2023.08.02.79
Files > Volume 8 > Vol 8 No 2 2023
Human Neutrophil Peptide 1- 3 and Vitamin D levels in periodontitis

Fatima Zidan Mahawi1,*, Batool Hassan Al-Ghurabi2,*
1 Master student, Department of Basic Science of Oral Microbiology, College of Dentistry, and the University of Baghdad.
2 Professor, Ph.D. in Microbiology/Immunology, Department of Basic Science College of Dentistry, University of Baghdad, Iraq.
* Correspondence: [email protected] , [email protected]
Available from: http://dx.doi.org/10.21931/RB/2023.08.02.79
ABSTRACT
Human neutrophil peptides 1-3 (HNP1-3) contribute to oral health by playing a role in innate response against oral diseases, owing to their antimicrobial, antiplaque and immunomodulatory activity. Vitamin D may reduce the risk of infection through multiple mechanisms and boosts innate immunity by modulating the production of antimicrobial peptides and cytokine response. This study was performed to evaluate the level of human HNP1-3 in periodontitis patients and controls and to study the effect of vitamin D on the levels of peptides in periodontitis. Eighty-five subjects were enrolled in this observational case-control study aged 20-59 years. The subjects were divided into fifty patients with periodontitis and 35 healthy controls. Periodontal parameters used in this study were plaque index, gingival index bleeding on probing, probing pocket depth and clinical attachment loss. Blood samples were collected from all subjects, and then ELISA was done to estimate the levels of HNP1-3 and vitamin D in patients and controls. The finding revealed a significant increase (P˂0.01) in the mean level of HNP1-3 among the patient's group compared to the control group.
Moreover, there is no significant correlation with all parameters except the gingival index, which has a considerable result (r=0.367, p=0.009). On the other hand, this study showed a significant decrease (P<0.01) in the mean level of vitamin D in patients compared to controls, and also that there was no significant decrease in the serum level of HNP1-3 among vitamin D insufficient group in comparison to vitamin D sufficient group. These results indicated that the increased levels of HNP1-3 and its correlation with GI provide evidence for the critical role of neutrophils in the inflammatory process in periodontitis and vitamin D, which plays a role in antimicrobial peptide production.
Keywords: periodontitis, antimicrobial peptides, human neutrophils peptides
INTRODUCTION
Periodontal diseases are disease processes involving the periodontium, a term used to describe the supportive apparatus surrounding the tooth, which includes the gingival tissue, alveolar bone, cementum and periodontal ligament 1. Gingivitis is the mildest form of periodontal disease and can be found in up to 90% of the population. It is a reactive condition that is reversible upon the improvement of oral hygiene. Periodontitis is when the periodontal condition has progressed beyond gingivitis into a chronic, destructive and irreversible inflammatory disease state. The bacteria then can penetrate deeper into the tissues and surrounding periodontium. This triggers a host response in an attempt to defend against the invading bacteria. However, during the process of protecting against the bacteria, the host defenses also destroy the periodontium. Periodontitis leads to loss of attachment of the periodontium, which subsequently progresses to alveolar bone loss, potentially resulting in loss of the affected tooth 2. Antimicrobial peptides (AMPs) are a class of active oligopeptides that are toxic to pathogens 3,4,5. AMPs are widely distributed in nature, with examples reported from microorganisms, plants, invertebrates, fish, amphibians, birds and mammals 6,7. Because they mostly have a net positive charge and also exhibit hydrophobicity, AMPs can combine with negatively charged surfaces due to electrostatic interactions, penetrate and destroy the membrane structure, resulting in the death of bacteria, fungi, parasites and viruses 8. AMPs can destroy pathogens by damaging multiple targets, which can greatly reduce the emergence of drug-resistant bacteria and make them one of the best alternatives for comprehensive antibiotics due to their broad-spectrum antibacterial properties 6,7,9. Human neutrophil peptides 1–3 (HNP1-3) are small cationic AMPs that provide the first line of host defense against a broad spectrum of microorganisms 10. HNP1-3 is expressed in ductal epithelial cells of submandibular salivary glands and secreted into saliva 11. They are also produced by neutrophils and released into gingival crevicular fluid 12.
