2023.08.04.95
Files > Volume 8 > Vol 8 no 4 2023
Chitin binding protein GbpA of Vibrio cholerae induces pro-inflammatory responses in the intestine
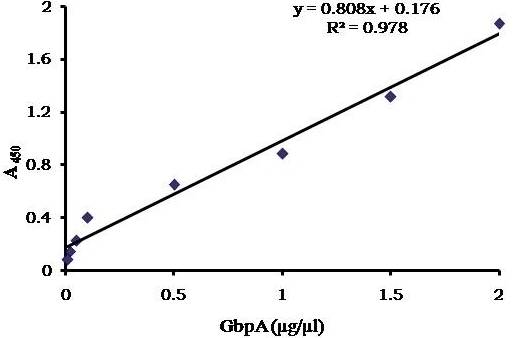
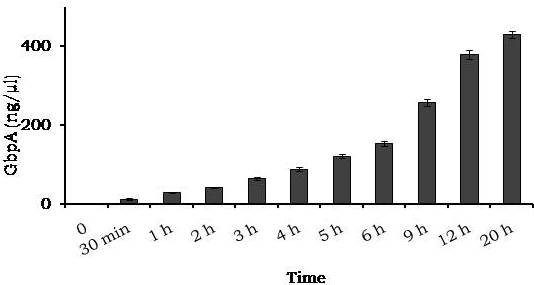
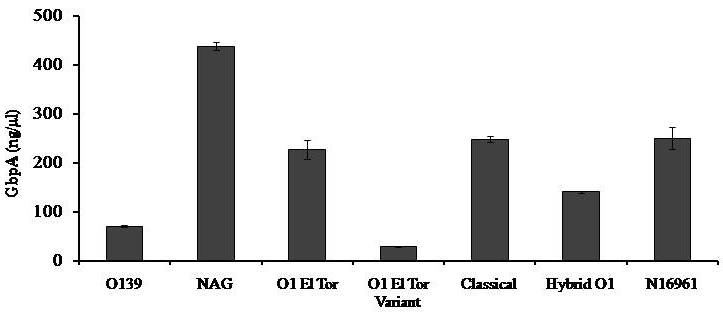
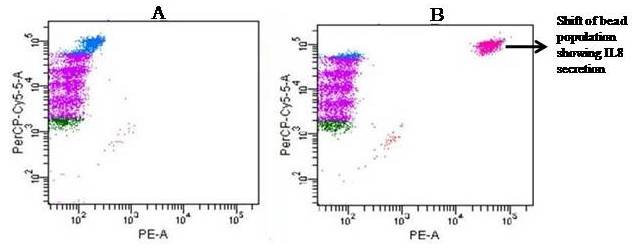
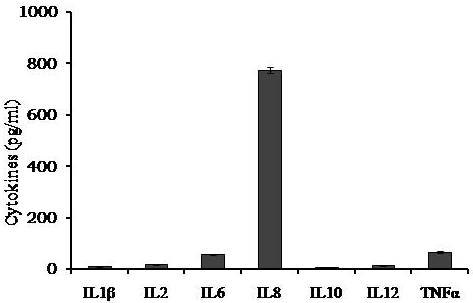
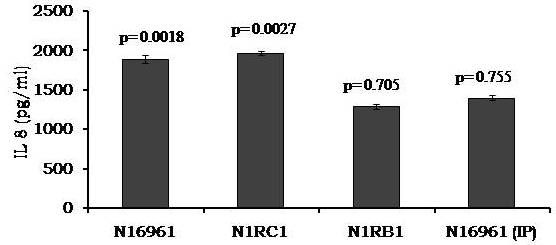
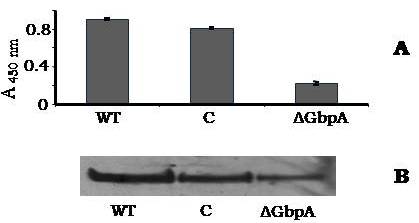
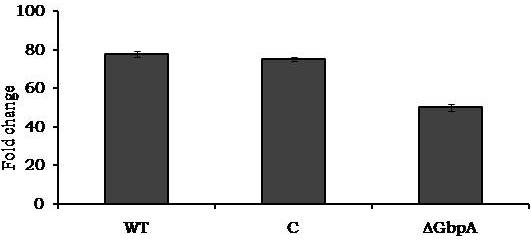
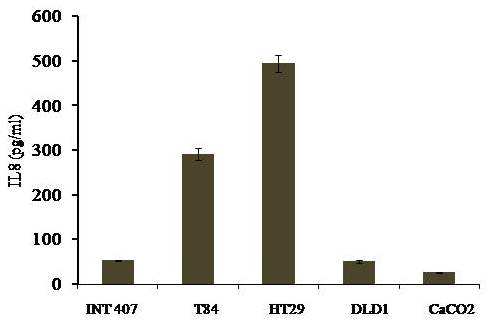
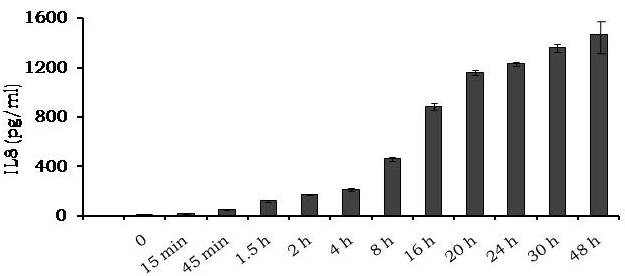
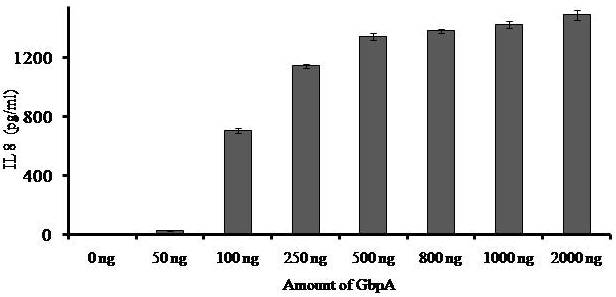
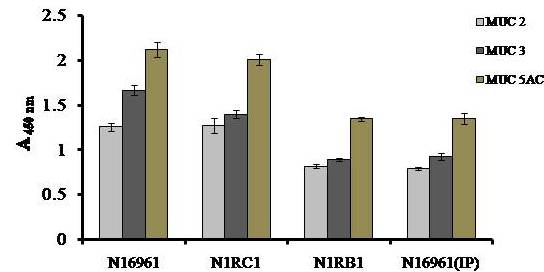
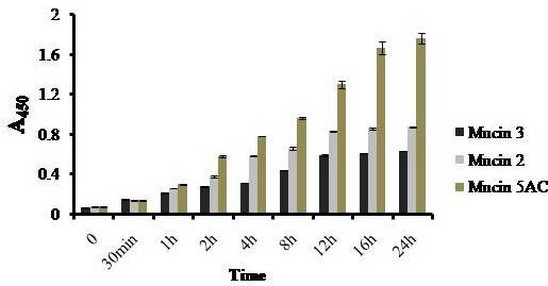
Avishek Ghosh1*1Department of Microbiology, Maulana Azad College, Kolkata, India*Corresponding author: [email protected];Available from. http://dx.doi.org/10.21931/RB/2023.08.04.95ABSTRACTChitin-binding protein GbpA is a secreted colonization factor of Vibrio cholerae. It has been observed to be secreted by various bio-variants of V. cholerae in substantial quantities. This specific protein is pivotal in triggering inflammation within the intestine by stimulating increased mucus production in vivo and in vitro. Following GbpA induction, there was a notable enhancement in the expression of critical mucins, including mucin 2, mucin 3, and mucin 5AC. Mucin secretion also enhanced GbpA secretion in vivo, as evidenced in the rabbit models. This increased mucin secretion was accompanied by a heightened pro-inflammatory response, as evidenced by the upregulation of the pro-inflammatory cytokine IL8 in intestinal epithelial cells and rabbit models. Among the seven cytokines examined, IL8 showed a particularly significant increase in expression. To establish the precise role of GbpA in these inflammatory responses, a ΔGbpA mutant strain was employed. These findings conclusively identify GbpA as a primary instigator of pro-inflammatory reactions within the intestinal environment and suggest that GbpA could be a potential target to open new ways of treating cholera.Keywords.Chitin binding protein, Pro-inflammatory cytokine, intestinal epithelial cells, colonization factor, IL8, mucinINTRODUCTIONThe attachment of Vibrio cholerae to intestinal epithelium is the first important step in the pathogenesis of cholera. This adherence to intestinal epithelium initiates the colonization and micro-colony formation of V. cholerae. Several cell surface factors are involved in this adhesion process1. Cholera pathogenesis is multifactorial and depends on factors like toxin-coregulated pilus2, outer membrane proteins1, and LPS3. N-acetylglucosamine binding protein A (GbpA), the gene product of locus VCA0811, plays a vital role in this adherence process. It is a chitin-binding protein (CBP) known as a common adhesin for chitinous surfaces and intestinal epithelium4.GbpA, a four-domain protein5, was considered an important member of the V. cholerae chitin utilization program, and it was essential for bacterial attachment to the chitinous surface for chitin utilization. This CBP was detected to be the most important factor of V. cholerae for specific attachment to chitin6, and deletion of this resulted in reduced interaction with Chitin, leading to diminished chitin utilization7. It was identified as an adhesion factor for the surfaces displaying N-acetylglucosamine (GlcNAc) and its oligomers7.GlcNAc is a crucial component of mucus. Mucin is a complex glycoprotein composed of this sugar moiety in large quantities8, which is also the monomer of Chitin. Using the chitin-binding protein, this mucin layer can act as the receptor for attachment of vibrios9 and other bacteria. CBP of Lactobacillus plantarum was also identified as the factor interacting with mucin for colonizing intestinal epithelial cells10, and the role of CBP21 of Serratia marcescens was determined to interact with mucin, too, during binding to colonic epithelial cells11. GbpA is the factor bridging V. cholerae and mucin in the host12. The structural analysis of GbpA has indicated that amongst its four domains, the first domain is responsible for direct binding to intestinal mucus during colonization. In contrast, domains 2 and 3 anchored the protein on the bacterial cell surface5.GbpA is the secretory colonization factor of V. cholerae and mainly remains in the culture supernatant of bacteria4. However, it is also found in bacterial surface-bound form5,12. Another study showed a coordinated correlation between GbpA and mucin secretion in the intestine 12. This suggests that V. cholerae adeptly employs GbpA as a strategic tool to facilitate its colonization of the intestinal epithelium, leveraging mucin as a foundational substrate for initial adherence. Secreted and bacteria-bound fractions of GbpA play an essential role in this coordinated interaction of intestinal mucus secretion and V. cholerae attachment. The upregulation of mucin secretion can be considered a significant indicator of the innate immune response, highlighting the intricate strategies employed by V. cholerae in its colonization and interaction with