2023.08.03.107
Files > Volume 8 > Vol 8 No 3 2023
The Role of CoQ10 and Selenium in Some Physiological Variables in Rabbits Under Oxidative Stress
1Department of Biology, College of Science, Mosul University, Mosul, Iraq
2 Department of Medical Laboratory Technologies/ Al Noor University College, Mosul, Iraq; E-mail: [email protected].
* Correspondence: [email protected].
Available from: http://dx.doi.org/10.21931/RB/2023.08.03.107
The current study was designed to investigate whether the use of coenzyme Q10 at a concentration of 200 mg/kg body weight and selenium at a concentration of 0.05 mg/kg improve the physiological and biochemical characteristics and reduce the adverse effects of H2O2 in domestic rabbits, Twenty local male rabbits, their ages ranged from (1 ±1.5) years. Their weights were (1500 ± 1700) grams. They were randomly Enrolled into four animals each, as follows: The first group was a control group, which was given a standard diet and regular water; for the second, third and fourth groups, they were given H2O2 at a concentration of 0.5%, coenzyme Q10 at a concentration of 200 mg/kg body weight, and sodium selenite at a concentration of 0.05 mg/kg, respectively, for 4 weeks.
The results of the Current study showed that there was a significant increase in the concentration of male sex hormones Spermatogenic Stimulating Hormone (SSH), Interstitial Cell Stimulating Hormone (ICSH), as well as Testosterone, accompanied by an increase in the concentration of Glutathione (GSH) and a decrease in the concentration of Malondialdehyde (MDA) for the third and fourth groups compared with the control group at the probability level (P≤0.05). The second group showed a significant decrease in the concentration of male sex hormones (SSH), (ICSH) and Testosterone, accompanied by a decrease in the concentration of Glutathione and a significant increase in the concentration of Malondialdehyde compared with the control group and the rest of the groups.
Keywords: Coenzyme Q10, Selenium, Males hormones, Oxidative Stress
INTRODUCTION
Domesticated rabbits are notable for their high reproductive capacity, fast growth, and short gestation periods compared to other domesticated animals1. If raised well in healthy conditions, rabbits are raised in typical farms that generate huge profits. They are a continuous source of income for farmers to improve the standard of living, and they do not need large areas in breeding if compared to the rest of the other animals2, and they are playing an ever-increasingly significant part in the production of meat in every region of the planet3. The imbalance between the production of free radicals and the ability of the body's defense system of antioxidants to detoxify or weaken oxidative damage to the DNA of proteins and fats is known as oxidative stress4. Antioxidants are the most important means of preventing oxidative stress. Oxidative stress is one of the most critical issues affecting the growth and production of animals. The various processes essential to life in the body will produce radicals; hence, these radicals serve as a defensive mechanism against the potentially damaging effects of free radicals5. Coenzyme Q10 is one of the essential antioxidants known as ubiquinone, naturally found in higher and microorganisms. It is a fat-soluble antioxidant and the only one that can be synthesized inside the body. Ubiquinone is naturally found in higher and microorganisms. Coenzyme Q10 is naturally found in higher and microorganisms6. An outstanding part will be played in energy production By one of the electron and proton transport compounds in the electron transport chain that occurs in the mitochondria7 and protects cell membranes from lipid peroxidation, as well as its role in determining other antioxidants such as vitamin E and C8.
Coenzyme Q10 has a unique structure, which gives it distinct properties and an apparent effect in physiological conditions. It is an essential cofactor for energy production, adenosine triphosphate (ATP), and has high antioxidant properties. It is an electron-transporting compound in oxidative phosphorylation in the mitochondria of cells9. Through its properties as an antioxidant, it works to restore the effective forms of work as an antioxidant for both vitamin E and C, as it reduces the inactive form, which is in the form of α-Tocopheryl radical resulting from the reaction with fats or in the form of an oxygen radical converting it into the active form α-Tocophero10. Thakur and colleagues found that increasing the concentration of Coenzyme Q10 in seminal plasma improved total TAC capacity and positively affected sperm concentration, motility, and normal morphology11. Selenium is one of the essential microelements, and it is a robust biological mineral12 and has several important physiological roles in many organisms13; selenium is considered one of the essential and highly efficient antioxidants that helps protect rabbits from oxidation of fats and proteins14. Also, selenium plays a significant role in increasing the antioxidant enzyme Gpx15.