Vitamin D (VD) is a steroid hormone obtained mainly from exposure to sunlight but also from diet and dietary supplements 13,14. VD is a generic name comprising VD2 and VD3. VD3 results from ultraviolet irradiation of 7-dehydrocholesterol from lanolin 15 exhibiting the biological activity of cholecalciferol (VD3), and it is synthesized in the human skin. Measuring serum 25-hydroxy VD (25[OH] D) is a widely accepted biomarker analysis for VD status 13,16. VD plays a role in the immune response against infections in the oral cavity 17. The deficiency of VD negatively impacts oral health and the patient's general well-being 18. Periodontitis and gum diseases are the most common oral diseases linked to VD deficiency 19. VD inhibits periodontal tissue damage from the excessive immune response induced by the presence of pathogenic microbes in an average healthy person 20,21. This study aims to evaluate the level of human HNP1-3 in periodontitis patients and controls and to study the effect of vitamin D on the levels of that peptides in periodontitis.
MATERIALS AND METHODS
Study groups: In this study, 50 periodontitis patients (25 males and 25 females) were included, ranging in age from 20 to 59 years. They were admitted to the Department of Periodontics /College of Dentistry / University of Baghdad clinic from November 2021 to January 2022. Patients were divided into two groups according to VD sufficiency; periodontal parameters used in this study were plaque index (PLI), gingival index (GI), bleeding on probing (BOP), probing pocket depth (PPD) and clinical attachment loss (CAL). Blood samples were collected from all patients and controls. ELISA was carried out to estimate the serum levels of HNP1-3 and VD in the studied groups. The control group consisted of 35 individuals (15 males and 20 females), and their ages ranged between (20-56) years.
Inclusion and Exclusion Criteria: The inclusion criteria included good general health with no history of systemic diseases, participants not having received periodontal treatment within the past 6 months, and at least 20 or more natural teeth. The exclusion criteria included patients with systemic diseases, pregnant women, smoking or alcohol dirking, previous periodontal therapy for the last 6 months, antibiotics and/or antiinflammatory medication in the last 3 months, intake of supplements and wearing orthodontic appliances or prosthodontics.
Oral examination: Clinical examination was performed by a specialist dentist for every subject who is under the study. The periodontal status of all teeth was assessed using a periodontal probe of William's graduation; the periodontal parameters include PLI, GI, BOP, PPD and CAL.
Sample collection: Three milliliters of venous blood was collected from all participants. Blood was transferred to a sterile plain tube, and serum was separated by centrifugation at 3000 rpm for 10 minutes, then divided into small aliquots and kept at -20ºC until used for analysis.
Measurement of HNP 1-3 and VD: Serum HNP1-3 and VD were estimated by ELISA and done according to the instructions on the kit's brochure (Shanghai/China).
Statistical analysis: The T-test and chi-square were utilized to calculate the statistical significance of the differences between groups, while the Pearson correlation coefficient tests were used to calculate correlations among different parameters. The P-values of P<0.01 and P<0.05 have been regarded as significant.
RESULTS
Table 1 shows the demographic features and periodontal parameters of the 85 participants in this study.
