2023.08.03.76
Files > Volume 8 > Vol 8 No 3 2023
Molecular detection and pathological modifications of Klebsiella pneumoniae in trachea and lung of rabbits after infected by intranasal instillation route
1Department of Veterinary Pathology and Poultry Diseases, College of Veterinary Medicine, University of Baghdad, Baghdad, Iraq
*Corresponding author. [email protected]
Available from: http://dx.doi.org/10.21931/RB/2023.08.03.76
ABSTRACT
Klebsiella pneumoniae, one of the more critical zoonotic pathogenic bacteria, causes many diseases, including pneumonia, urinary tract infections, bloodstream infections, and sepsis. This study aimed to insulate K. pneumoniae from sheep urine and identify it by microbiological method on MacConkey agar and confirmed with 16S-rRNA sequencing analysis, then study the pathological changes of bacteria in the trachea and lung of rabbits after infected by intranasal instillation route. A total of 24 adult healthy local male rabbits were divided randomly into 2 groups: in the control group, eight animals received 50 µL phosphate buffer saline via intranasal instillation. In contrast, sixteen animals received intranasally a single dose of K. pneumonia at 106 CFU/50 µL PBS/animal in the infected group. Two animals from the infected group and one from the control group were sacrificed at 3 hours, 6 hours, 12 hours, 24 hours, 3 days, 7 days, and 16 days post-infection. A postmortem examination was performed, and any gross lesions were reported. Trachea and lung specimens were then collected and fixed in 10% formalin. The results of the bacterial examination growing on the MacConkey media agreed with the results of molecular confirmed by 16S rRNA sequencing analysis. Macroscopic results highlighted severe lung congestion and white spot areas in the lung tissue. Microscopic changes in the trachea are characterized by acute tracheitis with infiltration of inflammatory cells within a fibro-cartilaginous layer, with sticky exudate consisting of fluid collection and inflammatory cells in the tracheal lumen. as well as bleeding and aggregation edematous fluid recorded in alveolar lumen, with alveolar epithelial sloughing, interstitial pneumonia, bronchiectasis, and bronchiolitis with emphysema. The conclusion of this study could be summarized by addressing the severity of infection at several different times from the Intranasal instillation route with the rapid and dangerous ascending progression of pathological lesions in tracheal and lung disease post-infected rabbits via Intranasal instillation.
Keywords: Klebsiella pneumoniae,16S-rRNA , trachea, lung, rabbit
INTRODUCTION
Klebsiella (K.) pneumoniae, a bacterial infection, causes lesions in the respiratory system, constituting an essential source of clinical signs and symptoms, primarily upper respiratory tract1,2. Furthermore, it is represented as a second cause of stream infections behind E. coli in animals and humans, like infection in the respiratory system, ankylosing spondylitis, and wound infections that lead to septicemia or bacteremia3-6. According to immunopathological research7, locally isolated K. pneumoniae have been found to form a disproportionately high proportion of ESKAPE bacteria, which includes Enterococcus faecium, Staphylococcus aureus, K. pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species. These bacteria also possess the K1 and K2 genes, demonstrating multi-antibiotic resistance.
Recent publications referred to the capability of K. pneumoniae to develop multidrug resistance to many antibiotics, which worsens the economic losses in animals due to the added value of the cost of treatment8,9. When K. pneumoniae enters the respiratory tract, it can lead to bacterial pneumonia or an infection of the lungs. Hence, symptoms include chest pain when you breathe or cough, fever and chills, shortness of breath, fatigue, a cough that may produce phlegm and changes in mental awareness10. Pathological effects of K. pneumoniae in the lung and kidney of Balb/c mice post infection intraperitoneally cause acute infection after a few hours, and it develops into a chronic infection after seven days of reinfection11. The Southern sea otters (a federally-listed threatened subspecies found almost exclusively in California, USA), which are infected by K. pneumonia, recorded bronchopneumonia, tracheobronchitis and pleuritis, also, enteritis, acanthocephalan peritonitis, septic peritonitis and septicemia 12,13. Therefore, and due to few studies about the pathogenesis of K. pneumoniae on the respiratory system, this study is designated to spot the light on the significant pathogenic effects of K. pneumoniae against the principal organs of the respiratory system (trachea and lungs) after infection via intranasal instillation route, through investigation of experimental infection which is evaluated by bacterial spread in the mentioned organs assessed by molecular detection and histopathological examination.