The field of the study Time and Place
The present study has been conducted in Laboratories of the College of Sciences, Department of Biology, University of Mosul.
Materials used in the study
animals under study
After ensuring that the rabbits were healthy, the 20 male local rabbits used in this research were confined in metal cages with dimensions of 60×40×50 cm in length, width and height, respectively, that had been purpose-built for this investigation. The rabbits' weights ranged from (1500 ± 1700) grams, and ages ranged from (1 ±1.5) years, and they were purchased from nearby markets. They were provided with a standard diet for rabbits with a protein content of 16.5%, and the National Research Council16 confirmed this percentage; the collective feeding lasted for a whole month. The appropriate conditions included temperatures ranging from (25-28) °C, lighting for approximately 14 hours, and adequate ventilation. The lighting lasted approximately 14 hours. The rabbits were subjected to a preparatory period of one week to acclimatize to the place and the diet before starting the experiment.
Experimental design
In this study, 20 male local rabbits were used, which were distributed into four groups 5 rabbits for each group, and the groups are as follows:
The control group: The rabbits were reared on Which diet and regular water.
The second group was given a Dose of H2O2 at a concentration of 0.5% with normal water and a standard diet.
The third group: Rabbits of this group were given COQ10 at a concentration of 200 mg/kg body with a standard diet and regular water.
Fourth group: The rabbits of this group were given a sodium selenate Dose of 0.05 mg/kg with a standard diet and regular water.
Sample collection
After the end of the treatment period, blood samples were obtained from rabbits by drawing blood from the heart directly with a heart stab. The blood was placed in tubes with tight, dry covers free of any anticoagulant and left at room temperature for 20 minutes to obtain the blood serum; the blood serum was kept freezing at -20 °C until hormonal and biochemical tests were performed.
Hormonal Evaluation
Each Spermatogenic Stimulating Hormone (SSH), Interstitial Cell Stimulating hormone (ICSH), and Testosterone were measured in the blood serum using the Accubind ELISA Microwells Analysis Kit, which was supplied by the American Monobind Inc. and using the German company that manufactured the semi-automated ELISA shaker-reader. This examination was based on the principle of Direct Sandwich at a wavelength of 450 nm17, and the final results were obtained through the values of the standard.
Biochemical examinations
The concentration of Glutathione will be determined in the blood serum
The modified method17 was used to determine the concentration of Glutathione in the serum. The concentration of Glutathione in the blood serum was calculated based on the following equation:
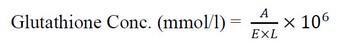
In the blood serum, the concentration of Malondialdehyde will be determined
The concentration of MDA in blood has been determined as one of the essential products of the peroxidation lipid18. The concentration of Malondialdehyde was calculated based on the following relationship:
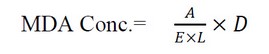
Statistical analysis
Results were statistically analyzed according to the simple process experiments system complete random design and used the Duncan multiple range test to find differences between the groups. The results are considered significant at the probability level (P≤0.05), using the statistical program SAS and Covariance test to extract way LSD19.
The results of this study indicated a significant increase in Spermatogenic Stimulating Hormone (SSH) in two groups treated with Coenzyme Q10 at a concentration of 200 mg/kg body weight and sodium selenate at a concentration of 0.05 mg/kg compared with the control group, as the mean of them reached 2.38 IU/ml and 2.38 IU/ml respectively, noting that there was no significant difference between the two groups. The rabbits exposed to oxidative stress induced using H2O2 at a concentration of 0.5% showed a significant decrease at the same probability level as the control group, as its mean was 0.88 IU/ml. Note that the mean of the control group is 1.74 IU/ml.
