2018.03.02.5
Files > Volume 3 > Vol 3 No 2 2018
INVESTIGATION / RESEARCH
Comparison of tropospheric ozone and nitrogen dioxide concentration levels in Ecuador and other latitudes
Manuel A. Andino-Enríquez 1 , Sandra P. Hidalgo-Bonilla1, Luis A. Ladino2,*
Available from: http://dx.doi.org/10.21931/RB/2018.03.02.5
ABSTRACT
In recent decades, problems related to anthropogenic activity have generated concern due to the accumulation of pollutants in the air, resulting in changes in the concentrations of greenhouse gases and particulate matter. In Ecuador and the rest of the world, air pollution represents a risk and a threat to health and the environment. To carry out an analysis on this subject, it is necessary to develop a dynamic process, followed by a permanent effort that allows improving the quality of the information. The studies developed in Ecuador on pollutant emissions are carried out since 2010; however, there are no comparisons made on this issue in the global context, which leads to the inability to make a real assessment of the air quality situation in the country, as well as the consequences that may result from not doing it. Thus, the main objective of this project is to analyze the concentration levels of NO2and O3 tropospheric in Ecuador and make a direct comparison with different cities around the world. Resulting that concentration of study gases varies with the number of the population of a sector in addition to the pollution that it produces.
In order to develop this research, the information collected comes from the World Meteorological Organization, belonging to WHO WDCGG DATA SUMMARY (World Meteorological Organization World Data Center for Greenhouse Gases) of the year 2015, while the data of the study cities of Ecuador (Ibarra and Quito) were provided by the municipality of Ibarra and by the Municipality of the Metropolitan District of Quito respectively.
Keywords: Anthropogenic pollution, nitrogen dioxide, tropospheric ozone, concentration of atmospheric gases.
INTRODUCTION
In recent decades, pollution problems due to the development of human activities have led to an increase in the concentration of greenhouse gases in the planet's atmosphere. Atmospheric pollution implies changes in the physical, chemical, and biological characteristics of atmospheric components, which can impair the health and survival of living beings 1.
The surface of the earth has had great natural temperature changes over the last 10000 years, which even surpass the current values. However, since the Industrial Revolution (1750) there has been a continuous and uncontrolled increase in the heating of the surface of the Earth due to the rise of the concentration of greenhouse gases as a result of anthropogenic pollution 2.
The sun is the greatest source of the radiation that Earth receives, transmitted by electromagnetic waves. When solar radiation comes into contact with the Earth's atmosphere, part of this radiation is absorbed by the earth's surface and then re-emitted as infrared radiation (IR). A fraction of the solar radiation and IR radiation is absorbed by the atmospheric components which result in an increase in the temperature of the planet. In the same way, part of the solar radiation is reflected back to space by clouds, aerosol particles, the atmosphere, and by the same terrestrial surface, which results in a cooling of the terrestrial surface 3. In order to maintain a stable temperature and climate, the Earth must have an energetic balance between the radiation it receives from the sun and the IR radiation it emits into space. This balance is known as effective temperature 4. When the IR radiation emitted by the earth is trapped (absorbed) by greenhouse gases, it is irradiated back to the terrestrial surface, resulting in the heating of this. This phenomenon is known as the greenhouse effect 4. The capture of terrestrial radiation by greenhouse gases is essential to maintain a temperature of the terrestrial surface sustainable for life since otherwise, the Earth's temperature would be near freezing point -18 °C 4. The problem arises because of anthropogenic pollution, as the uncontrolled emission of greenhouse gases can induce faster heating of the surface of the Earth. Currently, efforts to reduce its speed or magnitude have given moderate results but have failed to reverse adverse environmental changes 5.
The most common greenhouse gases are carbon dioxide (CO2), tropospheric ozone (O3), water vapor, and methane (CH4). Due to human activities, their concentrations have increased 6. The most tangible example is reported by CO2 as illustrated in Figure 1.
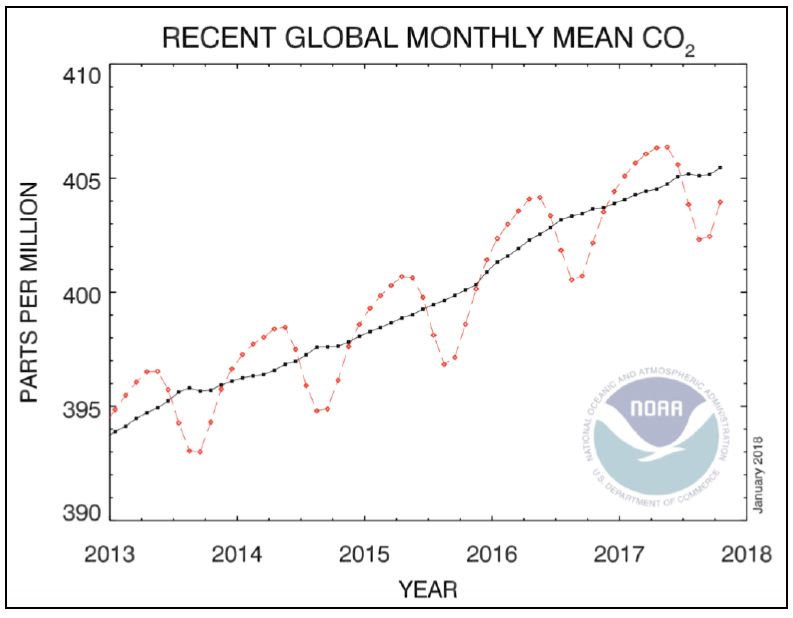
Figure 1. Increase in CO2 concentration with time 7
Nitrogen Dioxide (NO2)
Nitrogen dioxide is a poisonous, non-flammable, reddish-colored gas. It is a chemical compound consisting of two oxygen atoms and one nitrogen atom. Nitrogen dioxide is not a greenhouse gas, but in the atmosphere, it contributes to the photochemical formation of Tropospheric Ozone, in addition to being one of the biggest enemies of the cities as it poisons its inhabitants as explained below 8. Nitrogen oxides (NO2 and NO) are created from rays, soil microbial activity, biomass burning (both natural and anthropogenic fires) as well as the combustion of petroleum derivatives 9.