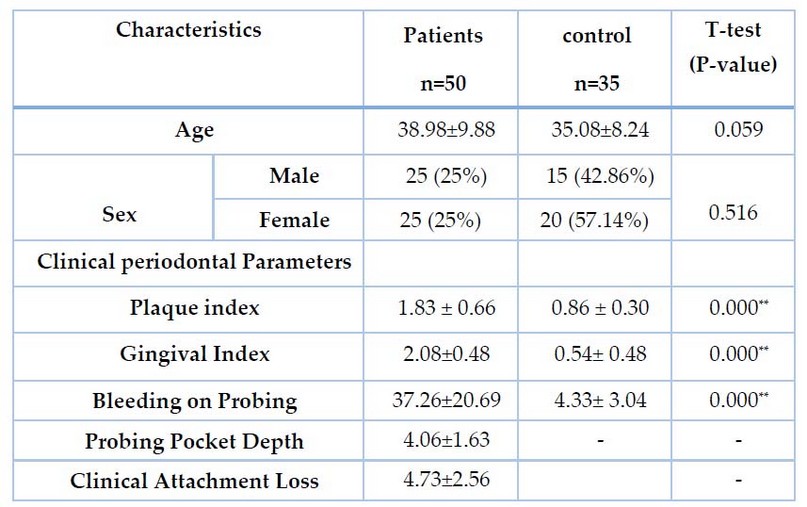
Table 1. Demographic and clinical parameters in the study and control groups
The present study showed a significant increase (P˂0.01) in the mean value of HNP1-3 among the patient's group (14.71±4.68 ng/ml) in comparison to the control group (7.14± 3.78 ng/ml), Table 2. Regarding the correlation between HNP1-3 and clinical parameters, this study found no significant correlation with all parameters except GI, which has a considerable result (r=0.367, p=0.009), as shown in Table 3.
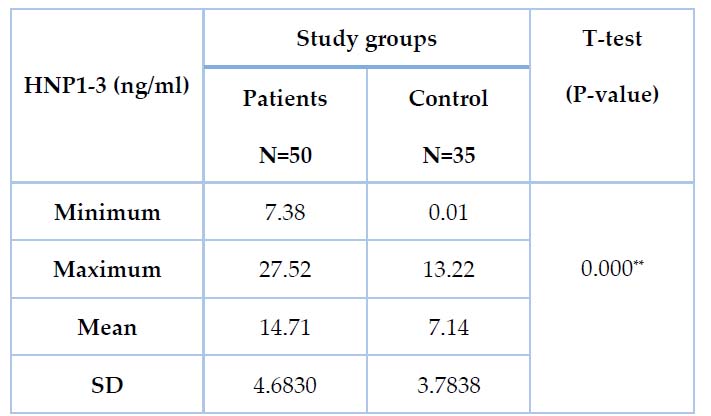
Table 2. Mean of HNP1-3 (ng/ml) Values in patients and Control Groups
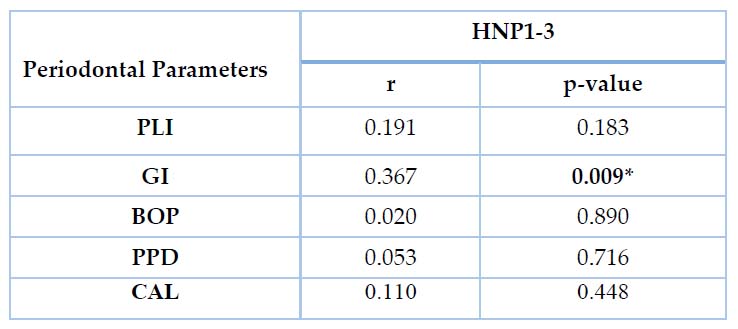
Table 3. Correlation Between HNP1-3 (ng/ml) and Periodontal Parameters in Patients Group
Analytic statistics for serum VD3 in periodontitis and control groups showed a significant decrease in the mean value of VD3 in periodontitis group compared to the control group at p-value ˂0.01 with a mean (40.65±11.46 ng/ml) for periodontitis group while it was (59.92± 21.67 ng/ml) for the control group as shown in table 4. The current study found a significant increase (P˂0.01) in the mean value of VD among VD sufficient group, who account for 28 (56%) of the total number of patients, compared to patients with insufficient VD, who account for 22 (44%) of the total number of patients. As shown in Table 5, the mean value of VD in the sufficient group was 47.81±9.99 ng/ml, whereas, in the insufficient group, it was 26.27± 2.36 ng/ml.