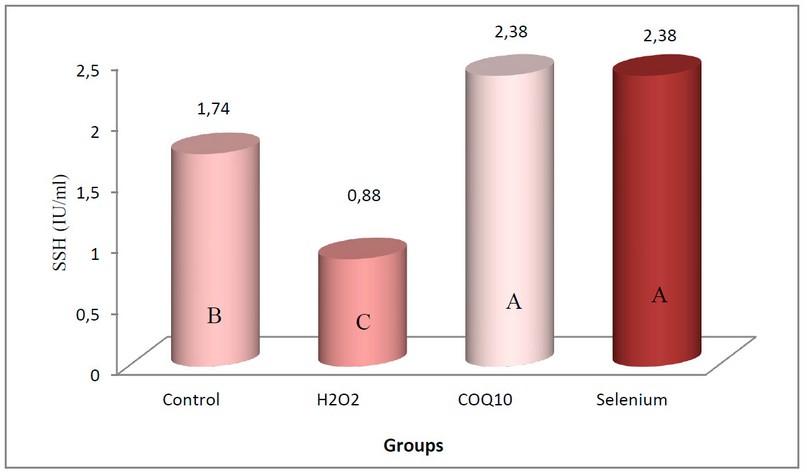
Figure 1. shows the effect of coenzyme Q10 and sodium selenate on the concentration of Spermatogenic Stimulating Hormone (IU/ml) in rabbits exposed to oxidative stress.
The values are expressed as the arithmetic mean (±) standard deviation and the number of rabbits/group = 5.
The different letters mean a significant difference at the level (P≤0.05).
Also significant increase in the concentration of Interstitial Cell Stimulating Hormone (ICSH) at P≤0.05 (Figure 2) in the two groups of rabbits treated with Coenzyme Q10 at a concentration of 200 mg/kg body weight and the group treated with a concentration of 0.05 mg/kg of sodium selenate compared with the control group, the mean of them reached 6.38 IU/ml and 6.37 IU/ml respectively, noting that there was no significant difference between the two groups. The group of rabbits exposed to oxidative stress induced by 0.5% of H2O2 showed a significant decrease at the same level of probability compared with the control group, as its mean was 3.25 IU/ml, knowing that the arithmetic mean of the control group was 5.79 IU/ml.
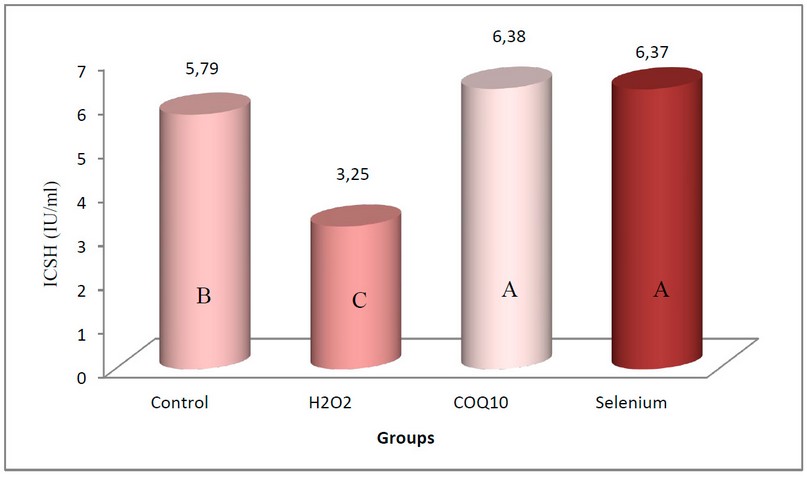
Figure 2. The effect is obvious coenzyme Q10 and sodium selenate on the concentration of Interstitial Cell Stimulating Hormone (IU/ml) in rabbits exposed to oxidative stress.