the host's intestinal environment8.The epithelial cells generally provide early mucosal inflammatory signals by releasing pro-inflammatory cytokines. According to some previous researchers, V. cholerae infection can induce intestinal pro-inflammatory responses13, and V. cholerae infection can specifically induce IL8 secretion by the intestinal epithelial cells14. Pro-inflammatory cytokine IL8 had also been shown to be released by intestinal epithelial cells during V. cholerae vaccine trials with reactogenic and non-reactogenic strains15,16. Enhanced IL8 secretion was also identified in cases of infections by toxin-producing variants of V. cholerae, such as the 0395 classical variant and other toxin-encoding variants of O1 and O139 group17. The induction of pro-inflammatory responses by V. cholerae is multifactorial, and flagellin is one of the essential factors 16,18 in inducing various pro-inflammatory reactions.In light of prior research demonstrating that V. cholerae infection triggers inflammatory reactions within the intestine, there is a need to investigate the specific role played by GbpA in mediating these pro-inflammatory responses within intestinal epithelial cells. This study aims to shed light on the involvement of chitin-binding protein, GbpA in provoking pro-inflammatory responses within intestinal epithelial cells by employing both in vitro and in vivo experimental systems to demonstrate intestinal cells' heightened secretion of pro-inflammatory cytokines and mucins.MATERIALS AND METHODSBacterial Cells and cultureVibrio cholerae N16961 (O1 El Tor Inaba), N1RB1 (ΔGbpAN16961) and N1RC1 (GbpA complemented)12 were cultured and maintained in Luria Bertani medium. V.chlolerae strains N1RB1 (ΔGbpAN16961) and N1RC1 (GbpA complemented) are generously gifted by Dr. Nabendu S. Chatterjee, ICMR-NICED, Kolkata, India.Cell culture and treatmentHuman colonic epithelial cell line HT29 was cultured in complete McCoy's 5A (Sigma, St Louis, USA) supplemented with 10% Fetal Calf Serum (Eurobio, Paris, France), Non-essential amino acids and Penicillin-Streptomycin (MP Biomedicals, USA). HEK293 and INT407 cell lines were cultured and maintained in DMEM supplemented with 10% Fetal Calf Serum (Eurobio, France), Non-essential amino acids and Penicillin-Streptomycin (MP Biomedicals, USA). CaCO2 cell line was cultured and maintained in MEM supplemented with 10% Fetal Calf Serum (Eurobio, France), Non-essential amino acids and Penicillin-Streptomycin (MP Biomedicals, USA). T84 cell line was cultured and maintained in DMEM added with Ham F12 (1:1) and supplemented with 5% Fetal Calf Serum (Eurobio, Paris, France), Non-essential amino acids and Penicillin-Streptomycin(MP Biomedicals, USA).Expression and purification of recombinant GbpARecombinant GbpA was expressed in pET 22b expression vector. This was done following a previously described protocol by Wong et al.5.Induction of Cytokine release in HT 29 cellsHT29 cells were induced with 250 ng of purified GbpA or its different domains to detect whether any cytokine is secreted or not. Induction by the culture supernatants of V. cholerae N16961, N1RB1 and N1RC1 were also carried out. The three different V. cholerae strains were grown up to 107Cfu/ml and the culture supernatants were collected for this experiment. These supernatants were used to infect the HT29 cells.Quantification of cytokinesThe quantification of cytokines released due to GbpA treatment was done using a colorimetric procedure by sandwich ELISA and by flow-cytometric procedure by cytometric bead array.Colorimetric quantification- The assay was performed with OptEIA kits (BD Biosciences, USA). In this process, the amount of different cytokines released was measured based on the standard curve obtained using the provided standard cytokine samples reading the absorbance in a microplate reader (BioRad 550, USA) at 450 nm.Flow cytometric quantification- The secreted inflammatory cytokines were measured using a cytometric bead array procedure by Cytometric Bead Array (CBA) kit (BD Biosciences, USA) following the manufacturer's protocol. Briefly, the standards given were reconstituted; negative control was prepared using only the assay diluent and 50 μl of each unknown sample to the appropriately labeled sample tube. All the tubes were incubated with 50 μl of the PE Detection Reagent for 3 h at room temperature, protected from light. The tubes were washed and read at the cytometer (BD FACS ARIA II) following the usual procedure for CBA analysis, and data was acquired and analyzed using BD Cellquest software.Rabbit ileal loop assay6-8 weeks New Zealand white rabbits (male, weighing 1.6 Kg) were fasted for 16h before the experiment and surgery was done under anesthesia. General anesthetic Ketamine 50 (35 mg/ Kg of body weight) was administered intravenously. The intestine was brought out through a mid-line incision in the abdomen, and a suitable length of ileum starting about 6-8 cm away from the ileocaecal junction was selected. The selected portion was washed with warm (37ºC), sterile PBS (pH 7.4) and intestinal loops were made with inner loops (2 cm) on both sides. 