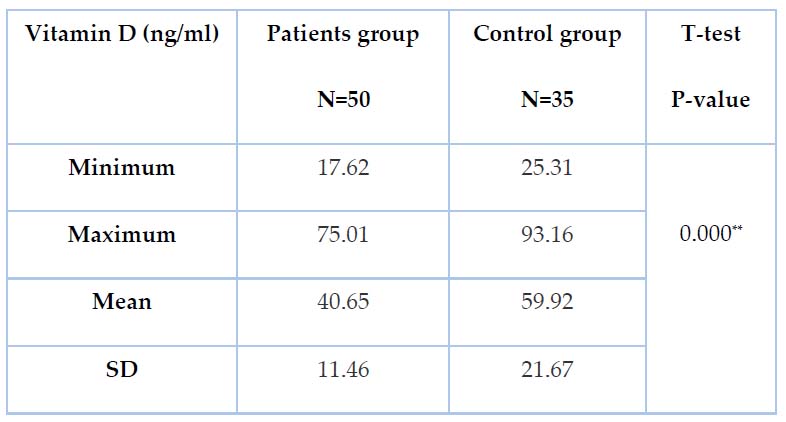
Table 4. Mean of VD3(ng/ml) Values in patients and Control Groups
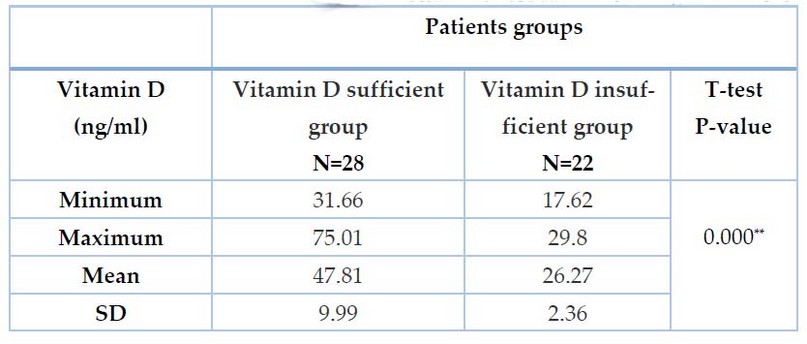
Table 5. Distribution of Patients According to Vitamin D Sufficiency
The current results found that there was a non-significant difference (P>0.05) in serum level of HNP1-3 between the two groups of patients; the mean value of HNP1-3 in the sufficient group was 13.49± 2.58 ng/ml, while in the insufficient group was (14.56±4.89 ng/ml), as shown in table 6.