The NO2 is generated mainly in the air burning fuel from emissions of cars, trucks, buses, power plants, among others. Along with other NOx, the gas NO2 reacts with other chemicals in the air to form both O3 tropospheric and particulate matter (PM) 10.
Due to the human contamination mentioned above, there has been an increase in the concentrations of NO2, mainly in the urban and industrial sectors 11. The daily cycle of NO2 begins in the first hours of the day where the maximum concentration is observed between 8 and 9 in the morning. Then, as this gas is a precursor of tropospheric ozone, it reacts chemically in the presence of solar radiation (290 nm < λ < 400 nm) with other gases, contributing to the formation of tropospheric O3, reducing its concentration in the atmosphere. As the intensity of solar radiation decreases, the concentration of tropospheric O3 will decrease and the concentration of NO2 will increase again (8-9 pm), but this concentration peak is not as high as the maximum in the early hours of the morning 12.
The nitrogen dioxide produces a great risk in the environment, as it is easily oxidized in the vapor of water of the clouds forming nitric acid (HNO3), one of the main constituents of acid rain 11. As the raindrops containing these acids precipitate, they react easily with organic and inorganic substances and modify them, damaging or destroying the soil, water, plants, animals and buildings 13. The precipitation of acid rain manages to subtract essential nutrients from the soil and release aluminum, which makes the absorption of water from trees difficult. Also, they damage the needles of the conifers and the leaves on trees 14.
The detrimental effects of NO2 on human health are focused on the respiratory system. According to the Department of Health of the region of Murcia, it has been found that NO2 causes damage to the pulmonary parenchyma. In addition, it determines the inhibition of mucociliary purification, phagocytosis and immune response in the lungs, resulting in a decrease in lung resistance to infections. Likewise, it increases pulmonary sensitivity to the bronchial-constricting, thus affecting especially the asthmatic 15.
The World Health Organization (WHO) established the guideline value of 20 ppb (40 μgm-3) as the annual average of NO2 to protect the population from the negative effects of this gas in health (guiding value: Numerical value related to the concentration of particles present in the air indicating that if this is exceeded, there is the possibility of having adverse effects in health). The rationale for this is that most concentration reduction methods are specific to NOx (NO2 and NO) and are not designed to control other contaminants that accompany them by increasing their emissions 16.
Tropospheric Ozone (O3)
Ozone is a colorless gas, whose molecular formula is O3. It is found in two layers of the atmosphere: in the stratosphere and in the troposphere. Ozone in the stratosphere acts as a protective layer against the negative effects in health and the environment because it is capable of absorbing the ultraviolet radiation from the sun, thus avoiding reaching the Earth's surface 17. Of the total ozone concentration present in the atmosphere, less than 10% is found in the troposphere. However, tropospheric ozone plays an important role in the environment due to its participation in different chemical processes. Between the geographic space comprised of the Tropic of Cancer (20 °N) and the Arctic Circle (60 °N), Tropospheric Ozone was the largest contributor to global warming during the twentieth century 2.
Tropospheric Ozone is a secondary pollutant, which means that it is not emitted directly to the atmosphere, but is the result of chemical reactions, inadequate conditions of solar radiation accompanied by other primary pollutants such as Volatile Organic Compounds (VOCs), carbon monoxide (CO), nitrogen oxides (NOx), and to a lesser extent methane (CH4). One of the training mechanisms is the interaction between solar radiation λ < 400 and NO2. This interaction produces O-, which acts as an intermediate in the reaction. This chemical species reacts with O2 and produces O3 tropospheric 18. Similarly, in its destruction, the O3 tropospheric reacts with nitrogen monoxide (NO) forming O2 and NO2 lowering the concentration of O3 in the atmosphere 19.
NO2 + hn(λ<400 nm) → O- + NO
O- + O2 → O3
NO + O3 → O2 + NO2
The daily cycle of this pollutant begins in the early morning hours, in which anthropogenic activities begin, emitting mainly: NOx, CH4, and CO2. Then, while the planet receives solar radiation, the concentration of ozone increases according to the presence of the compounds that form it. In the afternoon, solar radiation is not so intense causing the concentration of tropospheric ozone to decrease until it returns to its base concentration 12.
Tropospheric ozone originates mainly in urban areas by various emission sources (automobiles and industry). Elevated concentrations of tropospheric ozone cause this gas to react quickly by destroying or altering other molecules and then act as a toxic pollutant, causing many problems for human health (respiratory damage and pulmonary, chest pains, etc.) 12.
Exposure to ozone has been linked to premature mortality and a range of issues of morbidity and asthma symptoms 20. WHO established the guide value of ozone concentration levels at 100 µgm-3 for a daily average of eight hours; however, the data collected is insufficient to recommend an annual guide value. The unit of measurement used in this research work is ppb (parts per billion), the guiding value presented would be equivalent to 50 ppb 16.
Data collection
The Global Atmosphere Watch (GAW) is a program of the World Meteorological Organization (WMO) that owns a data center that archives and provides observations of the chemical composition of the atmosphere and its natural change, and it has more than 400 monitoring stations around the world 21.
Ibarra is the capital of the province of Imbabura. It is located in northern Ecuador, specifically 115 km northwest of Quito, 125 km south of the city of Tulcán, with an altitude of 2225 M.A.S.L (meters above sea level).
According to the national population census carried out in 2010, Ibarra has an approximate population of 181175 inhabitants. It has a variety of climates with an average temperature of 18 º C, ranging between the cold of the Andean elevations, passing through the warm humid of the area of Lita and Carolina, to the dry tropical of the Chota Valley 22.