108 CFU/ml of each of N16961, N1RB1 and N1RC1 was introduced into the loops. The rabbits were knitted and kept fasting overnight. Then the next day, the intestines were again opened and accumulated fluids were collected. The ileal loops were then slit open, and the epithelial cells were scrapped for RNA isolation. The fluid from different loops was used to measure the secretion of IL8 in the intestine.Primers usedβ-actin gene expression was used as the internal control for normalizing the mRNA expression in quantitative PCR of IL8 from rabbit intestinal cells. The primers used in this experiment are listed below:IL8- Forward 5ʹ- TGAAGCTGCAGTTCTGACACGG-3ʹ;Reverse 5ʹ- TTGGGATGAAAGGTGTGGGAGTG- 3ʹβ-actin- Forward 5ʹ- GGGCATGGGTCAGAAGGATT -3ʹReverse 5ʹ- GCAGCTCATTGTAGAAGGTGTGG -3ʹStatistical analysisWherever applicable, the results represented in this manuscript are mean ± SE (standard error) of at least three separate experiments. ANOVA analyzed statistical differences with level of significance being set at 5% (P<0.05).RESULTSGbpA is secreted from V. cholerae:Preparation of Standard curve of GbpA-The amount of GbpA present in the culture supernatant of V. cholerae was quantitatively determined from the standard curve of GbpA prepared using different concentrations of purified GbpA.The standard curve of GbpA was prepared using the indirect ELISA method. The concentrations used to prepare the standard curve were 0.01, 0.02, 0.05, 0.1, 0.5, 1, 1.5 and 2 µg/µl of purified GbpA [Figure 1]. The standard regression equation is y = 0.8087x + 0.1787.Figure 1: Standard curve of GbpA. Different concentrations of GbpA i.e. 0.01, 0.02, 0.05, 0.1, 0.5, 1, 1.5 and 2 µg/µl was coated in the wells of microtitre plate and amount of colour developed was quantified using polyclonal anti mouse GbpA at a dilution 1:500 by measuring the absorbance at 450 nm.Secretion of GbpA in culture supernatant-GbpA is a secretary colonization factor of V. cholerae, and it is found in the culture supernatant of the bacteria. V. cholerae N16961 was cultured for different periods, and the culture supernatant of each time point was concentrated up to 10% of the original culture volume by ultrafiltration. These culture supernatants were dialyzed in 20 mM Tris-HCl buffer pH 7.5 to remove the medium components. Then, 1 µg of total protein was coated with carbonate buffer in the wells of a microtitre plate. The amount of GbpA was determined by indirect ELISA using Anti GbpA antibody at a dilution of 1:500. The result showed that the amount of GbpA secreted increased with time, the highest being 429 ng/µl after 20 h of culture [Figure 2].Figure 2: Time dependent secretion of GbpA in bacterial culture supernatants. V. cholerae N16961 culture supernatant was concentrated by ultrafiltration and after dialysis coated in the microtitre plate wells. Each sample having 1 µg of total protein was coated with 0.1 M carbonate buffer in the well. Indirect ELISA measured the amount of GbpA.GbpA is secreted from different strains of V. cholerae:GbpA is secreted in the culture supernatant of N16961 and is valid for other Vibrio strains. To see the amount of GbpA that could be secreted by different strains of V. cholerae, O139, Non O1 non O139, O1 EL Tor, O1 EL Tor variant, Classical, and Hybrid O1 strains were assayed. 200 µl of overnight culture having 107CFU/ml of each strain was used to inoculate 200 ml broth culture and incubated at 37ºC for 12 h. The culture supernatants were concentrated up to 10% of the original volume and dialyzed in 20 mM Tris-HCl buffer pH 7.5 to remove the medium components. Then 1 µg of total protein was coated with carbonate buffer in the wells of a microtitre plate. The amount of GbpA was determined by indirect ELISA using Anti GbpA antibody at a dilution of 1:500. Highest expression of GbpA was shown by Non O1 non O139 strain, and O1 EL Tor variant & O139 strains could secrete a small amount of GbpA in the culture supernatant [Figure 3].Figure 3: Differential secretion of GbpA from different V. cholerae strains. Culture supernatants were collected from different strains i.e. O139, Non O1 non O139 (NAG), O1 EL Tor, O1 EL Tor variant, Classical, Hybrid O1 and N16961. Equal amount of bacteria was cultured for 12 h and culture supernatants were concentrated; dialysed, 1 µg total protein coated. Indirect ELISA measured the amount of GbpA.Induction of cytokines in GbpA-treated HT29 cellsTo begin, the study aimed to investigate the impact of GbpA treatment on a mammalian cell line. Specifically, HT29 cells cultured in 12-well plates were utilized, having approximately 107 cells/well, and subjected them to an overnight treatment involving 300 ng of GbpA. Subsequently, the culture supernatants of the treated cells were examined to assess the presence of cytokines using a CBA kit from (BD Biosciences). Among the seven pro-inflammatory cytokines that were discussed (namely, IL8, IL 1β, IL6, TNF-α, IL12, IL2, and IL10), the findings revealed that IL8 exhibited the highest secretion in the culture supernatants of the HT29 cell line. Notably, the shift in the bead population indicating a positive result for IL8 was markedly more pronounced compared to the other cytokines assessed.