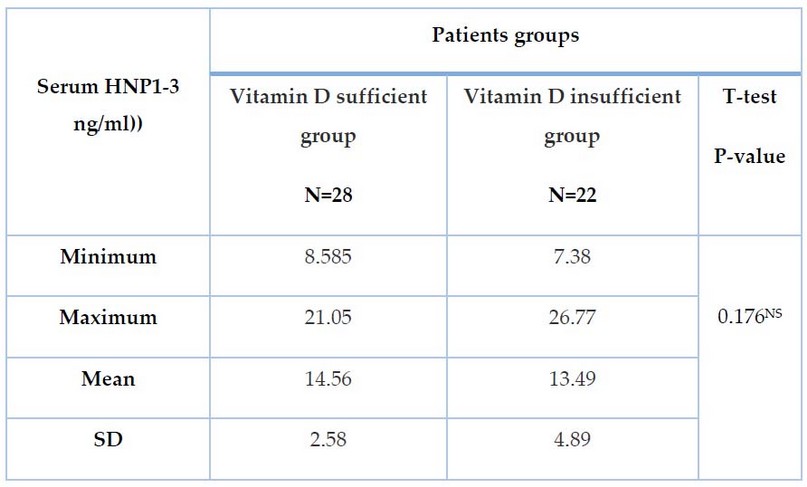
Table 6. Comparison of the levels of Serum HNP1-3 (ng/ml) in patient groups according to Vitamin D Sufficiency
DISCUSSION
In this study, non-significant differences were observed between periodontitis patients and healthy controls according to age and sex, thus depicting age and sex-matched individuals between the two study groups; this result agreed with other local studies reported by 22,23. Moreover, the present study showed significant elevation in the serum levels of HNP1-3 in the periodontitis patients compared to the control group. In agreement with this study, 24 reported that HNP1-3 was elevated in periodontitis patients compared to control 24. Similarly, 25 showed that the concentration of HNP 1 was higher in the saliva of patients with oral inflammation than in healthy subjects 25. In addition, 26 suggested that high levels of HNP1-3 in GCF indicate these AMPs' effects in controlling the gingival crevice microbiota 26. The possible explanation for the high level of HNP1-3 in periodontitis could be attributed to the fact that sources other than neutrophils are responsible for α-defensin production in inflamed periodontal/gingival tissue. Indeed, it has been shown that HNP1-3 is also expressed in lymphocytes and monocytes, which are abundant in periodontitis sites 27,28.
On the other hand, 29 examined the α-defensins (HNP1-3) in periodontitis and periodontally healthy and reported no significant difference between healthy and diseased sites. However, these defensins were more abundant in a higher proportion of the beneficial sites 29. The current results showed no significant correlation between HNP1-3 and clinical periodontal parameters except for GI, which has a considerable influence. This finding is confirmed by a study by Türkolu et al., who discovered no significant correlation between HNP1-3 and clinical periodontal parameters 30. The present study showed a significant decrease in serum levels of VD among periodontitis patients compared with healthy periodontium, similar to other results reported by 31,32. 33 observed an increased prevalence of VD deficiency in patients with periodontitis, suggesting that the decreased VD level could be a risk factor, and screening is recommended where deficiency is suspected 33.
In contrast to this result, 34 found that serum VD levels were higher in periodontitis patients compared to the control group 34. Mahmood et al. (2019) conducted a study in Erbil city and reported no significant relationship between VD deficiency and periodontitis 35. However, a higher serum concentration of 25(OH)D was shown to predict a lesser likelihood of bleeding on gingival probing, which suggests that vitamin D may reduce susceptibility to gingival inflammation through its antiinflammatory effects 31.
Vitamin D inhibits T-lymphocyte proliferation, immunoglobulin secretion, and B-lymphocyte transformation into plasma cells and protects the organism from an excessive specific immune response by reducing the secretion of cytokines such as IL-1, IL-6, IL-8, IL-12, and TNF-α, which are released during periodontitis pathogenesis and cause lymphocyte infiltration, bone resorption, and extracellular matrix deterioration 36, and due to this mechanism there may be relation between periodontitis and VD deficiency. Miley et al. (2009) observed improved periodontal health after taking VD supplements 37. 38, on the other hand, suggested that further controlled clinical trials be conducted to investigate the adjuvant usefulness of calcium and VD supplements in treating periodontal disease 38. This study revealed that the mean value of VD decreased in the insufficient group as compared to the sufficient group. Low serum concentrations of 25(OH)D are substantially related to periodontal disease, according to 39, suggesting that insufficient VD levels may be implicated in periodontal disease progression 39. 1,25(OH)2D has been demonstrated to potently down-regulate monocyte TLR2 and TLR4 expression, inhibiting inflammatory responses usually activated by these receptors, suggesting that VD may provide feedback modulation of immune activation pathways. VD may help stimulate proper innate immune responses while reducing over-shooting and tissue damage, which are usually linked with this 40. This study found a non-significant decrease in HNP1-3 in periodontitis patients with insufficient VD compared to the sufficient group. This finding supports the idea that VD plays a role in controlling these peptides. 41 found that VD induces gene expression of cathelicidin and defensin, which play a role in primary defense against infections 41. These proteins are also expressed by epithelial cells in the gingiva 42,43 and play a role in the first defense against bacteria by reducing the proportion of bacteria in the mouth. The presence of VD response element on promoter regions of cathelicidin and defensin supports the contribution of VD in regulating these peptides 41. The lower level of AMPs in insufficient groups may cause the failure to respond appropriately to invading pathogens in periodontal diseases 44.
