iodine, iron, zinc, copper and selenium 35. This component enhanced the antioxidant protective role by decreasing MDA, the end product of lipid peroxidation and a marker for tissue damage 14. The accumulation of MDA 36 evidenced the extensive lipid peroxidation. Also, another study reported that administering ginseng in humans for 8 weeks decreased MDA but increased SOD and CAT activities 37. So, a decrease in MDA indicates ginseng's antioxidant activity.
SOD defends against oxygen free radicals, which are responsible for damage to the plasma membrane and biological structures, causing an elevation in the intracellular Ca2+ ion concentration leading to irreversible conversion of xanthine dehydrogenase to xanthine oxidase 38. Also, it may be due to increasing antioxidant enzyme activities by increasing scavenger of H2O2, preventing the formation of free radicals 39. So, the formation of free radicals is inhibited due to the increased activity of SOD due to ginseng administration.
Catalase and GPx are essential in detoxifying H2O2 14 and indirectly protecting cells 40. The increase in CAT activity is thought to be due to degrade H2O2 produced by SOD activity. Also, ginseng enhanced sex hormone levels, testicular structure, and redox status and has more antioxidant activity than Tribulus Extracts and Pollen Grains 41. Generally, these results indicated that ginseng has protective effects against ciprofloxacin-induced oxidative stress by increasing antioxidant activity and gene expression 42. In the same respect, another study reported that ginseng induced a protective role against alcohol-induced hepatic injury in mice by upregulating the gene expression of the antioxidant enzymes 43. These results are also consistent with a previous study that suggested that ginseng inhibited cardiomyocyte apoptosis by inhibiting the expression of the pro-apoptotic Bax gene in rats 44.
In the present study, the group treated with ciprofloxacin beside vitamin E showed an increased antioxidant enzyme activity and upregulated its gene expression. Consistently, a study that treated 100 Parkinson's disease patients with vitamin E reported that it had a potential therapeutic target for disease-modifying treatments via activating cellular pathways involved in antioxidant and anti-inflammatory responses 45.
Furthermore, another study reported that despite ginseng 100 mg/kg/day have superior outcomes in liver protection than vitamin E 100 mg/kg/day, their antioxidant properties were similar, and this was evidenced by nearly absence of differences in liver tissue MDA, SOD or CAT levels in both treated groups 46. Also, rats treated with vitamin E or Panax ginseng concomitant with levofloxacin significantly improve biochemical and antioxidant parameters 47.
CONCLUSIONS
Ginseng has a protective effect against ciprofloxacin-induced oxidative stress, causing male infertility. It increases antioxidant enzyme activities (SOD, TAC, and GPX) and decreases MDA levels. These results are consistent with previous studies showing ginseng has antioxidant and anti-inflammatory properties.
Vitamin E also has antioxidant properties and can protect against ciprofloxacin-induced oxidative stress. However, ginseng and vitamin E have similar antioxidant properties, so it is unclear which is more effective in protecting against ciprofloxacin-induced male infertility.
More research is needed to determine the optimal dose and duration of ginseng or vitamin E treatment for protecting against ciprofloxacin-induced male infertility.
REFERENCES
1. MacLeod J. The role of oxygen in the metabolism and motility of human spermatozoa. American Journal of Physiology-Legacy Content. 1943;138(3):512-8.
2. Tosic J, Walton A. Formation of hydrogen peroxide by spermatozoa and its inhibitory effect on respiration. Nature. 1946;158(4014):485-.
3. Alvarez JG, Storey BT. Lipid peroxidation and the reactions of superoxide and hydrogen peroxide in mouse spermatozoa. Biology of Reproduction. 1984;30(4):833-41.
4. Henkel RR. Leukocytes and oxidative stress: dilemma for sperm function and male fertility. Asian journal of andrology. 2011;13(1):43.
5. Bui A, Sharma R, Henkel R, Agarwal A. Reactive oxygen species impact on sperm DNA and its role in male infertility. Andrologia. 2018;50(8):e13012.
6. Mokhimar HM, Kandiel MM, Amin AA, Elsawah HK, El Mahmoudy AM. Ciprofloxacin and levofloxacin adversely affect male infertility indicated by pharmacological, andrological and pathological evidence. International Journal of Basic & Clinical Pharmacology. 2020;9(2):353.
7. Albesa I, Becerra MC, Battán PC, Páez PL. Oxidative stress involved in the antibacterial action of different antibiotics. Biochemical and biophysical research communications. 2004;317(2):605-9.
8. Talla V, Veerareddy P. Oxidative stress induced by fluoroquinolones on treatment for complicated urinary tract infections in Indian patients. Journal of Young Pharmacists. 2011;3(4):304-9.