The city of Ibarra is located within two hydrographic sub-basins: Chorlaví River and Tahuando River. The topography of its soil corresponds to slopes that oscillate between 5 and 15% in the foothills of the Cerro Imbabura 22. The industrial activity of the city of Ibarra is formed by manufacturing, construction, mining, and energy industries. The latter focuses on the production of electricity, gas and water 23. In relation to the vehicular activity, each year there is an increase of 8% to 11% in the number of vehicles circulating the city 24.
Quito is the political capital of the Republic of Ecuador, currently with 2644145 inhabitants, at 2700 M.A.S.L. The city is located mainly on the valley of Quito, forming part of the Hoya de Guayllabamba. Its climate corresponds to the subtropical climate of Highlands, ranging from arid and temperate to humid and cold climates. Quito is divided into 3 zones: south, center, and north; the south is the coldest part in the city as it is the highest; the center is hot, always showing the highest temperatures, and the north is temperate. Quito, being the capital of the Republic of Ecuador, houses the largest number of industries in the country, becoming one of the main sources of development in the country. This has caused serious environmental impacts in the city and consequently problems in the health of its inhabitants 25. In 2014, the Automotive Park of Quito was 468777 vehicles. The occupancy levels of the vehicles have decreased from 1.7 people in 2013 to 1.2 people in the year 2014, which represents an increase in vehicular congestion in the city 26. At this time, there are 10450 companies installed in the Ecuadorian capital, of which 372 are industries and are located in urban areas. Due to this problem, parks and industrial areas are being reorganized, with the intent of having companies located in a specific sector through the creation of three industrial parks. Industries in Quito are related to structural products, heavy machinery for the electrical sector, the agricultural sector, construction, textiles, metalworking industries, etc. 27.
METHODOLOGY
The WHO WDCGG (World Meteorological Organization World Data Centre for Greenhouse Gases) DATA SUMMARY of the year 2015 belonging to the World Meteorological Organization(WMO), provided the data of the concentrations of NO2 and O3 of the different stations that it possesses, to help with the development of this research work. In addition, the Municipality of Ibarra and the Secretariatof the Environment of the Municipality of the Metropolitan District of Quito provided the data of their respective cities.The selection of the stations of the GAW was carried out with the purpose of being able to elaborate a direct comparison of the concentration of NO2 and O3 of theEcuadorian stations with different cities from around the world covering different latitudes and lengths. A selection of the study time was made by a review of the data provided by the different institutions, verifying that inthe selected time there are data from Ecuador from the cities of study (not all the stations belonging to the (WMO) have recent data on greenhouse gas concentrations) that have the longest possible duration. The foregoing resultedin the study time being from January 2013 to December of the same year for NO2, while for O3 tropospheric the study period was between April 2013 and September 2014. Once the cities of study and the data of concentrations ofgases of interest were obtained, information about their population was collected in order to have a more general vision of the capacity of emission of atmospheric pollutants. By formulating hypotheses, the comparison of the concentration of the study gases and the population of the study cities wasfinally carried out, obtaining the following results.
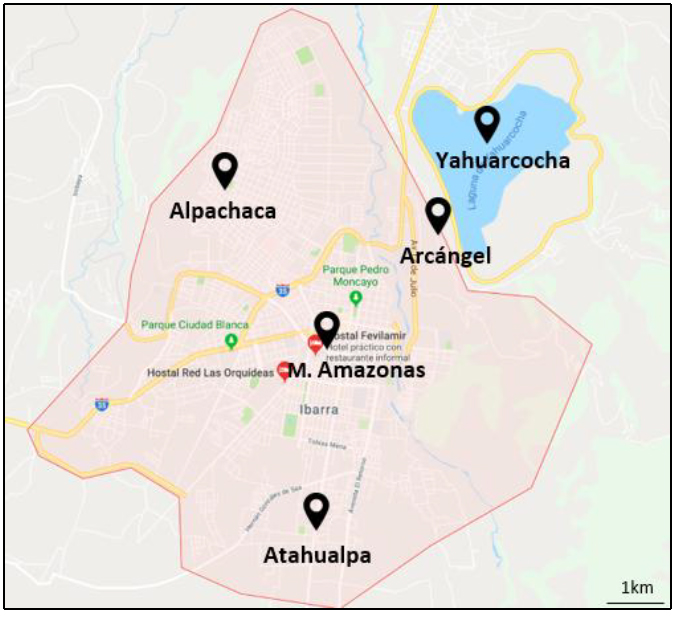
Figure 2. Map of the tropospheric ozone stations in Ibarra
Figure 2 shows the location of the stations in the city of Ibarra, on the outskirts and in the center of the city. The shortest distance between stations is about 2 km, while the farthest is approximately 4 km. Unfortunately, the data provided by the municipality of Ibarra do not detail the equipment used for the data taking of tropospheric O3.
RESULTS
Nitrogen Dioxide
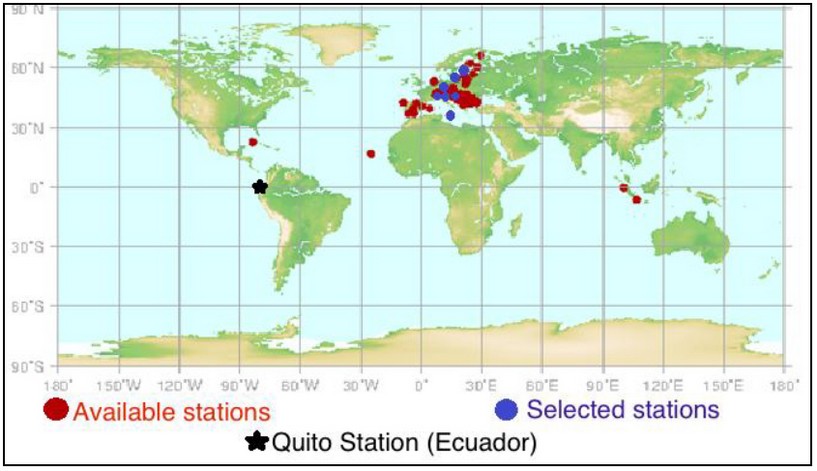
Figure 3. Selected NO2 Stations 2
Figure 3 shows the stations selected for nitrogen dioxide. Red marks represent all NO2 stations available from the WDCGG (World Data Centre for Greenhouse Gases). From there, the stations selected for the study of this gas are marked in blue. The exception is that the stations of Ecuador (Quito and Ibarra) are not part of the WDCGG. The hypotheses posed were:
· Air currents influence the concentration of nitrogen dioxide in a city/region.· The concentration of nitrogen dioxide is proportional to the increase in the population of a city/region.