MATERIALS AND METHODS
Experimental animals and bacterial isolation
A total of 24 adult local male rabbits were divided into two groups. Intranasal instillation gave the control groups (eight animals) 50 μL/animal phosphate buffer saline (PBS). In contrast, the second group (infected groups) included 16 animals, given by intranasal instillation route, as a dose (50 μL/animal) containing 106 CFU/K. pneumonia suspended in PBS. Bacteria were isolated from urine that accumulated in the urinary bladder of slaughtered sheep after culture on MacConkey agar. After harvesting growing bacteria, infected doses were determined using the Miles and Misra method15 to calculate the number of colony-forming units (CFU) per mL from the original sample dependent on the following equation. The infective dose which was used in our study (50 μL/contain 106 CFU) given by intranasal instillation (Average no. of colonies for dilution = CFU/µL × 50 × diluted factor). Two animals from the infected group and one from the control group were sacrificed at 3 hours, 6 hours, 12 hours, 24 hours, 3 days, 7 days, 12 days, and 16 days directly post-infection. A postmortem examination was performed, and any gross lesions were reported. Tissue samples from the trachea and lungs were then fixed in 10% formalin.
Histopathological examination
The tissue specimens collected from the chest region, trachea, and lung were fixed in 10% formalin for 72 hours and processed for slide preparation and staining with Hematoxylin and Eosin (H&E) stain16. Histopathological changes were observed under a light microscope.
Sequence of 16S rRNA

Table 1. According to Srinivasan et al. (2015)20. the sequence of primers used in this study
RESULTS
Bacterial identification
The bacterial isolates were isolated from urine that aggregated in the urinary bladder from 25 samples), in different slaughter sheep, appear in (Fig. 1) on MacConkey agar, and are characterized by large, convex, heavy mucoid colonies pinkish to reddish.
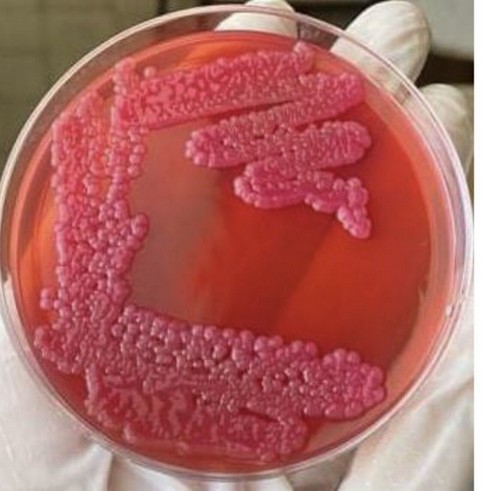
Figure 1. Klebsiella pneumoniae on Macconkey agar appears as a pinkish color, convex, heavy mucoid colonies colony (after 24 hrs., at 37 °C).
Molecular detection (16S rRNA) primer by PCR technique
Figure (2) shows the PCR product of the 16S-rRNA gene (1250 bp) from bacterial DNA, stained with Red safe stain; the product was electrophoresis on 1.5% agarose at (5 volt/cm2. 1x TBE buffer) for (1:30 hours). M: DNA ladder (100). M: marker (10000 base pair). Lane (1-7) positive16S-rRNA.