[Figure 4a] suggests that IL8 was the main proinflammatory cytokine produced on induction by GbpA in the HT29 cell line.Figure 4a: Flow cytometric assay showing IL8 as the main cytokine secreted in GbpA treated HT29 cells. GbpA treated and untreated HT29 culture supernatants were incubated with beads tagged with antibodies of cytokines IL8, IL 1β, IL6, TNF-α, IL12, IL2, IL10, washed in PBS and loaded in BD Flow cytometer and detected using Phycoerythrin (PE) and Per CP channels. A. Control- No shift of beads showing absence of secretion of any cytokine. B. Shift of beads incubated with culture supernatants of GbpA treated cells showing IL8 secretion.Indirect ELISA further confirmed the secretion of cytokines mediated by GbpA in HT29 cells. Here, IL8 was also shown to be induced most compared to other proinflammatory cytokines. Secreted IL8 was 774.8 ± 44.6 pg/ml, whereas other cytokines were below 100 pg/ml [Figure 4b]. This outcome undoubtedly suggested that GbpA induction triggers IL8 secretion as the chief cytokine in HT29 cells.Figure 4b: GbpA mediated secretion of different cytokines from HT29 cells. The culture supernatants of HT29 cells treated with GbpA for 16 h were incubated in microtitre wells pre-coated with antibodies of specific cytokines. The amount of each cytokine secreted was quantified by sandwich ELISA method using a pre-determined standard curve.Induction of IL8 secretion in HT29 cells by V. cholerae culture supernatants:The involvement of GbpA in IL8 secretion from HT29 cells was confirmed by incubating the concentrated culture supernatants of N16961, N1RC1 and N1RB1. The culture supernatant of N16961 was treated with an Anti GbpA antibody and was used as a control. Twenty milliliters of broth culture of each strain was grown till A600 nm = 1.0. Two milliliters of culture were used to inoculate 200 ml of overnight cultures. The overnight culture of each strain was centrifuged to obtain the cell-free supernatant and concentrated to 10% of the original volume by ultrafiltration. These supernatants were then dialyzed in PBS, and the total protein content of each supernatant was measured. HT29 cells were treated with an equal concentration of each dialyzed culture supernatant for 4 h. The IL8 secretion was measured using the sandwich ELISA method. The result showed that the culture supernatant from wild type and complemented strains induced the IL8 secretion 1890.8 ± 98.4 pg/ml & 1963.5 ± 84.7 pg/ml.In contrast, the amount of IL8 produced by the culture supernatant of the mutant strain was 1109.2 ± 64.7 pg/ml, which was lower than the amount of IL8 induced by the supernatant of wild type. Immunoprecipitated culture supernatant of the wild type could produce 1289.2 ± 84.7 pg/ml. This proves that GbpA played an active role in inducing the secretion of IL8 in HT29 cells [Figure 5].Figure 5: Secretion of IL8 from HT29 cells treated with culture supernatants. Equal number of bacteria of Wild type N16961, complemented N1RC1 and ∆GbpA mutant N1RB1 were cultured overnight and the culture supernatant of each strain was concentrated by ultrafiltration dialysed to remove the medium components and total protein concentration was measured. HT29 cells were treated with equal total protein amount of each culture supernatant. A portion of N16961 culture supernatant was immunoprecipitated with anti GbpA to remove the GbpA present in that, which is denoted by N16961 (IP). The amount of IL8 secreted was quantified by sandwich ELISA.Determination of amount of GbpA secreted in rabbit intestine From the regression equation obtained, the concentration of GbpA secreted by N16961 and N1RC1 was calculated as 338 ng/µl and 309 ng/µl respectively in the rabbit illeal loops [Figure 6].Figure 6: Secretion of GbpA in rabbit ileal loop. The fluid accumulated in the loop infected with V. cholerae N16961 and ΔGbpA V. cholerae N16961 was immunoblotted using anti-GbpA antibody at a dilution 1:2500.Induction of inflammatory cytokine IL8 in rabbit intestine-The GbpA-mediated induction of IL8 in rabbit intestines was studied using the ileal loop method. Eight loops were prepared in the ileum of a rabbit. Two loops were prepared for V. cholerae N16961, N1RB1, N1RC1 and negative control (PBS). Each loop was inoculated with 108 CFU of N16961 or, N1RB1 or N1RC1 in 1 ml PBS and two loops were added with 1 ml sterile PBS. Fluids accumulated inside the loops were collected for quantification of IL8 secreted. The secretion of IL8 was determined using an indirect ELISA method with a rabbit-IL8 antibody. The amount of IL8 secreted in the N16961-loop was significantly more significant than that of the N1RB1-containing loop. The N1RC1-containing loop showed almost the same IL8 secretion as wild-type N16961 [Figure 7].Figure 7: Secretion of IL8 in Rabbit illeal loops. WT- Wild type N16961, C- N1RC1, ΔGbpA- N1RB1 A. Quantitative estimation of rabbit IL8 secreted from illeal loop containing WT- Wild