As can be seen in Figure 3, among these selected stations are those of cities of the following countries: Latvia, Switzerland, Poland, Hungary, Czech Republic, Slovenia, Malta and Ecuador
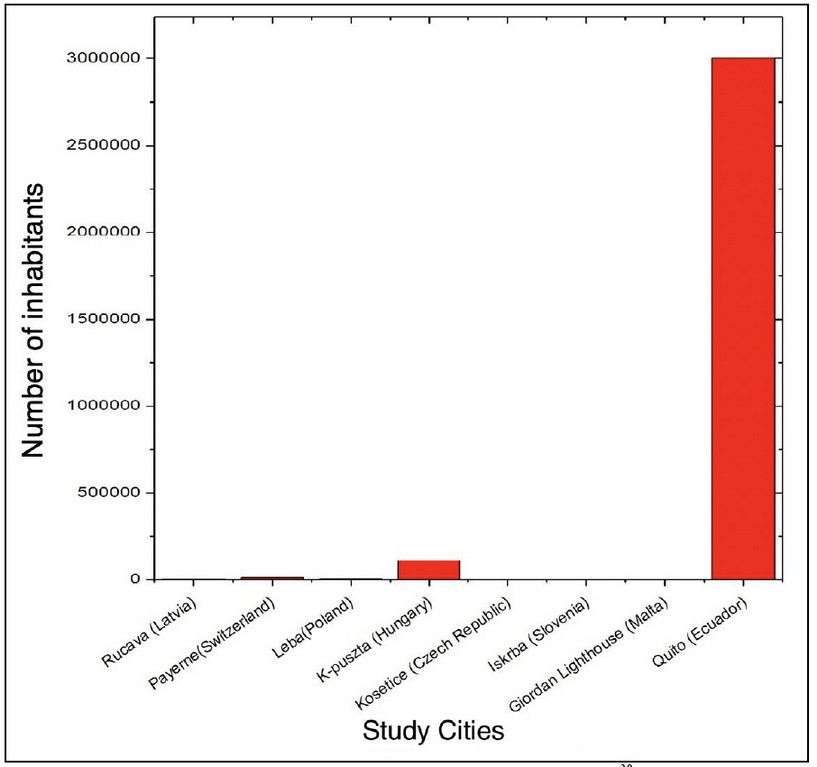
Figure 4. Population in the cities of study for NO2 28
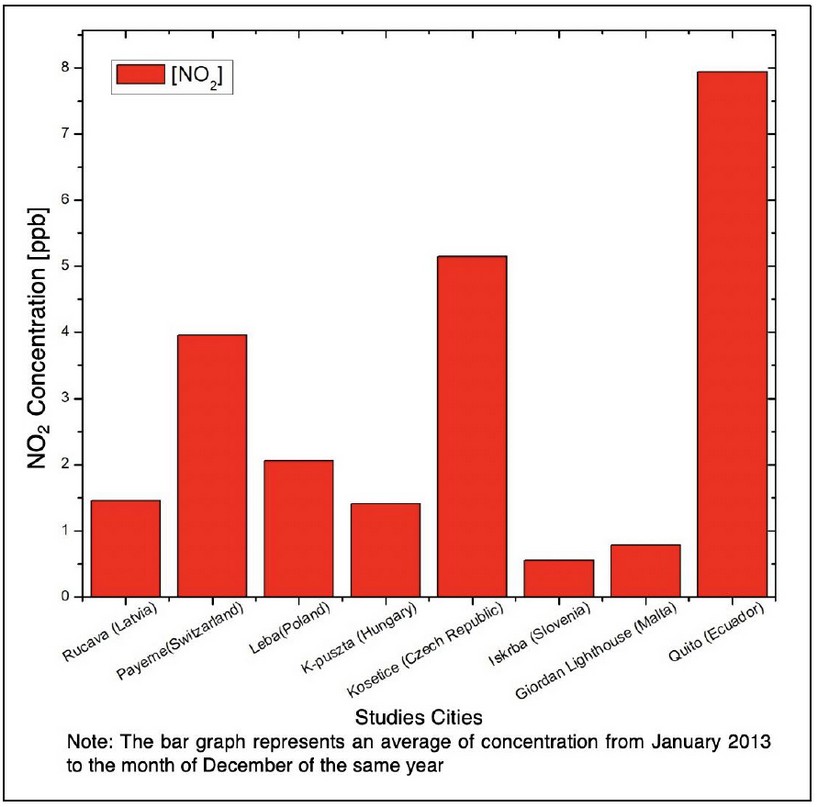
Figure 5. Comparison of NO2 concentration in selected stations
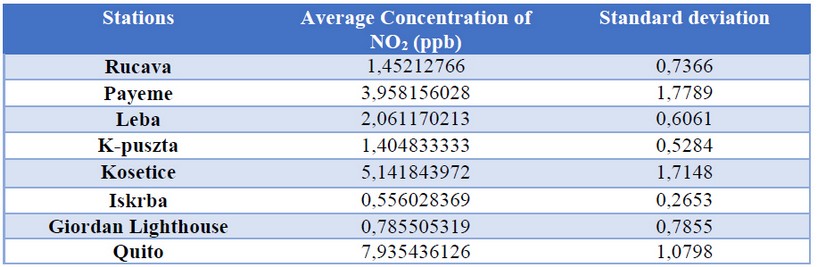
Table 1. Standard deviation of the concentration of NO2 and O3 in each study station
As can be seen in Figure 4, the city of Quito has the highest population density of the cities of study, in accordance with the levels of NO2, which are the highest (Figure 5 and Table 1). One of the main reasons for this level of concentration is the population density in this city and the pollution that it produces (vehicular and industrial pollution mainly), as can also be seen in the different cities where population density is high (city of Guayaquil, in 2017 estimated a population of 2644891 inhabitants) 29. This indicates that population density is directly proportional to the level of NO2 concentration.