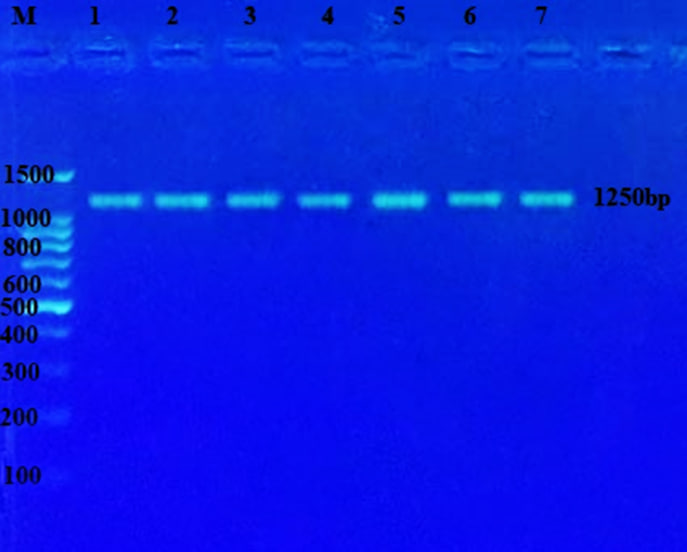
Figure 2. The result of the PCR technique revealed that all K. pneumoniae isolates owned the 16S rRNA genes.
Histopathological examination
No apparent pathological alteration in the trachea and lungs appeared in animals within the control group. Animals in the infected group, after observation of the trachea and lungs recorded a white spot area appeared in the lung and heart, with congestion in lung tissue, through gross appearance in the chest region of rabbit after 3 hours from infected by (50 μL/106 K. pneumonia) intranasal instillation. While under light microscope, histopathological section of rabbit trachea at 3 hours post infection by (50 μL/106 K. pneumonia) by intranasal instillation show, neutrophils also infiltration of inflammatory cells neutrophils and macrophages, within fibro-cartilaginous layer recorded at 6 hours (Fig.3), while at 24 hours post infection, trachea showed severe infiltration of inflammatory neutrophils and MNCs (Fig.4), and sticky exudate consist from fluid collection, and inflammatory cells in the tracheal lumen appeared after day 3 post bacterial infection (Fig.5), as well as, necrotic epithelial cells in the lumen of trachea with multifocal MNCs infiltration show after seven days of infection (Fig.6), but at the end of experiment congestion and large abscess formed from aggregation of necrotic neutrophils surrounded by fibrous connective capsule and ulceration of pseudo-stratified columnar epithelium after day 16 of infection (Fig.7). More distant, histopathological section of lung at 3 hours post infection by (50 μL/106 K. pneumonia) by Intranasal instillation show, hemorrhage and pink homogenous fluid in alveolar lumen, and inflammatory cell infiltrate within interstitial tissue (Fig.8). On the other hand, edematous fluid with dilation and alveolar epithelial sloughing, also infiltration of inflammatory cells neutrophils and MNCs was seen at 6 hours post infection (Fig.9), at 24 hour) also, at day 7, post-infection lung suffering from emphysema with fibrotic knobs with thickening of alveolar space and infiltration of inflammatory cells between alveolar septa (Fig.10)
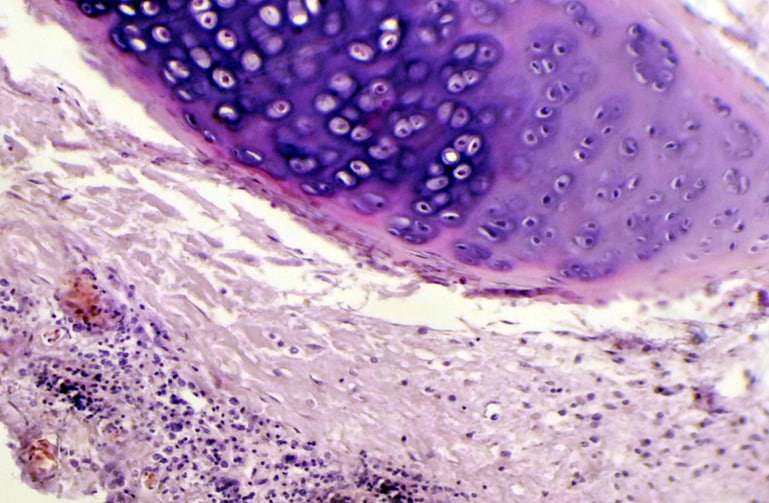
Figure 3. Histopathological section in rabbit trachea at (3 hours) post-infection by (50 μl/ 106 K.pneumonia) by intranasal instillation show acute tracheitis congestion B.V with infiltration of inflammatory cells mainly neutrophils (H&E stain 10X).