CONCLUSIONS
The increased level of HNP1-3 and its correlation with GI provide evidence for an important role of neutrophils in the inflammatory process in periodontitis. There are also low levels of HNP1-3 in periodontitis patients with insufficient VD, implying that VD plays a role in AMP production.
ETHICAL APPROVAL
All of the individuals were given thorough information about the study and the procedures involved, and their informed consent was acquired on a form approved by the ethics committee of the University of Baghdad College of Dentistry.
Competing interests
The authors declared that there is no competitor.
Acknowledgment
We want to thank all donors recruited in our present study.
REFERENCES
1 Gasner NS, Schure RS. Periodontal Disease. In: StatPearls. StatPearls Publishing, Treasure Island (FL); 2021. PMID: 32119477.
2 Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. 2017 Jun 22;3:17038.
3 G. Pen, N. Yang, D. Teng, R. Mao, Y. Hao, J. Wang, A review on the use of antimicrobial peptides to combat porcine viruses, Antibiotics (Basel) 9 (11) ,2020; 801.
4 G.E.B. Montalvo, L.P.S. Vandenberghe, V.T. Soccol, J.C. Carvalho, C.R. Soccol, The antihypertensive, antimicrobial and anticancer peptides from arthrosis with therapeutic potential: a mini-review, Curr. Mol. Med. 2020; 20 (8): 593–606.
5 T. Gong, J. Fu, L. Shi, X. Chen, X. Zong, Antimicrobial peptides in gut health: a review, Front. Nutr. 8,2021.
6 J.K. Lee, T. Luchian, Y. Park, New antimicrobial peptide kills drug-resistant pathogens without detectable resistance, Oncotarget, 2018; 9 (21) .15616–15634.
7 Q.Y. Zhang, Z.B. Yan, Y.M. Meng, X.Y. Hong, G. Shao, J.J. Ma, X.R. Cheng, J. Liu, J. Kang, C.Y. Fu, Antimicrobial peptides: mechanism of action, activity and clinical potential, Mil. Med. Res. 2021; 8 (1). 48, https://doi.org/10.1186/ s40779-021-00343-2.
8 T.V. Vineeth Kumar, G. Sanil, A review of the mechanism of action of amphibian antimicrobial peptides focusing on peptide-membrane interaction and membrane curvature, Curr. Protein Pept Sc, 2017; 18 (12).1263–1272, https://doi.org/ 10.2174/1389203718666170710114932.
9 Wei, D. X., & Zhang, X. W. Biosynthesis, Bioactivity, Biosafety and Applications of Antimicrobial Peptides for Human Health. Biosafety and Health. 2022.
10 Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–20.
11 Tao R, Jurevic RJ, Coulton KK, Tsutsui MT, Roberts MC, Kimball JR. Salivary antimicrobial peptide expression and dental caries experience in children. Antimicrob Agents Chemother. 2005;49:3883–8.
12 McKay MS, Olson E, Hesla MA, Panyutich A, Ganz T, Perkins S, et al. Immunomagnetic recovery of human neutrophil defensins from the human gingival crevice. Oral Microbiol Immunol. 1999;14:190–3.
13 Turck, D.; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Update of the tolerable upper intake level for vitamin D for infants. EFSA J. 2018, 16, 1–118.