type N16961, C- N1RC1, ΔGbpA- N1RB1. B. Secretion of IL8 in rabbit illeal loop detected by immunoblot analysis. Lane 1- Fluid Accumulated in the loop with Wild type N16961, Lane 2- Fluid Accumulated in the loop with complemented N1RC1 and Lane 3- Fluid Accumulated in the loop with ΔGbpA- N1RB1.The epithelial cells from the inner lining of each ileal loop were scraped, and total RNA was isolated from these cells to quantify the IL8 mRNA levels induced by GbpA treatment. This analysis showed that the loop containing the wild-type N16961 strain exhibited significantly higher IL8 mRNA levels than the loop containing the ∆GbpA mutant N1RB1 strain. Moreover, the loop infected with the complemented N1RC1 strain demonstrated a substantial increase in IL8 mRNA production compared to the negative control and N1RB1 [Figure 8]. Mainly, in the loops containing N16961 and N1RC1, IL8 mRNA production was observed to be 77 and 75 times higher than in the negative control, respectively. Conversely, in the N1RB1-infected loop, the increase in IL8 mRNA production was only 55-fold higher than in the negative control. This substantial reduction in secreted IL8 levels in the mutant bacteria-containing loop is noteworthy, particularly in light of the absence of GbpA.Figure 8: Expression of IL8 mRNA in rabbit intestine. WT- Wild type N16961, C- Complemented N1RC1, ∆GbpA- N1RB1. The cells from the inner side of each illeal loop were scrapped and total RNA was isolated. cDNA prepared by reverse transcription and amount of IL8 mRNA in each loop was quantified by qPCR using specific rabbit IL8 primers.Cell line-dependent secretion of IL8:The secretion of IL8 was cell-line dependent. HT29 cells could secrete 494.8±32 pg/ml IL8. In the case of INT407, CaCO2 and T84 cell lines 54±3 pg/ml, 27±2 pg/ml and 291.7±24 pg/ml IL8 secretion were observed, respectively, suggesting the cell line dependence of GbpA-induced IL8 secretion [Figure 9].Figure 9: Cell line dependent IL8 secretion induced by GbpA.105 each of INT 407, T84, HT29, DLD1 and CaCO2 cells were treated with 250 ng of GbpA for 8 h; the amount of IL8 secreted was measured by sandwich ELISA in each case.Time dependent secretion of IL8 in HT29 cells:Since IL8 was found to be the main pro-inflammatory cytokine secreted from GbpA-treated HT29 cells, we tried to estimate the level of secreted IL8 by treating HT29 cells with GbpA for different periods. HT29 cells were treated with 250 ng of GbpA for 0- 48 h. The culture supernatants were tested for the amount of IL8 secreted. In the untreated cells, the level of IL8 was found to be only 5.4 pg/ml, and it gradually increased with time; at the highest time point, it was found to be 1466 pg/ml [Figure 10].Figure 10: Time-dependent IL8 secretion from GbpA-treated HT29 cells. 250 ng of GbpA was treated to HT29 cells and incubated for different periods. The culture supernatants of each time point was used to quantify the amount of IL8 secreted by the sandwich ELISA method.Concentration dependent secretion of IL8 in HT29 cells:In another set of experiments, a different amount of GbpA was treated on HT29 cells for a fixed period, and IL8 secretion was quantified. The amount of GbpA used for infection in each 105 HT29 cells was 0- 2 µg. Here also, IL8 secretion gradually increased with the amount of GbpA and reached a plateau after 500 ng; the maximum IL8 secreted was 1493.3 ± 94.8 pg/ml where the amount of GbpA was 2000 ng [Figure 11].Figure 11: Concentration-dependent IL8 secretion from GbpA-treated HT29 cells. Different amounts of GbpA were treated to HT29 cells for an period 8 h, and each sample's culture supernatants were used to quantify the amount of IL8 secreted by sandwich ELISA.Secretion of mucin in HT29 cells:In addition to its ability to kindle IL8 secretion, GbpA has also been demonstrated to induce mucin secretion from HT29 cells. This observation was made when HT29 cells were exposed to concentrated culture supernatants from N16961, N1RC1, and N1RB1. As a control, the culture supernatant of N16961 was treated with a GbpA antibody. Twenty milliliters of broth culture for each strain were grown to prepare these supernatants until reaching an optical density (O.D.) of 600 nm = 1.0. Two milliliters of culture were used to inoculate 200 ml of overnight cultures. Afterward, the overnight cultures of each strain were centrifuged to obtain cell-free supernatants, which were concentrated to 10% of their original volume using ultrafiltration. To prepare the control i.e. immunoprecipatated culture supernatant of N16961, 1:250 Anti GbpA antibody tagged with Protein A Sepharose CL-4B was added to the culture supernatant, incubated overnight at 4°C to precipitate the secreted GbpA present in the culture supernatant. Subsequently, these supernatants were dialyzed in PBS, and the total protein content of each supernatant was quantified. HT29 cells were treated with an equal volume (300 µl) of dialyzed culture supernatant for 8 hours, and mucin secretion was assessed using an indirect ELISA method. The results indicated that the wild-type and complemented strains induced mucin secretion to a similar extent. In contrast, the mutant strain induced mucin secretion at a level comparable to that induced by the immunoprecipitated culture supernatant of the wild type. This compellingly demonstrates that GbpA actively contributes to MUC 2, MUC 3, and MUC 5AC secretion from HT29 cells [Figure 12a].Figure 12a: Secretion of Mucin 2, Mucin 3 & Mucin 5AC from GbpA treated HT29 cells. 