Another factor influencing the concentration of this gas is the wind currents. Air currents influence the dispersion of contaminants, specifically wind direction and velocity. The concentration of contaminants is inversely proportional to wind velocity. The dispersion of pollutants in the atmosphere is influenced by the direction of the wind. If the wind direction is constant, the same area will be continuously exposed to relatively high levels of contamination. If the wind direction varies, the concentrations will be relatively minor due to contaminants being scattered over a larger area 30. For these reasons, the concentrations of a contaminant may vary depending on: population density, air currents, topography, altitude, solar radiation, the height of the mixture layer 30.
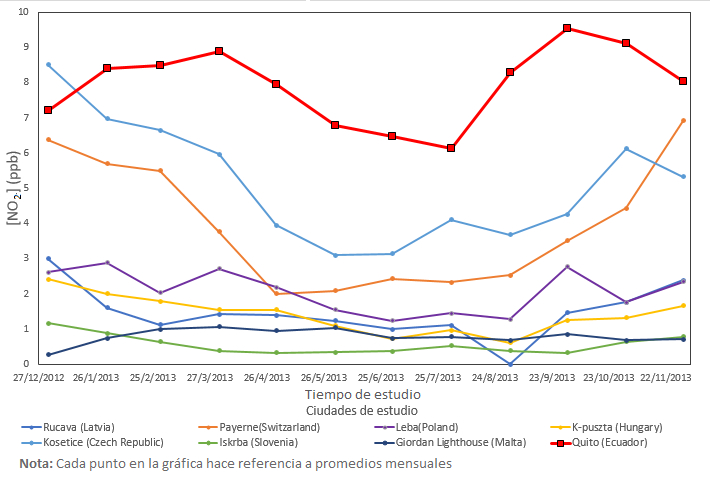
Figure 6. Comparison of NO2 levels in Ecuador with other latitudes, the year 2013
Figure 6 shows that during the time of the study, the levels of nitrogen dioxide for the city of Quito were higher during the majority of months compared to the other cities of study, showing that the population of that city produces an excessive emission of this gas, which is very alarming. In the same way, Figure 6 shows the trend line of each study city, presenting the monthly values of NO2 concentration. If the trend line over the time of study is high, then the city of study produces a considerable emission of NO2 regardless of the number of its inhabitants, such as the City of Kosetice (Czech Republic) which has little population density (Figure 4) but produces a high amount of NO2 that emanates into the atmosphere. The trend line of the city of Quito shows lower values mid-year and the highest values at the end and beginning of the year. This is because in mid-year there is more radiation in Ecuador so, the NO2 with solar radiation from O3 tropospheric, lowers the concentration of NO2 present in the city of Quito. On the contrary, at the end of the year, solar radiation is not as intense compared to mid-year months, which allows not to produce so much tropospheric O3, increasing the concentration of NO2.
According to WHO, the permissible levels of nitrogen dioxide concentration correspond to 40 μgm-3 (21.27 ppb), so the concentration of nitrogen dioxide in the city of Quito is below the limit value according to WHO standards. However, according to the data obtained, the concentration of this gas in the city of Quito is the highest of all, so it is recommended to reduce the burning of biomass (both natural and anthropogenic fires) as well as the burning of fuels as these activities are most likely the main cause of the level of concentration of this gas.
Tropospheric Ozone
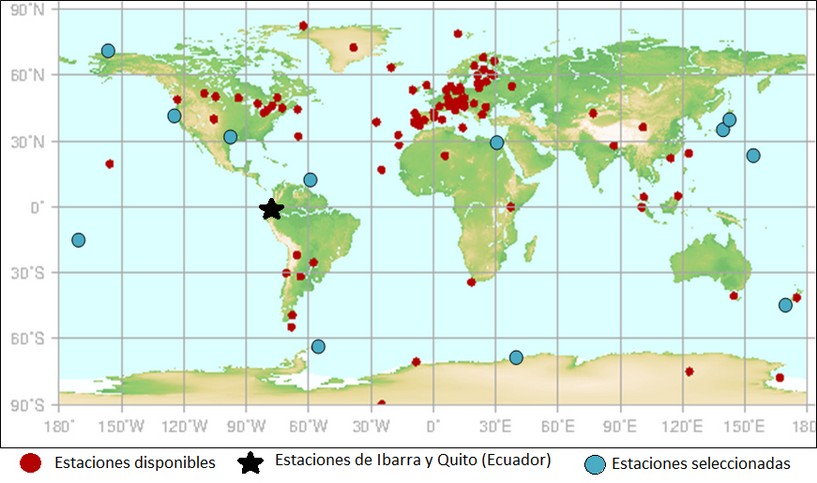
Figure 7. Selected tropospheric O3 stations
Unlike for nitrogen dioxide, for the analysis of tropospheric ozone, there was an additional station in Ecuador, the Ibarra station.
As in Figure 3, in Figure 7 the blue marks represent the stations that were used for the analysis of tropospheric ozone. The hypotheses posed for this gas are as follows:
· As long as the population of a city/region increases, the concentration of tropospheric ozone will increase.
· The tropospheric ozone concentration will be higher in a city/region if it receives higher solar radiation.