14 Jones, G. The discovery and synthesis of the nutritional factor vitamin D. Int. J. Paleopathol. 2018, 23, 96–99. [CrossRef]
15 Lanham-New, S.A.; Wilson, L.R. Vitamin D—Has the new dawn for dietary recommendations arrived? Nutr. Bull. 2016, 41, 2–5. [CrossRef]
16 Botelho, J., Machado, V., Proença, L., Delgado, A. S., & Mendes, J. J. Vitamin D deficiency and oral health: a comprehensive review. Nutrients, 2020; 12(5), 1471
17 M.G. Cagetti, T.G. Wolf, C. Tennert, N. Camoni, P. Lindstrom, ¨ G. Campus, The role of vitamins in oral health. A systematic review and meta-analysis, Int. J. Environ. Res. Public Health 17. 2020.
18 J. Botelho, V. Machado, L. Proença, A.S. Delgado, J.J. Mendes, Vitamin D deficiency and oral health: a comprehensive review, Nutrients 12. 2020.
19 E. Jagelaviˇciene, ˙ I. Vaitkeviˇciene, ˙ D. Silingait ˇ e, ˙ E. Sink ˇ unait ¯ e, ˙ G. Daugelait ˙ e, ˙ The relationship between vitamin D and periodontal pathology, Medicina (B. Aires). 2018; 54. 45
20 R.J. Schroth, R. Rabbani, G. Loewen, M.E. Moffatt, Vitamin D and dental caries in children, J. Dent. Res. 2016; 95, 173–179, https://doi.org/10.1177/ 0022034515616335.
21 Mumena, C. H., Mudhihiri, M. H., Sasi, R., Mlawa, M., Nyerembe, S., Akimbekov, N. S., & Razzaque, M. S. The relevance of vitamin D in the oral health of HIV infected patients. The Journal of steroid biochemistry and molecular biology, 2021; 211, 105905
22 Fadil Z, Al-Ghurabi B. Study the role of pro-and antiinflammatory cytokines in Iraqi chronic periodontitis patients. J Bagh Coll Dent. 2012;24:164-9
23 Mahmood HK, AL-Ghurabi BH. Association between anti-CMV IgG and salivary levels of IL-6 and TNF-α in chronic periodontitis. J Bagh CollDent. 2020;32(2):5-11.
24 Puklo, M., Guentsch, A., Hiemstra, P. S., Eick, S., & Potempa, J. Analysis of neutrophil‐derived antimicrobial peptides in gingival crevicular fluid suggests importance of cathelicidin LL‐37 in the innate immune response against periodontogenic bacteria. Oral microbiology and immunology, 2008;23(4), 328-335.
25 Mizukawa N, Sugiyama K, Ueno T, Mishima K, Takagi S, Sugahara T. Levels of human defensin-1, an antimicrobial peptide, in the saliva of patients with oral inflammation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999;87:539-543.
26 McKay MS, Olson E, Hesla MA, et al. Immunomagnetic recovery of human neutrophil defensins from the human gingival crevice. Oral Microbiol Immunol 1999;14:190-193.
27 Agerberth B, Charo J, Werr J et al. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood 2000: 96: 3086–3093.
28 Gamonal J, Acevedo A, Bascones A, Jorge O, Silva A. Characterization of cellular infiltrate, detection of chemokine receptor CCR5 and interleukin-8 and RANTES chemokines in adult periodontitis. J Periodontal Res 2001: 36: 194–203.
29 Lundy FT, Orr DF, Shaw C, Lamey PJ, Linden GJ. Detection of individual human neutrophil alpha-defensins (human neutrophil peptides 1, 2 and 3) in unfractionated gingival crevicular fluid – A MALDIMS approach. Mol Immunol 2005;42:575-579.