250 ng of GbpA was treated to HT29 cells for different periods. Culture supernatant of each sample collected and dialysed in phosphate buffer, total protein concentration was measured. 1 µg from each sample (diluted in 0.1 M carbonate buffer) was coated in the microtitre plate wells. Time dependent expression of Mucin 2, Mucin 3 & Mucin 5AC was determined by using anti Mucin 2, anti Mucin 3 and anti Mucin 5AC antibodies in indirect ELISA method.107 HT29 cells cultured in a 12-well plate were treated with GbpA for 0-24 h periods. Untreated cells were used as controls. MUC 2, MUC 3 and MUC 5AC were shown to be secreted in increased amounts due to GbpA treatment. MUC 5AC was found to be induced at the highest amount amongst the mucins [Figure 12b].Figure 12b: Secretion of Mucin 2, Mucin 3 & Mucin 5AC from GbpA treated HT29 cells. 250 ng of GbpA was treated to HT29 cells for different periods. Culture supernatant of each sample collected and dialysed in phosphate buffer, total protein concentration was measured. 1 µg from each sample (diluted in 0.1 M carbonate buffer) was coated in the microtitre plate wells. Time-dependent expression of Mucin 2, Mucin 3 & Mucin 5AC was determined by using anti Mucin 2, anti-Mucin 3 and anti Mucin 5AC antibodies in the indirect ELISA method.DISCUSSIONThe chitin-binding role of GbpA or its involvement in host-pathogen interaction is well established; however, the role of GbpA in the host's response remains relatively unexplored. In elucidating the host response induced by GbpA, the expression of GbpA in various strains was examined. This examination aims to ascertain the significance of GbpA from both the bacterial and host perspectives. The correlation between the GbpA expression by a particular strain of V. cholerae and the subsequent host response induced was considered here.An intriguing observation was made in the current research regarding the secretion of GbpA by V. cholerae. This secretion was detected both in the culture supernatant and within the intestine. A comprehensive analysis involving six distinctive V. cholerae strains, namely O139, Non-O1 Non-O139, O1 El Tor, O1 El Tor variant, Classical, Hybrid O1, and the laboratory strain N16961, to assess the secretion of GbpA into the culture supernatant was undertaken. Notably, the highest expression of GbpA was observed in the case of the Non-O1 Non-O139 strains. These strains do not possess active CT and TCP1. TCP plays a vital role in colonizing and adhering V. cholerae in the intestine1. The absence of TcpA in vibrios leads to decreased intestinal colonizing ability19. Therefore, the comparatively high level of GbpA secretion in case of these strains may compensate for the absence of TCP so that the involvement of GbpA is more in the colonisation and adherence of these strains than TCP-positive strains. Non O1 non O139 strains expressed GbpA up to ~ 450 ng/µl, and N16961 secreted ~ 260 ng/µl GbpA in the culture supernatant. N16961 secreted ~390 ng/µl of GbpA in the rabbit intestine. So, these studies have shown that V. cholerae secretes GbpA in vitro and in vivo. The amount of GbpA secreted in vivo is higher than in vitro. This phenomenon can be attributed to mucin within the rabbit intestine. When V. cholerae encounters mucin, it increases the expression of GbpA. This heightened GbpA expression can be accredited to a coordinated relationship between GbpA and mucin, where their levels correlate with each other12.Several other secretory proteins of V. cholerae were shown to have immunomodulatory effects. Cholera Toxin (CT) was shown to be pro-inflammatory as it could induce the production of TNFα from mouse macrophages20 by previous workers and V. cholerae flagellins were shown to have potent pro-inflammatory roles as inducer of IL8 secretion18. In this study, we looked into the host response induced by GbpA. Here we found that IL8 was induced at a significant level in HT29 cells mediated by GbpA. The IL8 secretion was dose and time-dependent. The cell-free culture supernatant of V. cholerae ΔGbpA showed a lower level of IL8 secretion compared to the amount of IL8 induced by the culture supernatant of wild-type V. cholerae. This confirmed the role of GbpA in inducing IL8 in HT29 cells. In vivo studies in rabbit ileal loop have also confirmed that GbpA could induce IL8 at a significant level. Previous studies have shown that TNF-α was induced at significant level by V. cholerae LPS21. Other pro-inflammatory cytokines like IL1β, IL6, IL2, and TNF-α tested here were not induced by GbpA at a substantial level.Induction of pro-inflammatory cytokines and chemokines during infection of non-toxigenic V. cholerae was previously