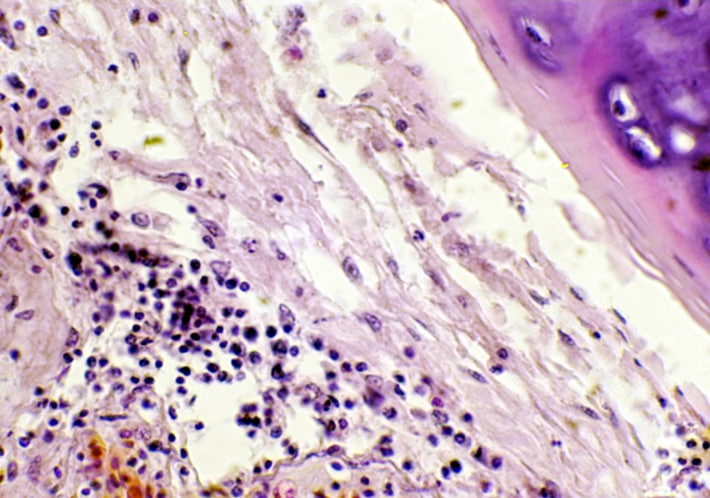
Figure 4. The histopathological section in rabbit trachea at (6 hours) post-infection by (50 μl/ 106 K. pneumonia) by Intranasal instillation shows infiltration of inflammatory cells neutrophils and macrophages within the fibro-cartilaginous layer (H&E stain 10X).
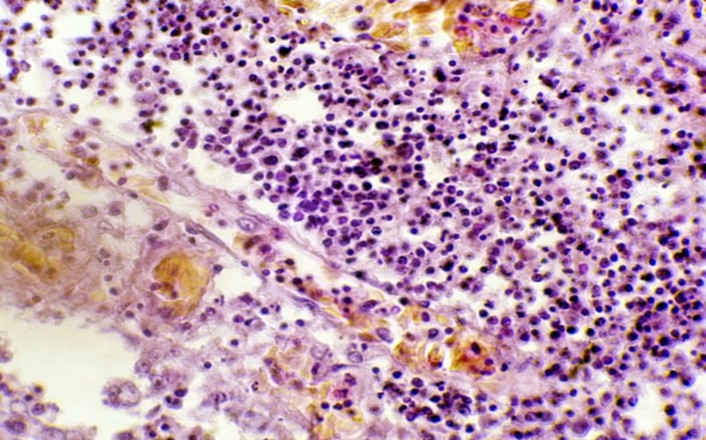
Figure 5. The histopathological section in rabbit trachea after (day 3) post-infection by (50 μl/ 106 K. pneumonia) by Intranasal instillation show sticky exudate consisting of fluid collection and inflammatory cells in the tracheal lumen (H&E stain 40X).
