2023.08.01.88
Files > Volume 8 > Vol 8 No 1 2023
Assessment of serum Pentraxin3 level in Iraq patients with and without Diabetic Retinopathy
Zena M Hassan 1,Rana A Hamdi 2 , Khalaf G Hussein Al.Mohammadaw3 , Rassmia H Basal4
1Basic Science, Department of Biochemistry, College of Medicine, University of Baghdad,
e-mail:[email protected]
2 Clinical Biochemistry, Department of Biochemistry, College of Medicine, University of Baghdad, e-mail:[email protected]
3Consultant physician Endocrinology Specialized Center for Endocrinology and Diabetes/Baghdad/Al-Rusafa
e-mail [email protected]
4M.B.C.HB. D.O.Internalmedicin, College of Medicine, University of Baghdad
Available from: http://dx.doi.org/10.21931/RB/2023.08.01.88
ABSTRACT
Diabetic retinopathy is the main cause of vision loss worldwide. It is considered one of the most severe diabetic microvascular complications affecting the retina's blood vessels due to prolonged hyperglycemia. Pentraxin 3 is an acute-phase glycoprotein that is correlated with inflammation. Inflammation is mechanistically involved in the development of diabetic retinopathy. This study aims to measure serum pentraxin3 levels in type 2 diabetic patients with and without retinopathy and compare their levels to controls. Also, investigate the relationship between circulating pentraxin3 and the development of diabetic retinopathy. This case–control study included one hundred and twenty (120) individuals aged 40 to 70 years. Individuals were divided into 3 groups: Group 1 included 40 types 2 diabetic patients with retinopathy, group 2 included 40 type 2 diabetic patients without retinopathy and group 3- included 40 persons as controls. Significant increase in the mean value of serum pentraxin3 in the diabetic patient with retinopathy as compared to diabetic patients with and without retinopathy as compared to and control(p=0.000) as well as a significant increase in the mean value of serum pentraxin3 in the diabetic patient with retinopathy as compared to diabetic patients without retinopathy (p=0.000). In addition, a significant positive correlation was found between serum pentraxin3 level and HbA1C in diabetic patients with retinopathy group (r=0.936, p= 0.0001). Higher serum level of pentraxin 3 in diabetic patients with retinopathy and its association with poor glycemic control, as well as pentraxin 3, is an acute-phase reactant, so serum pentraxin 3 levels may have a significant role in the initiation and development of diabetic retinopathy.
Keywords: diabetes mellitus, diabetic retinopathy, pentraxin‑3.
INTRODUCTION
Diabetes mellitus (DM) is one of the most common chronic metabolic diseases with many causes in the world. It is characterized by high glucose concentration in the blood. The body's inability causes this to produce insulin or fully use insulin 2. Diabetes mellitus is associated with microvascular complications, such as neuropathy, nephropathy and retinopathy, induced by chronic hyperglycemia and subsequent hypoxia 3. Diabetic retinopathy (DR) is a common microvascular complication that is one of the leading causes of adult visual impairment and blindness 4. The pathophysiology of DR suggests it is highly complex and multifactorial, involving the activation of several interrelated pathways, which all tie into several key mechanisms, namely increased oxidative stress, increased proinflammatory mediators and increased release of growth factors, including vascular endothelial growth factor (VEGF) 1. Pentraxin 3 (PTX3) is an acute-phase reactant 19.PTX3 is a 200 amino acid protein that is secreted from the endothelium, macrophages, myeloid cells, dendritic cells, and many other cells in response to cytokines and end toxins such as bacterial products, interleukin‑1, and tumor necrosis factor 11.PTX-3 is one of the endothelium-specific inflammatory cytokines, representing the tissue inflammatory response, especially the one involving the vascular bed, and may reflect the inflammatory status of the vasculature. PTX-3 enhances the procoagulant effect of the endothelial cells and reduces endothelial repair by disabling the effect of the fibroblast growth factor. It may modulate inflammation-associated tissue damage and offer protection against atherosclerosis (AS). The maximum plasma level of PTX-3 varies depending on the time of the vascular event 5.PTX-3 is produced in response to ischemic and proinflammatory signals and may directly reflect vascular inflammation 12.