9. Krcmery S, Naber K, German Ciprofloxacin U, Group US, Naber K, R Barth M, et al. Ciprofloxacin once versus twice daily in the treatment of complicated urinary tract infections. International journal of antimicrobial agents. 1999;11(2):133-8.
10. AbdEl-Aziz AA, Naguib Y, Zahran WA, Mahrous H, Khalil H, El-Nahas SA. Detection of oxidative stress induced by ciprofloxacin in immature rat. Research Journal of Applied Biotechnology. 2018;4(1):77-81.
11. Srinivasu M, Singh S, Ahmad A, Pathak A. Effect of enrofloxacin and ciprofloxacin on oxidative stress in rats. Journal Of Veterinary Pharmacology And Toxicology. 2022;21(1):80-2.
12. Choi M-K, Song I-S. Interactions of ginseng with therapeutic drugs. Archives of pharmacal research. 2019;42:862-78.
13. Eskandari M, Ghalyanchi Langeroudi A, Zeighami H, Rostami A, Kazemi M, Eyni H, et al. Co‐administration of ginseng and ciprofloxacin ameliorates epididymo‐orchitis induced alterations in sperm quality and spermatogenic cells apoptosis following infection in rats. Andrologia. 2017;49(3):e12621.
14. Ramesh T, Kim S-W, Hwang S-Y, Sohn S-H, Yoo S-K, Kim S-K. Panax ginseng reduces oxidative stress and restores antioxidant capacity in aged rats. Nutrition research. 2012;32(9):718-26.
15. Council NR. Nutrient requirements of laboratory animals: 1995. 1995.
16. Mokhimar H, Elsawah HK, Kandiel MM, Amin A, Abdallah FE, El-Mahmoudy A. The protective potential of ginseng on ciprofloxacin-induced male gonadotoxicity. Benha Veterinary Medical Journal. 2022;42(2):114-9.
17. Lee M, Lee S-H, Kim M-S, Ahn K-S, Kim M. Effect of Lactobacillus dominance modified by Korean Red Ginseng on the improvement of Alzheimer's disease in mice. Journal of ginseng research. 2022;46(3):464-72.
18. Blackshaw J, Fenwick D, Beattie A, Allan D. The behaviour of chickens, mice and rats during euthanasia with chloroform, carbon dioxide and ether. Laboratory Animals. 1988;22(1):67-75.
19. O'Shaughnessy P. Age-dependent differences in testicular inactivation of FSH and in inhibition of FSH binding to rat testis. Biology of Reproduction. 1979;20(5):1009-14.
20. Gasparino E, Del Vesco A, Voltolini D, Nascimento CD, Batista E, Khatlab A, et al. The effect of heat stress on GHR, IGF-I, ANT, UCP and COXIII mRNA expression in the liver and muscle of high and low feed efficiency female quail. British poultry science. 2014;55(4):466-73.
21. Swift ML. GraphPad prism, data analysis, and scientific graphing. Journal of chemical information and computer sciences. 1997;37(2):411-2.
22. Becerra M, Albesa I. Oxidative stress induced by ciprofloxacin in Staphylococcus aureus. Biochemical and biophysical research communications. 2002;297(4):1003-7.
23. Badawy S, Yang Y, Liu Y, Marawan MA, Ares I, Martinez M-A, et al. Toxicity induced by ciprofloxacin and enrofloxacin: oxidative stress and metabolism. Critical Reviews in Toxicology. 2021;51(9):754-87.
24. Agarwal A, Prabakaran SA. Mechanism, measurement, and prevention of oxidative stress in male reproductive physiology. 2005.
25. Kefer JC, Agarwal A, Sabanegh E. Role of antioxidants in the treatment of male infertility. International Journal of Urology. 2009;16(5):449-57.
26. Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertility and sterility. 2003;79(4):829-43.
27. Griveau J, Dumont E, Renard P, Callegari J, Le Lannou D. Reactive oxygen species, lipid peroxidation and enzymatic defence systems in human spermatozoa. Reproduction. 1995;103(1):17-26.
28. Lewis S, Aitken R. DNA damage to spermatozoa has impacts on fertilization and pregnancy. Cell and tissue research. 2005;322(1):33-41.
29. Bungum M, Bungum L, Lynch KF, Wedlund L, Humaidan P, Giwercman A. Spermatozoa DNA damage measured by sperm chromatin structure assay (SCSA) and birth characteristics in children conceived by IVF and ICSI. International journal of andrology. 2012;35(4):485-90.
30. Aitken RJ, Gibb Z, Baker MA, Drevet J, Gharagozloo P. Causes and consequences of oxidative stress in spermatozoa. Reproduction, Fertility and Development. 2016;28(2):1-10.