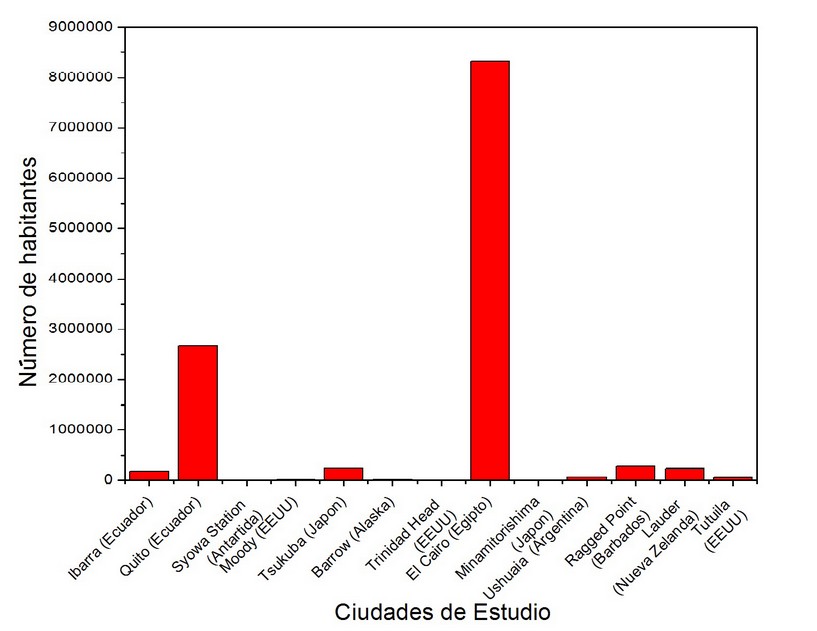
Figure 8. Populations in different study cities 28
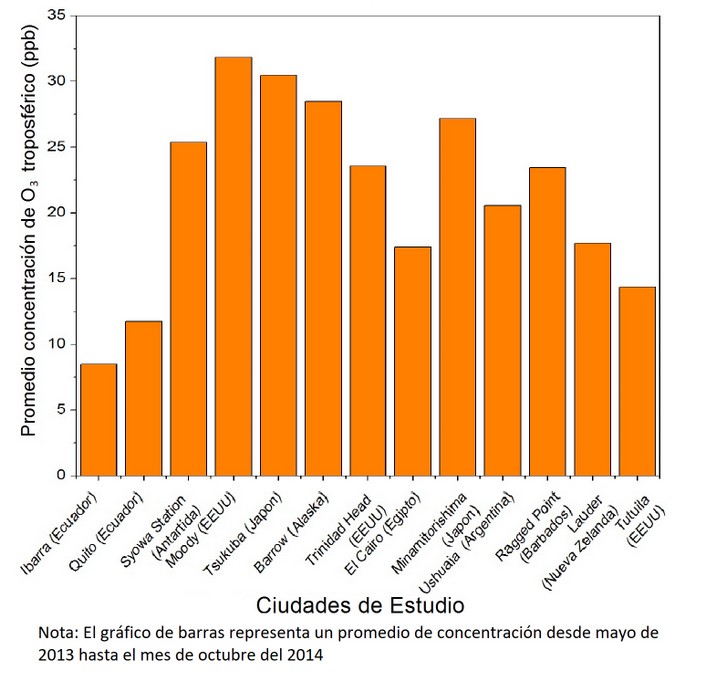
Figure 9. The average concentration of tropospheric O3 in the cities of study
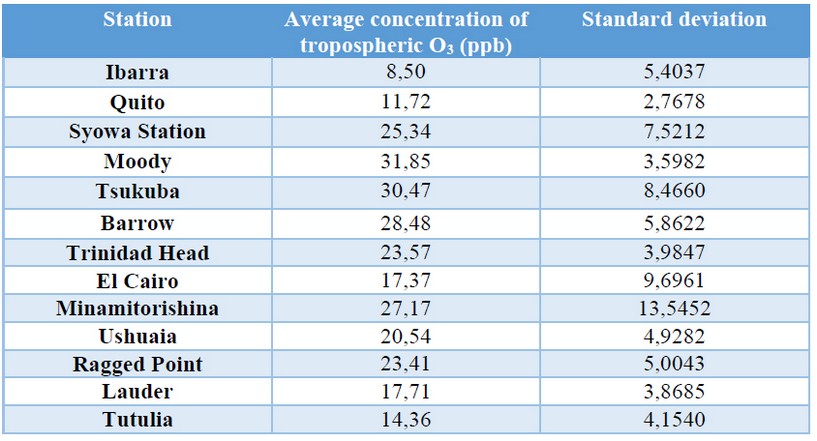
Table 2. Standard deviation of the concentration of tropospheric O3 in each study station
It can be seen in Figure 8 that the population of Cairo far exceeds the other cities. Figure 9 and Table 2 show that Cairo's tropospheric ozone concentrations are much lower than expected, considering its population (very high) and its geographical position (prone to high solar radiation). The concentration of this gas in Ecuador is the lowest of all the cities of study, however, the sum of the population of the two Ecuadorian stations results in the second highest population among the cities of study (Figure 8).