demonstrated in a murine-pulmonary cholera model22. The enhanced secretion of IL6, TNF-α and MIP-2 were shown. However, interestingly, the deletion of hapA & hlyA genes could still induce the pro-inflammatory cytokines, indicating that there was some other bacterial mediator for the induction of pro-inflammatory responses. In earlier work, V. cholerae cytolysin/hemolysin was shown to induce Il-1β as the significant pro-inflammatory cytokine in macrophages involving the MAPK pathway23. The other studies by Mandal et al. also reported the inflammatory and necrotic changes induced by GbpA by mitochondrial dysfunction24. In the case of Int407 cells, it was identified that non-toxigenic V. cholerae could induce enhanced expression of pro-inflammatory chemokines like IL-1, IL6, GM-CSF, and MCP-125. IL8 was identified as the significant pro-inflammatory cytokine in V. cholerae OmpU-induced polarized HT29 cells26, indicating that HT29 cells are candidates for IL8 secretion by V. cholerae antigens which matches with this particular study where IL8 was found as the major cytokine induced in HT29 cells by GbpA. So, it can be said that, non-toxigenic V.cholerae strains have some specific inducers of pro-inflammatory responses other than CT and TCP and this fact is also confirmed in this study since the secretion of GbpA was highest in case of non O1/nonO139 (NAG) V. cholerae strains which are devoid of cholera toxin. The property of induction of IL8 response was unique to this colonization factor GbpA of V. cholerae as previously no colonization factor of V. cholerae was shown to induce IL8 as a marker for intestinal inflammation.The colonization factor GbpA was found to have the capability to stimulate mucin secretion, both in vivo and in vitro. Previous studies had hinted at a coordinated relationship between mucin secretion and GbpA12. However, in this study, the role of GbpA in mucin secretion was further substantiated. This was strongly supported by the notable disparity in mucin secretion between the culture supernatants of O1 N169161 V. cholerae and ΔGbpA V. cholerae within the rabbit intestine. Additionally, this finding was reinforced by the enhanced mucin secretion observed in HT29 cells treated with GbpA. Consequently, confirming GbpA's role in inducing pro-inflammatory responses was solidified.CONCLUSIONThe secretory colonization factor GbpA from V. cholerae emerges as a promising candidate for triggering substantial pro-inflammatory responses within the intestine. Notably, the heightened induction of IL8, a key cytokine in the human system, by GbpA unequivocally underscores its involvement in intestinal inflammation. However, further studies will be essential in the future to gain a comprehensive understanding of the mechanisms underlying IL8 induction.Authors' ContributionThe study was conceived, designed and performed by AG. The manuscript was written and formatted by AG.FundingThis study was partially supported by CSIR project No. 37(1410)/10/EMR-II.Institutional Review Board StatementThe study was conducted according to the guidelines of the Declaration of Helsinki and approved by the College Research Review Board (ECR/1327/MC/WB/2020/RQ-37, Dated 12/03/2020).Informed Consent StatementNot applicable.AcknowledgmentsThe author sincerely acknowledges Dr. Nabendu S. Chatterjee, Dr. Sagar Acharya and Dr. Subrata Sabui for their support and encouragement in the study. V.chlolerae strains N1RB1 (ΔGbpAN16961) and N1RC1 (GbpA complemented) are generously gifted by Dr. Nabendu S. Chatterjee, ICMR-NICED, Kolkata, India. Recombinant GbpA constructs were kindly gifted by Prof. D.M.F. van Aalten, Dundee University, Scottland and Dr. E. Wong, The National Institute for Medical Research, London, United Kingdom, for helping write the manuscript.Conflicts of InterestNot applicable.REFERENCES1. Kaper JB, Morris JG, Levine MM. Cholera. Clin Microbiol Rev. 1995;8(1):48-86. doi:10.1128/CMR.8.1.482. Attridge SR, Manning PA, Holmgren J, Jonson G. Relative significance of mannose-sensitive hemagglutinin and toxin-coregulated pili in the colonization of infant mice by Vibrio cholerae El Tor. Infect Immun. 1996;64(8):3369-3373. doi:10.1128/iai.64.8.3369-3373.19963. Chitnis DS, Sharma KD, Kamat RS. Role of the somatic antigen of Vibrio cholerae in adhesion to intestinal mucosa. J Med Microbiol. 1982;15(1):53-61. doi:10.1099/00222615-15-1-534. Kirn TJ, Jude BA, Taylor RK. A colonization factor links Vibrio cholerae environmental survival and human infection. Nature. 2005;438(7069):863-866. doi:10.1038/nature042495. Wong E, Vaaje-Kolstad G, Ghosh A, et al. The Vibrio cholerae colonization factor GbpA possesses a modular structure that governs binding to different host surfaces. PLoS Pathog. 2012;8(1):e1002373. doi:10.1371/journal.ppat.10023736. Montgomery MT, Kirchman DL. Role of Chitin-Binding Proteins in the Specific Attachment of the Marine Bacterium Vibrio harveyi to Chitin. Appl Environ Microbiol. 