MATERIAL AND METHODS
This case–control study included one hundred and twenty (120) individuals with an age range from 40 to 70 years. Participants were selected from the Diabetic Control Clinic and Diabetes of Specialized Center for Endocrinology and Ibn-Al Haitham Teaching Hospital in Rusafa city in Baghdad from November 2021 to January 2022. Informed consent was taken from each participant. The Scientific Committee of the College of the Medicine/ University of Baghdad approved the study. Individuals were divided into 3 groups: Group 1 included 40 type 2 diabetic patients with retinopathy, group 2 included 40 type 2 diabetic patients without retinopathy and group 3- included 40 persons as controls. Patients with a history of cardiac disease, malignancies, end-stage renal disease, current or past history of immune-modulating drugs, IV drug users, severe eye disease or retinal detachment, and typ1DM have been excluded from this study. The diagnosis of DM depended on the presence of a history of type2 DM, fasting serum glucose(FSB>126) and Glycated hemoglobin (HbA1 >6.5%) according to WHO criteria. In addition, the diagnosis of DR was made by a senior Ophthalmologist after history taking, clinical examination, and ophthalmological examination. The ophthalmological examination included Fundus examination which was carried out by slit lamp biomicroscope and indirect ophthalmoscopy with volk 90D lens, funds color photograph centered on the macula, and Optical coherence tomography (OCT). Blood involved glycated hemoglobin (HA1C) was measured by autoanalyzer (cobs) C2, and serum lipid profile including total cholesterol, triglycerides, HDL-C, LDL-C and VLDL-C were measured by autoanalyzer (cobs) C3. In addition, serum PTX3 levels were determined by an enzyme-linked immunoassay.
Statistical analysis
Analysis of data was carried out using the available statistical package of SPSS-27 (Statistical Packages for Social Sciences- version 27). Data were presented in simple measures mean, standard deviation, and range (minimum-maximum values). The significance of the difference between different means (quantitative data) was tested using Students-t-test for the difference between two independent means or ANOVA test for the difference between more than two independent means. Statistical significance was considered whenever the P value was equal to or less than 0.05. Correlation and regression were applied for the relationship between two quantitative variables, taking P≤ 0.05 lowest limit of significance
RESULTS
Significant increase in the mean value of serum pentraxin3 in the diabetic patient with retinopathy as compared to diabetic patients with and without retinopathy as compared to and control (p=0.000) as well as a significant increase in the mean value of serum pentraxin3 in the diabetic patient with retinopathy as compared to diabetic patients without retinopathy (p=0.000) as shown in table 1.

Table 1. Mean value of serum pentraxin3 level in all studied groups.
Moreover, a significant increase in the mean value of fasting blood sugar(p=0.0001), HbA1c (p=0.0001), TC (p=0.02), TG (p=0.0001), LDL-C (p=0.03) and VLDL-C (p= 0.0001) in diabetic patients with and without retinopathy as compared to control as demonstrated in table 2.
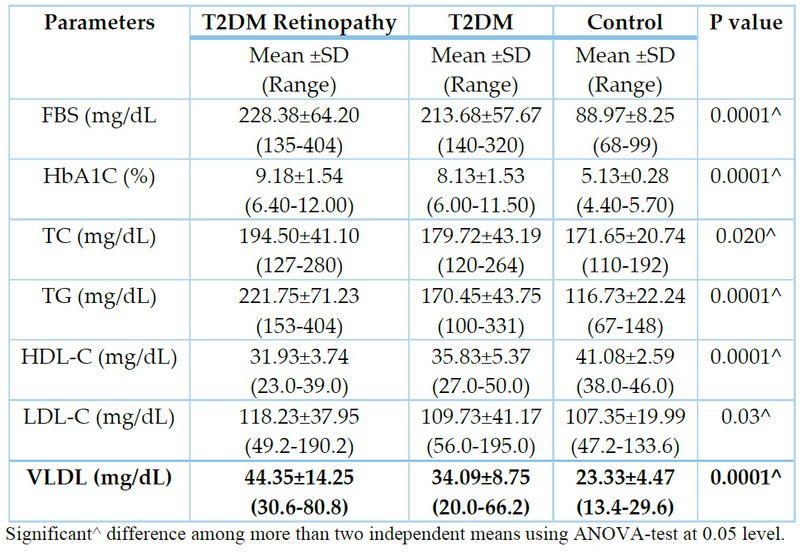
Table 2. Mean value of FBS, HBA1C, TC, TG, HDL-C, LDL-C and VLDL in all studied groups.
In addition, a significant positive correlation was found between serum pentraxin3 level and HbA1C in diabetic patients with retinopathy group (r=0.936, p= 0.0001), as shown in figure 1.