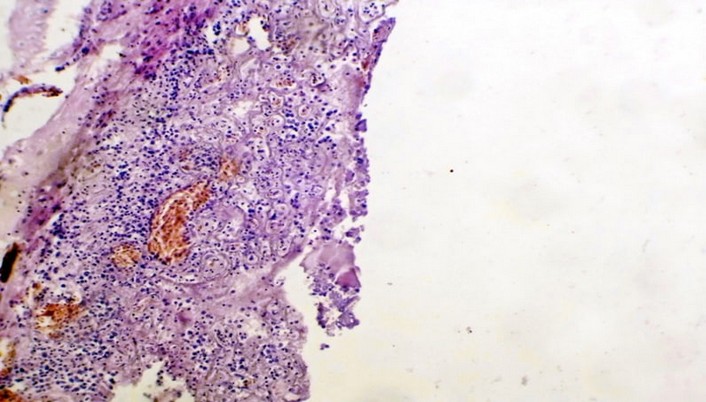
Figure 6. The histopathological section in rabbit trachea at (day 16) post-infection by (50 μl/ 106 K. pneumonia) Intranasal instillation shows congestion, a large abscess formed from the aggregation of necrotic neutrophils surrounded by fibrous connective capsule a, and ulceration of pseudo-stratified columnar epithelium (H&E stain 10X)
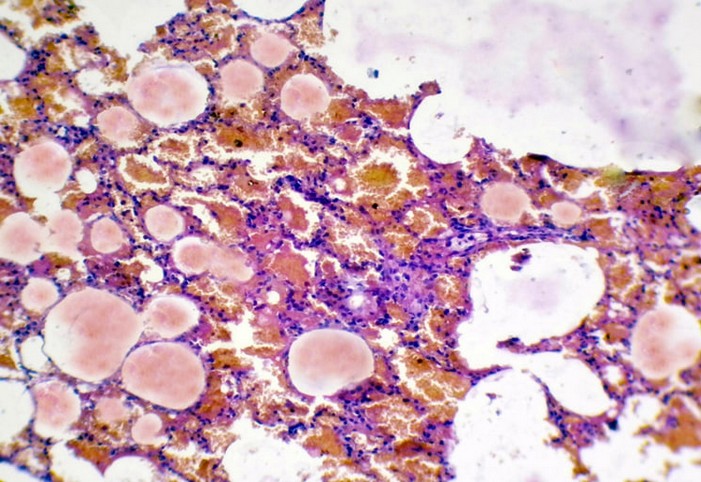
Figure 7. The histopathological section in rabbit lung at (3 hours) post-infection by (50 μl/ 106 K. pneumonia) by (Intr.Ins.) show hemorrhage and pink homogenous fluid in the alveolar lumen,& inflammatory cell infiltrate within the interstitial tissue
(H&E stain10X).
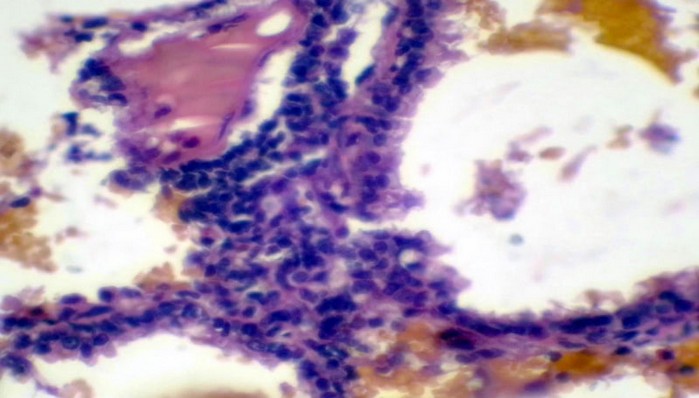
Figure 8. The histopathological section in rabbit lung at (6 hours) post-infection by (50 μl/ 106 K. pneumonia) by (Intr.Ins.) show edematous fluid with dilation and alveolar epithelial sloughing, as infiltration of inflammatory cells neutrophils, and
MNCs (H&E stain 40X).