The values are expressed as the arithmetic mean (±) standard deviation and the number of rabbits/group = 5.
The different letters mean that no significant difference can be observed at the level (P≤0.05).
Also, a significant increase in the concentration of Testosterone hormone at P≤0.05 (Figure 3) in the two groups of rabbits treated with Coenzyme Q10 at a concentration of 200 mg/kg body weight and the group treated with a concentration of 0.05 mg/kg of sodium selenate compared with the control group, where the mean was 4.52 ng/ml and 4.39 ng/ml, respectively, noting that there was no significant difference between the two groups. In contrast, the group of rabbits exposed to oxidative stress induced by using 0.5% of H2O2 showed a significant decrease in the concentration of the hormone at the same level of probability compared with the control group, as its mean was 1.70 ng/ml, knowing that the arithmetic mean of the control group was 2.65 ng/ml.
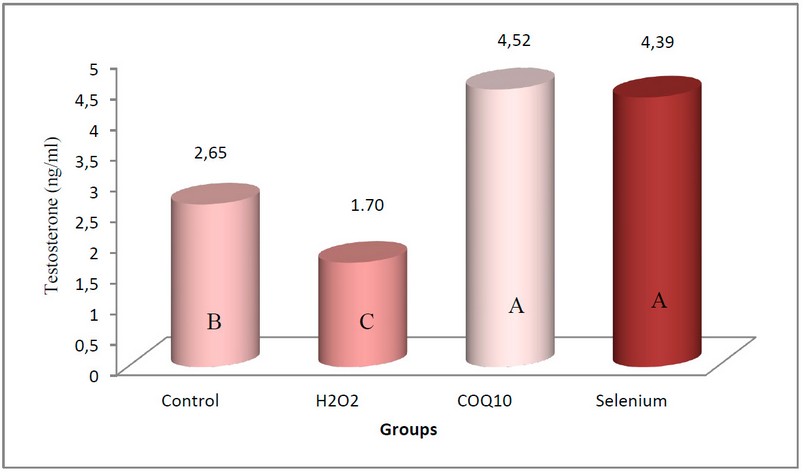
Figure 3. shows the effect of coenzyme Q10 and sodium selenate on Testosterone concentration (ng/ml) in rabbits exposed to oxidative stress.
The values can be expressed as the arithmetic mean (±) standard deviation and the number of rabbits/group = 5.
The different letters mean a significant difference at the level (P≤0.05).
Figure (4) showed a significant increase in the concentration of Glutathione (GSH) in the blood serum of local male rabbits at the probability level (P≤0.05) in the two groups of rabbits treated with Coenzyme Q10 at a concentration of 200 mg/kg body weight and sodium selenate at a concentration of 0.05 mg/kg compared with the control group, the mean was 4.60 µmol/L and 4.52 µmol/L, respectively, with no significant difference between them. At the same time, the group of rabbits exposed to oxidative stress induced by using 0.5% of H2O2 showed a significant decrease in the concentration of Glutathione at the same level of probability compared with the control group, which gave an arithmetic mean of 1.90 µmol/L. Note that the mean of the control group is 3.30 μmol/L.
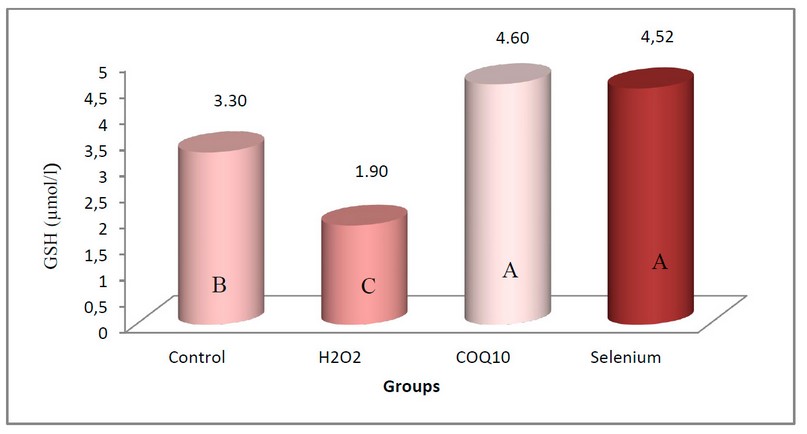
Figure 4. shows the effect of coenzyme Q10 and sodium selenate on the concentration of Glutathione (ng/ml) in rabbits exposed to oxidative stress.