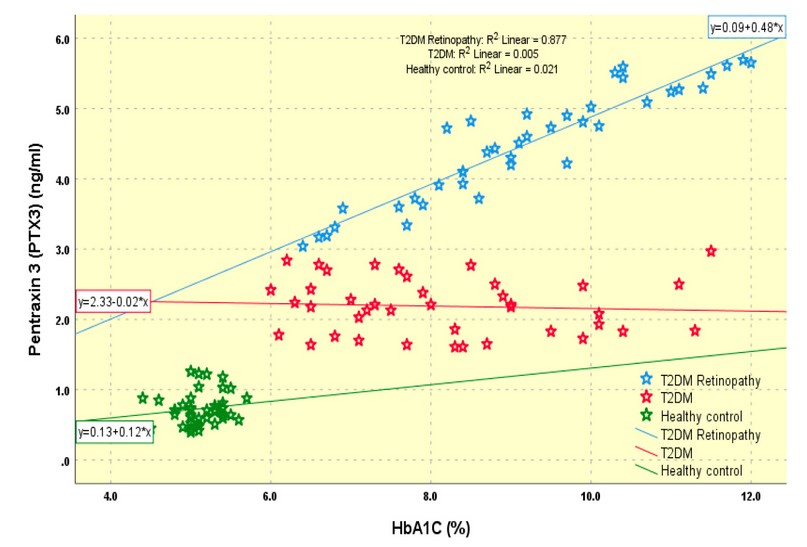
Figure 1. Significant positive correlation between serum pentraxin 3 level and HbA1C in diabetic patient with retinopathy group (r=0.936, p= 0.0001)
DISCUSSION
Inflammation is one potential mechanism of diabetic retinopathy 18. Hyperglycemia-induced vascular damage is the initial mechanism of retinopathy. Vascular endothelial injury and permeability alteration trigger the inflammatory process 15. Pentraxin 3 is reported to be a vascular inflammation marker providing prognostic information of vasculopathy16. Inflammation is mechanistically involved in the development of DR14. PTX3 are acute phase reactants involved in inflammation and endothelial dysfunction. As they are increased in DM, they can be used as prognostic factors for vascular complications such as diabetic retinopathy 17. Diabetic retinopathy is usually diagnosed too late; therefore, identifying novel biomarkers associated with diabetic retinopathy may facilitate the early detection of individuals at risk and further prevent or reverse diabetic retinopathy. The result of this study revealed higher levels of serum pentraxin3 level in DR patients. This finding was in agreement with previous studies suggesting that increased serum pentraxin 3 concentration may be implicated in the development of DR. This reflects that diabetic retinopathy in T2DM is an inflammatory process associated with increased inflammatory reactants 17,18,0. Similar results were done by 20, who suggested that the retinal pigment epithelium and vascular tissues can express PTX3 locally, reflecting that it can be used as a biomarker of vascular inflammation 20. As well as 10 stated that PTX3 levels are associated with the development and progression of DR in Korean patients with T2DM 10. This study demonstrated a positive correlation between pentraxin3 plasma concentration and HBA1c in the diabetic retinopathy group in agreement with other recent studies, which reflects that poor glycemic control increases the risk of the development of microvascular complications of DM 22,21 .
CONCLUSIONS
Higher serum level of pentraxin 3 in diabetic patients with retinopathy and its association with poor glycemic control and pentraxin 3 is an acute-phase reactant, so serum pentraxin 3 levels may have a significant role in the initiation and development of diabetic retinopathy.
REFERENCE
1. Pusparajah, P., Lee, L.H. and Abdul Kadir, K. Molecular markers of diabetic retinopathy: potential screening tool of the future?. Frontiers in physiology, 2016, 7, p.200.
2. Wang, Y., Zhai, WL and Yang, Y.W. Association between NDRG2/IL-6/STAT3 signaling pathway and diabetic retinopathy in rats. European Review for Medical and Pharmacological Sciences, 2020, 24(7), pp.3476-3484.
3. Chatziralli, I.P., Theodossiadis, G., Dimitriadis, P., Charalambidis, M., Agorastos, A., Migkos, Z., Platogiannis, N., Moschos, M.M., Theodossiadis, P. and Keryttopoulos, P. The effect of vitamin E on oxidative stress indicated by serum malondialdehyde in insulin-dependent type 2 diabetes mellitus patients with retinopathy. The open ophthalmology journal, 2017, 11, p.51.
4. Dave, A., Kalra, P., Gowda, BR and Krishnaswamy, M. Association of bilirubin and malondialdehyde levels with retinopathy in type 2 diabetes mellitus. Indian journal of endocrinology and metabolism, 2015, 19(3), p.373.
5. Güngel, H., Erdenen, F., Pasaoglu, I., Sak, D., Ogreden, T. and KilicMuftuoglu, I., 2021. New Insights into Diabetic and Vision-Threatening Retinopathy: Importance of Plasma Long Pentraxine 3 and Taurine Levels. Current Eye Research, 46(6), pp.818-823.
6. Wu, H., Wang, M., Li, X. and Shao, Y., 2021. The Metaflammatory and Immunometabolic Role of Macrophages and Microglia in Diabetic Retinopathy. Human Cell, 34(6), pp.1617-1628.-
7. Hussien, S. .; Doosh, K. S. . Production And Characterization Of Β-Galactosidase Enzyme In The Plant Extract From (Ziziphus Spina-Christi) And Its Application In Milk. ). Journal of Life Science and Applied Research. 2021, 2, 1-8..