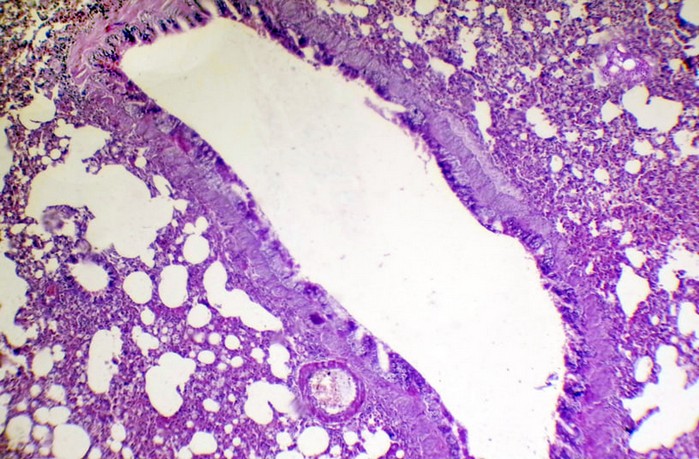
Figure 9. Histopathological section in rabbit lung at (24 hours) post-infection (50 μl/ 106 K.pneumonia) by(Intr.Ins.) show, active hyperemia with interstitial pneumonia also bronchiectasis (H&E stain 10X).
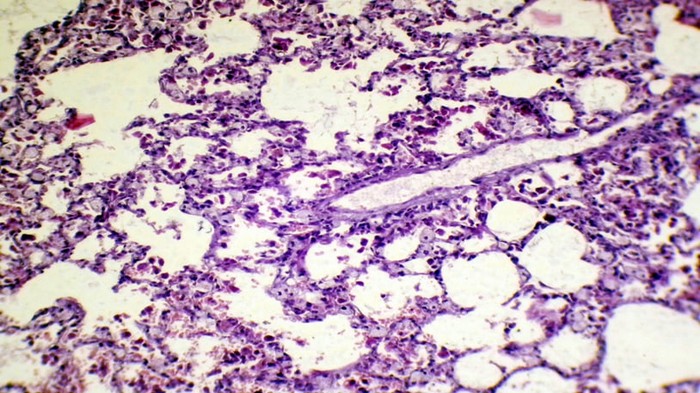
Figure 10. The histopathological section in rabbit lung at (day 7) post-infection by (50 μl/ 106 K. pneumonia) by (Intr.Ins.) shows emphysema with fibrotic knobs with thickening of alveolar space and infiltration of inflammatory cells between alveolar septa (H&E stain 10X).
DISCUSSION
The genus Klebsiella, in general, was extensively studied in both animals and humans. However, there was little research done about pathogenesis and complications of the respiratory system, especially during infection (trachea and lung ) by K. pneumoniae through the intranasal instillation route, and the role of PCR amplification is done among heterogeneous adjacent elements to get DNA fingerprints that can be easily for analyzed by using a software program to recognize patterns13. As a lactose-fermenting species, K. pneumoniae produces pinkish-colored and mucoid colony growth on MacConkey agar. In a study using 50 human and 50 sheep urine samples, Al-Rubaei17 also found that K. pneumoniae could be isolated and diagnosed by culturing the samples on MacConkey, blood, EMB, XLD, and BHI agars.
Histopathological examination of lesions in the trachea and lungs of rabbits infected with K. pneumonia via intranasal instillation revealed cellular degeneration and an inflammatory response of the respiratory system between three and six hours after infection. Mitochondrial damage caused by K. pneumoniae infection plays a crucial role in various forms of cellular damage, especially in apoptosis epithelial cells, as evidenced by changes in mitochondrial function or damage markers (Mitochondrial membrane potential, oxidative stress, and Ca2+ concentrations), which in turn induced cell damage17,18. These concepts are in agreement with the observation of Abdullah et al.2, who reported intensive lung effects after 48 and 72 hours of infection that exhibited congestion of blood vessels and infiltration of polymorphonuclear cells, occurring after infected mice with 0.2 mL of 7.5×104 CFU K. pneumoniae isolated from urine of human and cattle in Baghdad city.