30 Türkoğlu, O., Emingil, G., Kütükçüler, N., & Atilla, G. Evaluation of gingival crevicular fluid adrenomedullin and human neutrophil peptide 1–3 levels of patients with different periodontal diseases. Journal of Periodontology, 2010; 81(2), 284-291.
31 Dietrich T, Nunn M, Dawson-Hughes B, and Bischoff Ferrari H. Association between serum concentrations of 25-hydroxyvitamin D and gingival inflammation. Am J Clin Nutr. 2005;82:575–80. [Article]
32 Alzahrani, A. A. H., Alharbi, R. A., Alzahrani, M. S. A., Sindi, M. A., Shamlan, G., Alzahrani, F. A., ... & Sindi, A. A. A. Association between periodontitis and vitamin D status: A case-control study. Saudi Journal of Biological Sciences, 2021;28(7), 4016-4021.
33 Anbarcioglu, E., Kirtiloglu, T., Öztürk, A., Kolbakir, F., Acıkgöz, G., Colak, R. Vitamin D deficiency in patients with aggressive periodontitis. Oral Dis. 2019; 25, 242–249.
34 Pradhan, S., & Agrawal, S. Serum Vitamin D in Patients with Chronic Periodontitis and Healthy Periodontium. Journal of Nepal Health Research Council, 2021;18(4), 610-614.
35 Mahmood G, Hussein M and Aziz AH. The relationship between serum vitamin D level and chronic periodontitis in patients attending Khanzad center in Erbil city. J Kurdistan Board Spec. 2019;5(2):102-8. [Article]
36 Bikle DD. Vitamin D and the immune system: role in protection against bacterial infection. Curr Opin Nephrol Hypertens 2008;17:348-52. [Article] [PubMed]
37 Miley, D.D., Garcia, M.N., Hildebolt, C.F., Shannon, W.D., Couture, R.A., Anderson Spearie, C.L., Dixon, D.A., Langenwalter, E.M., Mueller, C., Civitelli, R. Cross‐sectional study of vitamin D and calcium supplementation effects on chronic periodontitis. J. Periodontol. 2009; 80, 1433–1439.
38 Hildebolt CF. Effect of vitamin D and calcium on periodontitis. J Periodontol 2005;76:1576-1587.
39 Laky, M., Bertl, K., Haririan, H., Andrukhov, O., Seemann, R., Volf, I., ... & Rausch-Fan, X. Serum levels of 25-hydroxyvitamin D are associated with periodontal disease. Clinical oral investigations, 2017; 21(5), 1553-1558.
40 Hewison M. Vitamin D and the immune system: new perspectives on an old theme. Endocrinol Metab Clin N Am 39(2): 365–379 table of contents. 2010.
41 Wang, T.-T., Nestel, F. P., Bourdeau, V., Nagai, Y., Wang, Q., Liao, J., ... Mader, S. Cutting edge: 1, 25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. The Journal of Immunology, 2004; 173(5), 2909–2912.
42 Dale BA, Fredericks LP. Antimicrobial peptides in the oral environment: Expression and function in health and disease. Curr Issues Mol Biol 2005;7:119-133.
43 Greer, A., Zenobia, C., & Darveau, R. P. Defensins and LL‐37: A review of function in the gingival epithelium. Periodontology 2000, 63(1), 67–79. 2013.
44 Bayirli BA, Öztürk A, Avci B. Serum vitamin D concentration is associated with antimicrobial peptide level in periodontal diseases. Arch Oral Biol. 2020 Sep;117:104827. doi 10.1016/j.archoralbio.2020.104827. Epub 2020 Jul 9. PMID: 32673820.
Received: May 15, 2023/ Accepted: June 10, 2023 / Published: June 15, 2023
Citation: Mahawi, F.Z.; Al-Ghurabi, B.H. Human Neutrophil Peptide 1- 3 and Vitamin D Levels in Periodontitis. Revis Bionatura 2023;8 (2) 79. http://dx.doi.org/10.21931/RB/2023.08.02.79