The values Were expressed as the arithmetic mean (±) standard deviation and the number of rabbits/group = 5.
The different letters mean to indicate that there is a fundamental difference at this level.
Figure (5) showed a significant decrease in the concentration of Malondialdehyde (MDA) in the blood serum of local male rabbits at the probability level (P≤0.05) in the two groups of rabbits treated with Coenzyme Q10 at a concentration of 200 mg/kg body weight and sodium selenate at a concentration of 0.05 mg/kg compared to the control group. The decrease was more significant in the group treated with Coenzyme Q10, whose mean was 312.37 nmol/gm, while the group treated with sodium selenate gave a mean of 336.12 nmol/gm. In contrast, the group of rabbits exposed to oxidative stress induced by using 0.5% H2O2 showed a significant increase in the concentration of MDA compared with the control group, as its mean was 665.63 nmol/g. Note that the arithmetic mean of the control group is 529.83 nmol/gm.
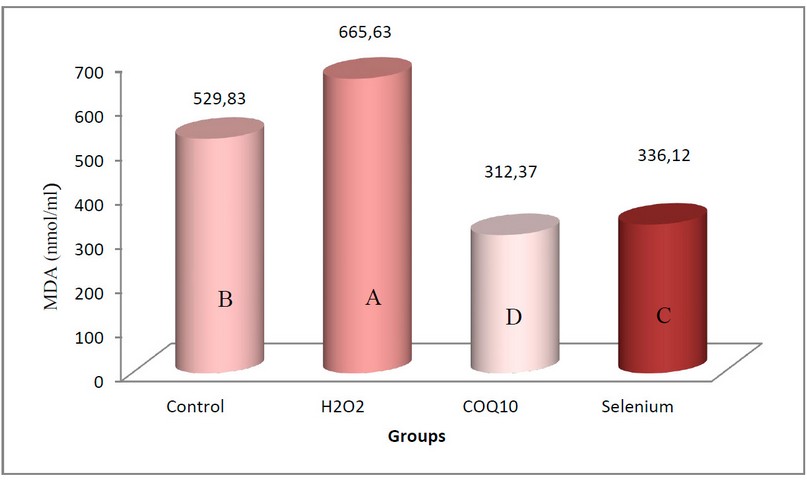
Figure 5. Shows the effect of coenzyme Q10 and sodium selenate on the concentration of Malondialdehyde (nmol/ml) in rabbits exposed to oxidative stress.
The values were expressed as the arithmetic mean (±) standard deviation and the number of rabbits/group = 5.
The different letters mean to indicate that there is a fundamental difference at this level.
A vital increase has been already shown in the present study concentrations of SSH, ICSH, and Testosterone in the two groups of rabbits treated with Coenzyme Q10 and sodium selenate compared to the control group and the group of rabbits exposed to oxidative stress induced by using 0.5% of H2O2; the reason may be because CoQ10 and selenium are antioxidants that directly or indirectly affect free radicals and enhance the antioxidants in the testicular tissue that positively affect the germ layers and reduce oxidative damage20, also the ability of CoQ10 and selenium to inhibit the secretion of stress hormones such as cortisol and cortisone, in addition to their ability to stimulate the secretion of the noradrenaline hormone, which affects steroid hormones21. In addition, selenium is necessary for the activity of the glutathione peroxidase enzyme22, thus protecting the testicle from oxidative damage caused by free radicals and increasing the secretion of testosterone23.