The severe interstitial pneumonia, accompanied by MNC infiltration at 6 hours post-infection, as well as severe inflammatory reactions represented by infiltration of MNCs into tissue parenchyma at 24 hours post-infection, which developed into necrosis to broad areas of trachea and lung, in addition to scattered hemorrhages and congestion with acute to chronic inflammation. It has been reported that the respiratory response against Klebsiella infection may occur by lung tissue stimulation to release caspase-11, which is required for limiting the outgrowth of bacteria in lung parenchyma11. Additionally, the present study is supported by, who reported severe acute inflammatory changes a few hours post-infected mice by 1×109 K. pneumonia, which is characterized by dilated congestion of blood vessels associated with the presence of edematous fluid in interstitial tissues, accompanied by fibrin and infiltration of PMNs mainly neutrophils.
The aggregates of swirling MNCs, with streaming fibroblasts and fibrin, fill and conform to the airway. This may occur due to response to chronic inflammation against bacterial infection, causing an increase in macrophage activation as well as cell recruitment to the site of infection that leads to an increase in pro-inflammatory cytokine , mainly TNF, that represents the primary signal important in granuloma formation, as an immune response against K. pneumonia13. The current finding may support the idea of7, which revealed that pro-inflammatory cytokines release in K. pneumonia infection could lower the host's innate immune response.
CONCLUSION
In conclusion, rabbits were used to investigate the pathogenesis of intranasal instillation dosing with K. pneumonia, which was found to spread from the trachea to the lungs at 3, 6, 12 and 24 hours, 3, 7, and 16 days after infection with 50 μL/106 K. pneumonia, as determined by bacterial isolation, molecular identification, and histopathological pictures.
Acknowledgments
The authors would like to acknowledge the College of Veterinary Medicine, University of Baghdad, for supporting this work.
Funding
There is no external financial source.
Author contributions
Both authors contributed equally to the experiment's design and performance, the results analysis, and the current submission's drafting, editing, reviewing, and revising.
Conflict of Interest
The authors declare that there is no conflict of interest.
Ethical Approval
The experimental design and all procedures performed in this study were reviewed and approved following animal welfare ethical standards by the Department of Veterinary Pathology and Poultry Diseases, College of Veterinary Medicine, University of Baghdad, in its session held on October 25, 2021, and the Scientific Committee at the University of Baghdad's College of Veterinary Medicine.
REFERENCES
1. Sushmitha, S.; Patil, G. S.; Patil, S. B. A histological analysis of urinary bladder specimens with elaboration of various neoplastic lesions. Ind J Pathol Oncol. 2019, 6(4), 688–694.
2. Abadullah, S. M.; Zghair, Z. R. Isolation of Klebsiella pneumoniae from the urine of human and cattle in Baghdad city with histopathological study experimentally in mice. Int. J. Adv. Res. Biol. Sci. 2016, 3(10), 38–45.
3. Podschun, R.; Ullmann, U. Klebsiella spp. as nosocomial pathogens: Epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 1998, 11, 589–603.
4. Winnett, V.; Sirdaarta, J.; White, A.; Clarke, F. M.; Cock, I. E. Inhibition of Klebsiella pneumoniae growth by selected Australian plants: Natural approaches for the prevention and management of ankylosing spondylitis. Inflammopharmacol. 2017, 25(2), 223–235.
5. Sabouni, F.; Movahedi, Z.; Mahmoudi, S.; Pourakbari, B.; Valian, S. K.; Mamishi, S. High frequency of vancomycin-resistant Enterococcus faecalis in children: An alarming concern. J Prev Med Hyg. 2016, 57(4), E201–E204.
6. Keynan, Y.; Rubinstein, E. The changing face of Klebsiella pneumoniae infections in the community. Int J Antimicrob Agents. 2007, 30(5), 385–389.
7. Mohammed, A. M. Immunopathological study of Klebsiella pneumoniae as major ESKAPE pathogens in AL Qadisiyah province [Ph.D. dissertation]. Baghdad, Iraq: University of Baghdad; 2019.