8. Pusparajah, P., Lee, L.H. and Abdul Kadir, K., 2016. Molecular markers of diabetic retinopathy: potential screening tool of the future?. Frontiers in physiology, 7, p.200.
9. Nouh, M.Z., Sonbol, A. and Mogahed, M. Evaluation of pentraxin-3 level in patients with diabetic retinopathy. Menoufia Medical Journal, 2018, 31(3), p.928.
10. Abdulateef, S.M. ,O.K. Atalla, MQ. Al-Ani, TH. T. Mohammed, F.M. Abdulateef, O.M. Abdualmajeed , K. Mahmod. The effect of the electric shock on embryonic development and neurophysiological traits in the chick's embryo . IOP Conference Series: Earth and Environmental Science.2021, 761(1), 012090.
11. Bottazzi B, Bastone A, Doni A, Garlanda C, Valentino S, Deban L, et al. The long pentraxin PTX3 as a link among innate immunity, inflammation, and female fertility. J LeukocBiol 2006; 79:909–912
12. Inoue K, Kodama T, Daida H. Pentraxin 3: A novel biomarker for inflammatory cardiovascular disease. Int J Vasc Med 2012;2012:657025
13. Hudzik B, Szkodzinski J, Pietka‑Rzycka A, Danikiewicz A, Wojnar R, Lekston A, et al. Plasma pentraxin 3 may be a more sensitive marker of inflammatory response than high‑sensitivity C‑reactive protein after bare metal stent compared to drug‑eluting stent implantation. J Interferon Cytokine Res 2013; 33:280–2
14. Zhou, W. and Hu, W., 2016. Serum and vitreous pentraxin 3 concentrations in patients with diabetic retinopathy. Genetic testing and molecular biomarkers, 20(3), pp.149-153.
15. Mutlu, M., Yuksel, N., Takmaz, T., Dincel, A.S., Bilgihan, A. and Altınkaynak, H., 2017. Aqueous humor pentraxin-3 levels in patients with diabetes mellitus. Eye, 31(10), pp.1463-1467.
16. Takashi, Y., Koga, M., Matsuzawa, Y., Saito, J., Omura, M. and Nishikawa, T., 2018. Circulating pentraxin 3 is positively associated with chronic hyperglycemia but negatively associated with plasma aldosterone concentration. PloS one, 13(5), p.e0196526.
17. Nouh, M.Z., Sonbol, A. and Mogahed, M. Evaluation of pentraxin-3 level in patients with diabetic retinopathy. Menoufia Medical Journal, 2018, 31(3), p.928.
18. Alkubaisy,S.A., A.A. Majid, S.M. Abdulateef, F.A. Al-Bazy, O.K. Attallah, O.M. Abdualmajeed, Th. T. Mohammed, F.M. Abdulateef, K.I. Mahmud. Effects of In-Ovo injection of Biotin on chick's embryonic development and physiological traits. IOP Conference Series: Earth and Environmental Science.2021, 761(1), 012111.
19. Yang, H.S., Woo, J.E., Lee, S.J., Park, S.H. and Woo, J.M., 2014. Elevated plasma pentraxin 3 levels are associated with development and progression of diabetic retinopathy in Korean patients with type 2 diabetes mellitus. Investigative ophthalmology & visual science, 55(9), pp.5989-5997
20. Alrseetmiwe, D. S. .; Almayah, A. A. .; Nasser, A. A. .; Alnussairi, M.; Zadeh, H. A.; Mehrzi, F. A. . Cloning And Expression Of An Optimized Interferon Alpha 2b In Escherichia Coli Strain Bl21 (De3). Journal of Life Science and Applied Research. 2020, 1, 40-44.
21. Chodkowski, A., Nabrdalik, K., Kwiendacz, H., Tomasik, A., Bartman, W. and Gumprecht, J. Pentraxin 3 and retinopathy among type 2 diabetic patients in relation to carotid atherosclerosis and systolic and diastolic cardiac function—a pilot study. Clinical Diabetology, 2018, 7(4), pp.196-202.
22. Elbana KA, Salem HM, Abdel Fattah NR, Etman E. Serum Pentraxin 3 level as a recent biomarker of diabetic retinopathy in Egyptian patients with diabetes. Diabetes MetabSyndr. 2019 Jul-Aug;13(4):2361-2364.
Received: January 15, 2023 / Accepted: February 25, 2023 / Published:15 March 2023
Citation. Hassan , Z.H.; Hamdi , R.A.;Al.Mohammadaw, J.G. Basal. RH Assessment of Serum Pentraxin3 Level in Iraq Patients with and without Diabetic Retinopathy. Revis Bionatura 2023;8 (1) 88 http://dx.doi.org/10.21931/RB/2023.08.01.88