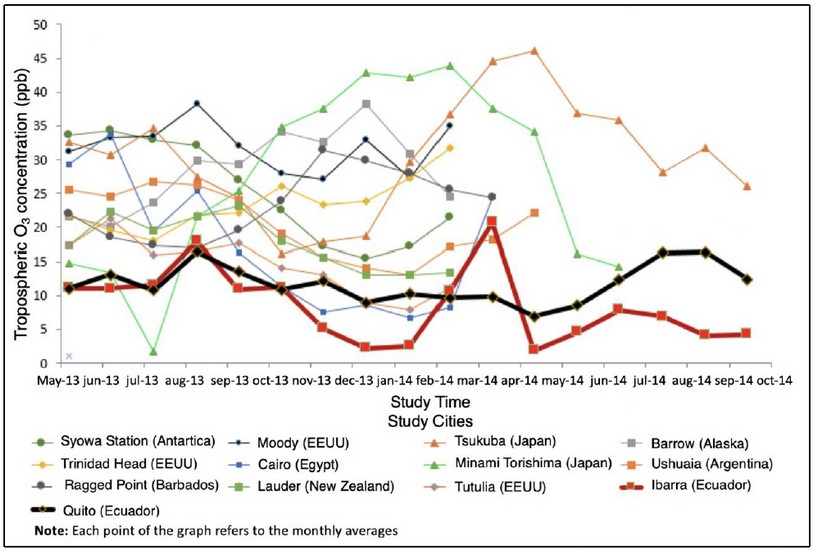
Figure 10. Comparison of levels of concentration of tropospheric O3 in Ecuador and the cities of study, year 2013
O3 Trends in Ibarra
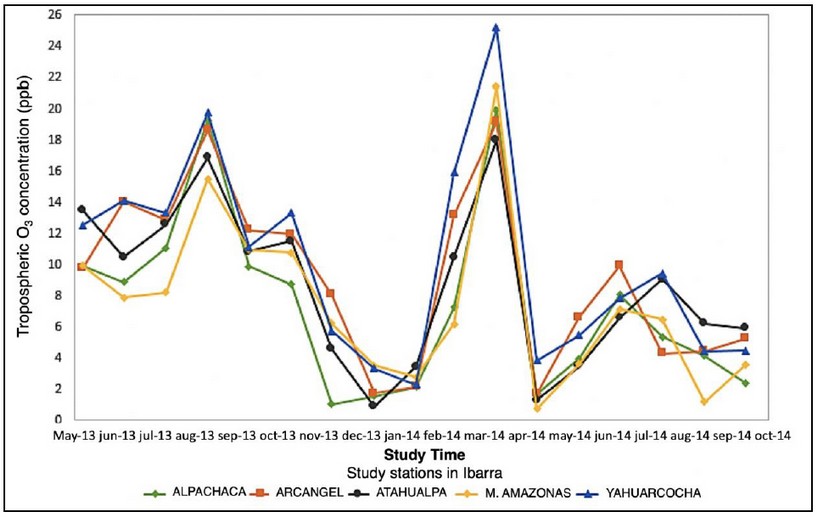
Figure 11. Measurements of tropospheric O3 in the city of Ibarra
Figure 11 represents the concentration of tropospheric O3 in the city of Ibarra where each line represents a station in the city. The tendency of all concentration lines of tropospheric O3 is closely related, although some differences can be noted, which may be due to an increase in anthropogenic activity in the sector where the station is located. In the month of August 2013 and in the month of August 2014 there is an increase in the concentration of the gas of study probably due to an increase in the solar radiation in the city of Ibarra, which explains that the lines of tendency of all the stations have an increase in its value during the months mentioned.
Since the stations of Ibarra do not perform the measurement of solar radiation, data from the city of Quito of solar radiation were used assuming that this value does not vary much between both cities. However, it should be noted that the two cities are separated by a distance of 116 km and that the height of Ibarra and Quito are 2215 and 2700 M.A.S.L., respectively. The r2 (0.0032) found in this analysis indicates that solar radiation is not the determinant factor in the formation of tropospheric O3 in Ibarra. This is because their values are relatively constant throughout the year, day after day. It is very probable that those who control the formation and concentration of O3 are their precursors. Unfortunately, the only precursor that is measured in Ibarra is the NO2 which also does not show a good correlation with tropospheric O3 (r2 = 0.0136).
The Madden-Julian Oscillation (MJO) consists of large-scale patterns coupled with atmospheric circulation and deep convection. These spread slowly eastwards (~ 5 m*s-1) through the portion of the Indian and Pacific oceans where the sea surface is warm. MJO constantly interacts with the underlying ocean and influences many meteorological and climatic systems 31. Tropospheric ozone concentrations may be decreased or increased by MJO; however, given the lack of studies in this direction in Ecuador, it is not possible to say that the MJO has a direct effect on the levels of O3 in Ibarra.
Tropospheric ozone trends in Quito
As mentioned above, the amount of solar radiation can affect the concentration of tropospheric ozone, so the following illustrations will be based on the relationship between these two factors. For the case of Quito, the r2 (0.4793) found shows that solar radiation has a relationship in the formation of tropospheric O3 in this city. The amount of solar radiation is adapted in a suitable way with the concentration of tropospheric O3, generating a possible tendency. Like in Ibarra, it was found that the nitrogen dioxide despite being a precursor of tropospheric ozone is not a factor that influences in great measure because its r2 (0.0045) is very low.According to WHO, there is a fixed value in the tropospheric ozone guidelines of 100 μgm-3 of a mean of 8h equivalent to 50 ppb, unfortunately, there is no annual value reported by WHO for this gas 32. Despite the absence of this data, several institutions grant approximate values in which 60 ppb of objective value year should not be exceeded for the protection of human health 33.
Following this data and Figure 9, the tropospheric ozone concentration of the two stations in Ecuador (Quito and Ibarra) is under the permitted value. However, it is recommended that not only authorities help to reduce the concentration of this gas, but also the community at large because although it is under the authorized value, it can still be detrimental to human health.
CONCLUSIONS
With these considerations, it is concluded in the present research work that those who control the formation and concentration of O3 are their precursors. Unfortunately, the only precursor that is measured in Ibarra and Quito is the NO2 which does not show a good correlation with O3 tropospheric. The amount of solar radiation is also a factor influencing the concentration of tropospheric ozone; however, although a good correlation was found with the levels of O3 in Quito, this does not seem to be the one that controls the levels of O3. It is necessary to be able to measure the other precursors of O3 in order to understand their sources and the influence of these in the quality of local air.
The levels of concentration of nitrogen dioxide and tropospheric ozone in the two stations of Ecuador (Quito-Ibarra) have values below those permitted according to the World Health Organization and the Directorate General for Sustainability and Environmental Control respectively, therefore, the air quality of Ecuador has adequate values in NO2 concentration and tropospheric ozone compared to the different cities taken into account for this study.