Sex hormones are among the hormones most affected by oxidative stress, as Owen24 pointed to an imbalance in the levels of sex hormones in rabbits exposed to oxidative stress, and from our results, it was concluded that hydrogen peroxide treatment caused a significant decrease in the concentrations of male sex hormones, the reason may be due to the stressful hydrogen peroxide effect25 and that stress often will have some consequences as it is expressed in this research. It stimulates many Hormones like (CRH), Hormone (ACTH), and The adrenal cortex to secrete cortisol26 and the increase in the secretion of ACTH, in turn, inhibits the secretion of gonadotropin-releasing hormone (GnRH), which negatively affects the secretion of SSH and ICSH27.
The decrease in the concentration of Glutathione in cases When there is this oxidative stress due to a reduction of the effectiveness of the Pentose shunt in these cases28, as the enzyme activity G-6-PDH, which is necessary for the activity of its conversion to Pentose, and thus the formation of NADPH decreases. GSH-RD is unable to reduce the oxidized form of GSSG. Consequently, there will be a decrease in the concentration reduced GSH occurs. It is noted from the results that the GSH decreased as a result of treatment with hydrogen peroxide, and this was accompanied by the noticed concentration increase in the MDA as a result of the increase in lipid peroxidation, which rises due to the imbalance between antioxidants and oxidants.
The decrease in MDA concentration when treated with coenzyme Q10 and sodium selenate indicates a decrease in the hydrolysis of unsaturated fatty acids in biological membranes, the reason for the improvement in the concentration of Glutathione in the group of rabbits treated with coenzyme Q10 and sodium selenate may be due to the fact that they are antioxidants and play a significant role in scavenging free radicals, in addition to the role of coenzyme Q10 in the respiratory chain, being one of the essential compounds for energy production during the process of electron transfer and oxidative phosphorylation, Coenzyme Q10 also possesses antioxidant properties due to the nature of its composition and content of phenols, because of its close location to unsaturated fatty acids in the cell membrane29 and because of its fat-soluble nature, it inhibits the lipid oxidation process in the cell membrane and keeps the LDL-c proteins present in the blood circulation from oxidation, which is the primary transporter in the blood, as 80-90% of coenzyme Q10 in the blood serum is in the form of Ubiquinol is dissolved in lipoprotein molecules30 thus, it may provide the necessary protection for DNA from the harmful effects of free radicals, including preventing mutations or damage to DNA and cell death31.
The present study investigated the effects of oxidative stress induced by hydrogen peroxide (H2O2) on testicular function in rabbits and the protective effect of Coenzyme Q10 (CoQ10) and sodium selenate. The results showed that H2O2 treatment caused a significant decrease in the concentrations of sex hormones (SSH, ICSH, and testosterone), glutathione (GSH), and an increase in the concentration of malondialdehyde (MDA). Treatment with CoQ10 and sodium selenate significantly reversed the effects of H2O2, increasing the concentrations of sex hormones, GSH, and decreasing the concentration of MDA. CoQ10 and sodium selenate have a protective effect on testicular function against oxidative stress induced by H2O2. They suggested that this protective effect may be due to the antioxidant properties of CoQ10 and selenium, which can scavenge free radicals and protect against lipid peroxidation.
Is also discussed the role of stress hormones in the effects of H2O2 on testicular function. They suggested that the increase in stress hormones caused by H2O2 may inhibit the secretion of gonadotropin-releasing hormone (GnRH), which negatively affects the secretion of SSH and ICSH.
Overall, the present study provides valuable information on the effects of oxidative stress on testicular function and the protective effect of CoQ10 and sodium selenate. The findings suggest that these antioxidants may be useful for protecting the testicles from oxidative damage and improving testicular function in men.
Funding: "This research received no external funding,"
Acknowledgments: The author would like to thank the Dean and Head of the Department of Biology at the College of the Science / University of Mosul for providing all facilities to complete this research (grant no. ST-2017-008).