8. Liu, A.; Dai, J.; Shen, R.; Zhong, F.; Sheng, X.; Huang, H. Correlation between drug resistance of Klebsiella pneumonia and antimicrobial drug usage. Evidence-Based Complementary and Alternative Medicine. 2022, 2022, 2691134, 6.
9. Fatima, S.; Liaqat, F.; Akbar, A.; Sahfee, M.; Samad, A.; Anwar, M.; Iqbal, S.; Khan, S. A.; Sadia, H.; Makai, G.; Bahadur, A. Virulent and multidrug-resistant Klebsiella pneumoniae from clinical samples in Balochistan. Int Wound J. 2021, 18(4), 510–518.
10. Welch, A. What is a Klebsiella pneumoniae infection? Symptoms, causes , diagnosis, treatment and prevention. Medically Reviewed by Sanjai Sinha, MD. Reviewed: October 31, 2022.
11. Perlee, D.; de Beer, R.; Florquin, S.; van der Poll, T.; van 't Veer, C.; de Vos, A. F. Caspase-11 contributes to pulmonary host defense against Klebsiella pneumoniae and local activation of coagulation. Am J Physiol Lung Cell Mol Physiol. 2020, 319(1), L105-L114.
12. Ibrahim, Z. I.; Jawad, Z. J. (2018). Histopathologic study in lung and kidney of mice post Infection with Klebsiella pneumoniae. The Ninth International Scientific Academic Conference. Istanbul. Turkey.
13. Chang, E. K.; Miller, M.; Shahin, K.; Batac, F.; Field, C. L.; Duignan, P.; Struve, C.; Byrne, B. A.; Murray, M. J.; Greenwald, K.; Smith, W. A.; Ziccardi, M.; Soto, E. Genetics and pathology associated with Klebsiella pneumoniae and Klebsiella spp. Isolates from North American Pacific coastal marine mammals. Vet Microbiol. 2022, 265, 109307.
14. Alsanie, W. F. Molecular diversity and profile analysis of virulence-associated genes in some Klebsiella pneumoniae isolates. Pract Lab Med. 2020,19, e00152.
15. Miles, A. A.; Misra, S. S.; Irwin, J. O. The estimation of the bactericidal power of the blood. J. Hyg. (Lond). 1938, 38, 732–749.
16. Luna, L. G. Manual of histologic staining methods of the Armed Forces Institute of Pathology. 3rd ed. McGraw-Hill, New York, USA. 1968.
17. Al-Rubaei, A. M. Extraction of some antigens of Klebsiella pneumoniae isolated from the urinary system of humans and sheep and study its pathogenicity and immunogenicity in rabbits [Ph.D. dissertation]. Baghdad, Iraq: University of Baghdad; 2011.
18. Cheng, J.; Zhang, J.; Yang, J.; Yi, B.; Liu, G.; Zhou, M.; Kastelic, J. P.; Han, B.; Gao, J. Klebsiella pneumoniae infection causes mitochondrial damage and dysfunction in bovine mammary epithelial cells. Vet Res. 2021, 52(1), 17.
19. Hussain, S. Measurement of nanoparticle-induced mitochondrial membrane potential alterations. Methods Mol Biol. 2019, 1894, 123–131.
20. Srinivasan, R.; Karaoz, U.; Volegova, M.; MacKichan, J.; Kato-Maeda, M.; Miller, S.; Nadarajan, R.; Brodie, E. L.; Lynch, S. V. Use of 16S rRNA gene for identification of a broad range of clinically relevant bacterial pathogens. PloS one. 2015, 10(2), e0117617.
Received: 25 June 2023/ Accepted: 26 August 2023 / Published:15 September 2023
Citation: Tawfeeq Jassim S, Mohammed Jwad B.Molecular detection and pathological modifications of Klebsiella pneumoniae in trachea and lung of rabbits after infected by intranasal instillation route, Revis Bionatura 2023;8 (3) 76. http://dx.doi.org/10.21931/RB/2023.08.03.76