1993;59(2):373-379. doi:10.1128/aem.59.2.373-379.19937. Meibom KL, Li XB, Nielsen AT, Wu CY, Roseman S, Schoolnik GK. The Vibrio cholerae chitin utilization program. Proc Natl Acad Sci U S A. 2004;101(8):2524-2529. doi:10.1073/pnas.03087071018. Moncada DM, Kammanadiminti SJ, Chadee K. Mucin and Toll-like receptors in host defense against intestinal parasites. Trends Parasitol. 2003;19(7):305-311. doi:10.1016/s1471-4922(03)00122-39. Alam M, Miyoshi S, Tomochika K, Shinoda S. Vibrio mimicus attaches to the intestinal mucosa by outer membrane hemagglutinins specific to polypeptide moieties of glycoproteins. Infect Immun. 1997;65(9):3662-3665. doi:10.1128/iai.65.9.3662-3665.199710. Sánchez B, González-Tejedo C, Ruas-Madiedo P, Urdaci MC, Margolles A. Lactobacillus plantarum extracellular chitin-binding protein and its role in the interaction between Chitin, Caco-2 cells, and mucin. Appl Environ Microbiol. 2011;77(3):1123-1126. doi:10.1128/AEM.02080-1011. Kawada M, Chen CC, Arihiro A, Nagatani K, Watanabe T, Mizoguchi E. Chitinase 3-like-1 enhances bacterial adhesion to colonic epithelial cells through the interaction with bacterial chitin-binding protein. Lab Invest. 2008;88(8):883-895. doi:10.1038/labinvest.2008.4712. Bhowmick R, Ghosal A, Das B, et al. Intestinal adherence of Vibrio cholerae involves a coordinated interaction between colonization factor GbpA and mucin. Infect Immun. 2008;76(11):4968-4977. doi:10.1128/IAI.01615-0713. Kuchta A, Rahman T, Sennott EL, et al. Vibrio cholerae O1 infection induces proinflammatory CD4+ T-cell responses in blood and intestinal mucosa of infected humans. Clin Vaccine Immunol. 2011;18(8):1371-1377. doi:10.1128/CVI.05088-1114. Ou G, Rompikuntal PK, Bitar A, et al. Vibrio cholerae cytolysin causes an inflammatory response in human intestinal epithelial cells that is modulated by the PrtV protease. PLoS One. 2009;4(11):e7806. doi:10.1371/journal.pone.000780615. Rodríguez BL, Rojas A, Campos J, et al. Differential interleukin-8 response of intestinal epithelial cell line to reactogenic and nonreactogenic candidate vaccine strains of Vibrio cholerae. Infect Immun. 2001;69(1):613-616. doi:10.1128/IAI.69.1.613-616.200116. Zhou X, Gao DQ, Michalski J, Benitez JA, Kaper JB. Induction of interleukin-8 in T84 cells by Vibrio cholerae. Infect Immun. 2004;72(1):389-397. doi:10.1128/IAI.72.1.389-397.200417. Sarkar M, Chaudhuri K. Association of adherence and motility in interleukin 8 induction in human intestinal epithelial cells by Vibrio cholerae. Microbes Infect. 2004;6(7):676-685. doi:10.1016/j.micinf.2004.02.01818. Harrison LM, Rallabhandi P, Michalski J, et al. Vibrio cholerae flagellins induce Toll-like receptor 5-mediated interleukin-8 production through mitogen-activated protein kinase and NF-kappaB activation. Infect Immun. 2008;76(12):5524-5534. doi:10.1128/IAI.00843-0819. Rollenhagen JE, Kalsy A, Cerda F, et al. Transcutaneous immunization with toxin-coregulated pilin A induces protective immunity against Vibrio cholerae O1 El Tor challenge in mice. Infect Immun. 2006;74(10):5834-5839. doi:10.1128/IAI.00438-0620. Viana CFG, Melo DH, Carneiro-Filho BA, et al. Pro-inflammatory effects of cholera toxin: role of tumor necrosis factor alpha. Toxicon. 2002;40(10):1487-1494. doi:10.1016/s0041-0101(02)00170-821. Zughaier SM, Zimmer SM, Datta A, Carlson RW, Stephens DS. Differential induction of the toll-like receptor 4-MyD88-dependent and -independent signaling pathways by endotoxins. Infect Immun. 2005;73(5):2940-2950. doi:10.1128/IAI.73.5.2940-2950.200522. Fullner KJ, Boucher JC, Hanes MA, et al. The Contribution of Accessory Toxins of Vibrio cholerae O1 El Tor to the Proinflammatory Response in a Murine Pulmonary Cholera Model. J Exp Med. 2002;195(11):1455-1462. doi:10.1084/jem.2002031823. Escartín-Gutiérrez JR, Ponce-Figueroa M, Torres-Vega MÁ, Aguilar-Faisal L, Figueroa-Arredondo P. Transcriptional Activation of a Pro-Inflammatory Response (NF-κB, AP-1, IL-1β) by the Vibrio cholerae Cytotoxin (VCC) Monomer through the MAPK Signaling Pathway in the THP-1 Human Macrophage Cell Line. Int J Mol Sci. 2023;24(8):7272. doi:10.3390/ijms2408727224. Mandal S, Chatterjee NS. Vibrio cholerae GbpA elicits necrotic cell death in intestinal cells. J Med Microbiol. 2016;65(8):837-847. doi:10.1099/jmm.0.00029825. Bandyopadhaya A, Das D, Chaudhuri K. Involvement of intracellular signaling cascades in inflammatory responses in human intestinal epithelial cells following Vibrio cholerae infection. Mol Immunol. 2009;46(6):1129-1139. doi:10.1016/j.molimm.2008.11.00326. Yang JS, Jeon JH, Jang MS, et al. Vibrio cholerae OmpU induces IL-8 expression in human intestinal epithelial cells. Mol Immunol. 2018;93:47-54. doi:10.1016/j.molimm.2017.11.005
Received: 26 September 2023 / Accepted: 15 April 2023 / Published:15 December 2023Citation: G Avishek Chitin binding protein GbpA of Vibrio cholerae induces pro-inflammatory responses in the intestine. Revis Bionatura 2023;8 (4) 95. http://dx.doi.org/10.21931/RB/2023.08.04.95Publisher's Note: Bionatura stays neutral concerning jurisdictional claims in published maps and institutional affiliations.Copyright: © 2023 by the authors. Submitted for possible open-access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).