Conflicts of Interest: "The authors declare no conflict of interest."
1. Ibrahim, F. K., Shahbaa K. I. & Aisha, K.A. Study of the blood components of female rabbits fed with a forage mixed with cinnamon. Iraqi Journal of Veterinary Sciences,2009: 23(2):271-274.
2. Bivolarski, B. L., Vachkova, E. G., & Ribarski, S. S. Effect of weaning age upon rabbit meat's slaughter and physicochemical traits. Veterinarski arhiv,2011: 81(4), 499-511.
3. Biagini, D., Gasco, L., Rosato, R., Peiretti, P. G., Gai, F., Lazzaroni, C., ... & Ginepro, M. Compost-sourced substances (SBO) as feedstuff additives in rabbit production. Animal Feed Science and Technology,2016: 214, 66-76.
4. Prasad, A. S., & Bao, B. Molecular mechanisms of zinc as a pro-antioxidant mediator: clinical therapeutic implications. Antioxidants,2019 8(6), 164.
5. Law, B. A., Liao, X., Moore, K. S., Southard, A., Roddy, P., Ji, R., ... & Cowart, L. A. Lipotoxic very‐long‐chain ceramides cause mitochondrial dysfunction, oxidative stress, and cell death in cardiomyocytes. The FASEB Journal,2018: 32(3), 1403-1416.
6. Littarru, G. P., Bruge, F., & Tiano, L. Biochemistry of coenzyme Q10. In Antioxidants in andrology,2017: (pp. 23-34). Springer, Cham.
7. Shukla, S., & Dubey, K. K. CoQ10 a super-vitamin: review on application and biosynthesis. 3 Biotech,2018: 8(5), 1-11.
8. Gvozdjáková, A., Kucharská, J., Dubravicky, J., Mojto, V., & Singh, R. B. Coenzyme Q10, α-tocopherol, and oxidative stress could be critical metabolic biomarkers of male infertility: disease markers, 2015.
9. Salvio, G., Ciarloni, A., Cutini, M., & Balercia, G. Hyperhomocysteinemia: Focus on endothelial damage as a cause of erectile dysfunction. International Journal of Molecular Sciences,2021: 22(1), 418.
10. Feher, G., Koltai, K., Alkonyi, B., Papp, E., Keszthelyi, Z., Kesmarky, G., & Toth, K.Clopidogrel resistance: role of body mass and concomitant medications. International Journal of Cardiology,2007: 120(2), 188-192.
11. Thakur, M. P., Milcu, A., Manning, P., Niklaus, P. A., Roscher, C., Power, S., ... & Eisenhauer, N. Plant diversity drives soil microbial biomass carbon in grasslands irrespective of global environmental change factors. Global Change Biology,2015: 21(11), 4076-4085.
12. El-Hack, A., Mohamed, E., Mahrose, K., Arif, M., Chaudhry, M. T., Saadeldin, I. M., ... & Rehman, Z. U. Alleviating the environmental heat burden on laying hens by feeding on diets enriched with certain antioxidants (vitamin E and selenium) individually or combined. Environmental Science and Pollution Research,2017: 24(11), 10708-10717.
13. Choi, H. S., Choi, J. H., Jung, D. Y., Kim, J. O., Shin, J. H., & Min, B. I. Effect of Selenium on the Thyroid gland Antioxidative Metabolisms in Rat Model by Ionizing Radiation. Journal of radiological science and technology,2017: 40(1), 135-142.
14. Müller, A. S., Pallauf, J., & Most, E. Parameters of dietary selenium and vitamin E deficiency in growing rabbits. Journal of trace elements in medicine and biology,2002: 16(1), 47-55.
15. Davood Abadi Farahani, M. H., Ma, D., & Nazemizadeh Ardakani, P. Nanocomposite membranes for organic solvent nanofiltration. Separation & Purification Reviews,2020: 49(3), 177-206.