According to the results obtained in this research, for the concentration of NO2 there is an influence of the number of inhabitants of the city/region as a larger population will produce an increase in pollution. Taking the same factor (number of inhabitants), the concentration of tropospheric O3 is not influenced by the population of a sector, since the main factor of influence in the concentration of this gas is the amount of solar radiation reported. Finally, the concentrations of both gases can be altered by population density, air currents, topography, altitude, solar radiation, the height of the mixture layer 30.
Although, as already mentioned, the concentrations of NO2 and tropospheric ozone are below those allowed according to the UN, it is recommended that industries and society, in general, become aware of the great problem of atmospheric pollution because it is in our hands that the following generations can enjoy a clean air and consequently a dignified quality of life as we all deserve. Similarly, it is recommended that municipalities and Governments propose to improve the monitoring of both gases and atmospheric particles to have a better uncertainty about the quality of the air we breathe and thus generate laws that allow reducing the emission of these pollutants.
THANKS
The authors thank the Global Atmosphere Watch for providing much of the data used in this study. In the same way, the economic support of the General Directorate of Academic Affairs (DGAPA) of the UNAM through the project PAPIIT IA108417 is highly appreciated.
REFERENCES
1 Silva Arroyave SM, Correa Restrepo FJ. Análisis de la contaminación del suelo: revisión de la normativa y posibilidades de regulación económica. Semest Económico 2009; 12: 13–34.
2 Centro mundial de datos para gases de efecto invernadero. WMO WDCGG DATA SUMMARY. 39th ed. Japan Meteorological Agency: Tokyo, 2015.
3 Restrepo C. ¿Cómo el deshielo del Ártico afecta el desarrollo ambiental y humano? Col. MARYMOUNT. 2014; : 28.
4 Jacob D. Introducción a la Química Atmosférica. Princeton University: Princeton, 1999.
5 United Nations Environment Programme. GEO5 (Global Environment Outlook). Nairobi, 2012.
6 Fox S. The Top Ten Greenhouse Gases. Pop. Sci. 2009.
7 National Oceanic and Atmospheric Administration. Recent Global Monthly Mean CO2. Glob. Monit. Div. 2018.
8 Martín B. Qué es el dióxido de nitrógeno y por qué afecta tanto a la salud y el medio ambiente. D. La Inf. 2016.
9 U.S. Greenhouse Gas Inventory Program. Greenhouse Gases and Global Warming Potential Values. Washington D. C., 2002.
10 United State Environmental Protection Agency. Basic Information about NO2. Nitrogen Dioxide Pollut. .
11 Fundación Crana. Óxidos de nitrógeno (NOX = NO + NO2). Fund. Crana. .
12 IDEAM. OZONO TROPOSFÉRICO.
13 Universidad Complutense de Madrid. Lluvia ácida. .
14 Redacción National Geographic. Lluvia ácida. Natl. Geogr. Mag. 2010.
15 Consejería de Salud de la Región de Murcia. Dióxido de Nitrógeno. Cons. Salud la Región Murcia. .
16 Organización Mundial de la Salud. Guías de calidad del aire de la OMS relativas al material particulado, ozono, el dióxido de nitrógeno y el dióxido de azufre. 2005.
17 GreenFacts. Ozono. GreenFacts. .
18 González Kirchner B. Ozono troposférico. 2009.
19 Consejería de Sanidad y Consumo de la Región de Murcia. El ozono troposférico y sus efectos sobre la salud. Cons Sanid y Consum la Región Murcia; : 26.
20 Secretariado de la Comisión para la Cooperación Ambiental. Ozono troposférico. El mosaico América del Norte Panor los Probl Ambient más Relev.
21 Organización Mundial de Meteorología. GAW stations network and other measurements. Glob. Atmos. Watch. .
22 Gobierno Autónomo Descentralizado Municipal San Miguel de Ibarra. Ibarra, ciudad a la que siempre se vuelve.
23 Gobierno Autónomo Descentralizado de San Miguel de Ibarra. PLAN DE DESARROLLO Y ORDENAMIENTO TERRITORIAL DEL CANTÓN IBARRA.
24 Bolaños C. Cada año aumenta un 8 % el parque automotor en Ibarra. 2016.
25 Rosero M. Actividades profesionales y comercios hacen que Quito sea la capital económica. El Comer. 2014.
26 Pacheco M. 50 000 automotores nuevos circulan en las vías de Quito. El Comer. 2014.
27 Redaccion Quito. La nueva zona industrial de Quito empieza a edificarse. Rev. Líderes. 2013.
28 Population.City. Poblaciónes de países y ciudades en todo el mundo. Population.City.
29 Instituto Nacional de Estadística y Censos. Guayaquil en cifras, 2017.
30 Venegas LE, Mazzeo NA. LA VELOCIDAD DEL VIENTO Y LA DISPERSIÓN DE CONTAMINANTES EN LA ATMÓSFERA. .
31 Zhang C. Madden-Julian Oscillation. Miami, Florida, USA, 2005.
32 World Health Organization. Ambient (outdoor) air quality and health. World Health. Organ. 2016.
33 Dirección general de sostenibilidad y control ambiental. Calidad del Aire 2016. 2016.
Received: 20 febrero 2018
Accepted: 21 mayo 2018
Manuel A. Andino-Enríquez 1 orcid.org/0000-0003-0493-9077
Sandra P. Hidalgo-Bonilla1 orcid.org/0000-0001-8905-8716
Luis A. Ladino2,* orcid.org/0000-0002-4941-7945
1Escuela de Ciencias Químicas e Ingeniería, Universidad de Investigación de Tecnología Experimental YachayTech, Urcuquí, Ecuador
2Centro de Ciencias de la Atmósfera, Universidad Nacional Autónoma de México, México City, México
*Corresponding author: Luis A. Ladino, [email protected]