31. Naguib YM, Motawea SM, Ameen O. Replenishing glutathione counters ciprofloxacin-induced acute liver failure via possible gene modifying mechanism. Bulletin of Egyptian Society for Physiological Sciences. 2021;41(4):525-36.
32. Won Y-J, Kim B-k, Shin Y-K, Jung S-H, Yoo S-K, Hwang S-Y, et al. Pectinase-treated Panax ginseng extract (GINST) rescues testicular dysfunction in aged rats via redox-modulating proteins. Experimental Gerontology. 2014;53:57-66.
33. Lim KH, Ko D, Kim J-H. Cardioprotective potential of Korean Red Ginseng extract on isoproterenol-induced cardiac injury in rats. Journal of Ginseng Research. 2013;37(3):273.
34. Kim M-H, Lee E-J, Cheon J-M, Nam K-J, Oh T-H, Kim K-S. Antioxidant and hepatoprotective effects of fermented red ginseng against high fat diet-induced hyperlipidemia in rats. Laboratory animal research. 2016;32(4):217-23.
35. Choi M-K, Kang M-H, Kim M-H. The analysis of copper, selenium, and molybdenum contents in frequently consumed foods and an estimation of their daily intake in Korean adults. Biological trace element research. 2009;128(2):104-17.
36. Ali MB, Hahn EJ, Paek K-Y. Protective role of Panax ginseng extract on lipid peroxidation and antioxidant status in polyethylene glycol induced Spathiphyllum leaves. Biochemical Engineering Journal. 2006;32(3):143-8.
37. Kim S-H, Park K-S. Effects of Panax ginseng extract on lipid metabolism in humans. Pharmacological Research. 2003;48(5):511-3.
38. Kim H-J, Lee S-G, Chae I-G, Kim M-J, Im N-K, Yu M-H, et al. Antioxidant effects of fermented red ginseng extracts in streptozotocin-induced diabetic rats. Journal of Ginseng Research. 2011;35(2):129.
39. Halliwell B. Antioxidants in human health and disease. Annual review of nutrition. 1996;16(1):33-50.
40. Kitahara A, YAMAZAKI T, ISHIKAWA T, CAMBA EA, SATO K. Changes in activities of glutathione peroxidase and glutathione reductase during chemical hepatocarcinogenesis in the rat. GANN Japanese Journal of Cancer Research. 1983;74(5):649-55.
41. Mansour AT, Alsaqufi AS, Omar EA, El-Beltagi HS, Srour TM, Yousef MI. Ginseng, Tribulus extracts and pollen grains supplementation improves sexual state, testes redox status, and testicular histology in Nile Tilapia Males. Antioxidants. 2022;11(5):875.
42. Naval M, Gomez-Serranillos M, Carretero M, Villar A. Neuroprotective effect of a ginseng (Panax ginseng) root extract on astrocytes primary culture. Journal of ethnopharmacology. 2007;112(2):262-70.
43. Li Y-G, Ji D-F, Zhong S, Shi L-G, Hu G-Y, Chen S. Saponins from Panax japonicus protect against alcohol-induced hepatic injury in mice by upregulating the expression of GPX3, SOD1 and SOD3. Alcohol & Alcoholism. 2010;45(4):320-31.
44. Liu Z, Li Z, Liu X. Effect of ginsenoside Re on cardiomyocyte apoptosis and expression of Bcl-2/Bax gene after ischemia and reperfusion in rats. Journal of Huazhong University of Science and Technology [Medical Sciences]. 2002;22(4):305-9.
45. Schirinzi T, Martella G, Imbriani P, Di Lazzaro G, Franco D, Colona VL, et al. Dietary Vitamin E as a protective factor for Parkinson's disease: clinical and experimental evidence. Frontiers in Neurology. 2019;10:148.
46. Abdelfattah-Hassan A, Shalaby SI, Khater SI, El-Shetry ES, Abd El Fadil H, Elsayed SA. Panax ginseng is superior to vitamin E as a hepatoprotector against cyclophosphamide-induced liver damage. Complementary Therapies in Medicine. 2019;46:95-102.
47. Ibrahim H, Mahmoud N, Abd El-Mottleb D, Khatab H. Ameliorative effect of vitamin e and panax ginseng against some adverse effects of levofloxacin in male rats. J Anim Health Prod. 2021;9(4):512-23.
Received: 28 September 2023/ Accepted: 15 November 2023 / Published:15 December 2023
Citation: Mokhimar H M, Elsawah H K, Kandiel M M,. Elsaid F E, El-Mahmoudy A M. Study of the protective effect of ginseng against testicular oxidative stress biomarkers and its gene expression induced by ciprofloxacin. Revis Bionatura 2023;8 (4) 20. http://dx.doi.org/10.21931/RB/2023.08.03.20