16. National Research Council. Nutrient requirements of poultry, National Academies Press.1994.
17. AL–Zamely, O. M. Y. Ischemic heart disease via oxidative hypothesis.2001 (Doctoral dissertation, Ph. D. Thesis).
18. Muslih, R., Al-Nimer, O., & Al-Zamely, M. The level of Malondialdehyde after activation with H2O2 and CuSO4) is inhibited by Desferoxamine and Molsidomine in the serum of patients with acute myocardial infection. J. Chem,2002: 5, 148.
19. Antar, S.H. Statistical analysis in scientific research and program SAS. Directorate Library for Printing and Publishing, University of Mosul- Iraq,2010: pp. 33- 41.
20. E Abdel Moneim, A. Citrus peel extract attenuates acute cyanide poisoning-induced seizures and oxidative stress in rats. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders),2014: 13(4), 638-646.
21. Hong, M. Y., Seeram, N. P., & Heber, D. Pomegranate polyphenols down-regulate expression of androgen-synthesizing genes in human prostate cancer cells overexpressing the androgen receptor. The Journal of Nutritional Biochemistry,2008: 19(12), 848-855.
22. Ónody, A., Csonka, C., Giricz, Z., & Ferdinandy, P. Hyperlipidemia induced by a cholesterol-rich diet leads to enhanced peroxynitrite formation in rat hearts. Cardiovascular research,2003: 58(3), 663-670.
23. Hamzaa, R. G., El Shahat, A. N., & Mekawey, H. M. S. The antioxidant role of mulberry (Morus alba L.) fruits in ameliorating the oxidative stress induced in γ-irradiated male rats. Biochem Anal Biochem, 2012:1(122), 2161-1009.
24. Owien, F. Effect of branched-chain amino acids on some physiological and productive characteristics of oxidatively stressed rabbits. Ph.D. thesis,2018: College of Agriculture, Tikrit University.
25. Coyle, C. H., Martinez, L. J., Coleman, M. C., Spitz, D. R., Weintraub, N. L., & Kader, K. N. Mechanisms of H2O2-induced oxidative stress in endothelial cells. Free Radical Biology and Medicine, 2006:40(12), 2206-2213.
26. Charmandari, E., Tsigos, C., & Chrousos, G. Endocrinology of the stress response 1. Annual review of physiology,2005: 67(1), 259-284.
27. Tsutsui, K., Saigoh, E., Ukena, K., Teranishi, H., Fujisawa, Y., Kikuchi, M., ... & Sharp, P. J. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochemical and biophysical research communications,2000: 275(2), 661-667.
28. Martins, R. N., Stokes, G. B., & Masters, C. L. Regulation of the multiple molecular forms of rat liver glucose 6-phosphate dehydrogenase by insulin and dietary restriction. Biochemical and biophysical research communications,1985: 127(1), 136-142.
29. Awad, A. M., Bradley, M. C., Fernández-del-Río, L., Nag, A., Tsui, H. S., & Clarke, C. F. Coenzyme Q10 deficiencies: pathways in yeast and humans. Essays in biochemistry,2019: 62(3), 361-376.
30. Bhagavan, H. N., & Chopra, R. K. Plasma coenzyme Q10 response to oral ingestion of coenzyme Q10 formulations. Mitochondrion,2007: 7, S78-S88.
31. Liu, Q., Huang, Y., Zhang, R., Cai, T., & Cai, Y. Medical application of Spirulina platensis derived C-phycocyanin. Evidence-Based Complementary and Alternative Medicine, 2016.
Received: 25 June 2023/ Accepted: 26 August 2023 / Published:15 September 2023
Citation: Al-Kattan M M , Alhayani A J. The Role of CoQ10 and Selenium in Some Physiological Variables in Rabbits Under Oxidative Stress. Revis Bionatura 2023;8 (3) 107 http://dx.doi.org/10.21931/RB/2023.08.03.107
