2023.08.03.29
Files > Volume 8 > Vol 8 No 3 2023
The direct microscopic examination of somatic cell count to detect sub-clinical mastitis in cows of Diyala province
Middle Technical University, Technical Institute of Baqubah, Diyala-Iraq;
*Coresponding auhotr. [email protected]
Available from: http://dx.doi.org/10.21931/RB/2023.08.03.29
Background Udder Inflammation is still one of the most significant economic issues that may affect milk production.
Methods: Several tests were used to detect the pH of milk, such as the aerial mastitis test, Whiteside test, chloride test, Bovi-mastitis test (Card test), Somatic cell count, and bacterial culturing.
Results showed that 32% of cows suffered abnormalities in the consistency of milk, whereas 68% of cows passed through subclinical mastitis. Furthermore, the results also showed that 80 % of cows were affected by Staph. Aureus, compared to other bacterial species
Conclusions: According to our findings, subclinical and clinical mastitis is significant in Diyala Province, and infectious and environmental pathogens are involved. Moreover, locally manufactured Arial mastitis test, Whiteside test, and the imported Bovi card test are appropriate tests for the early diagnosis of subclinical mastitis in cattle. Staph. Aureus was more prevalent than other inflammatory infections and ambient pathogens in cases of bovine mastitis.
Keywords: Detection; mastitis clinical and sub-clinical; Dairy cows; Diyala Province.
INTRODUCTION
A parenchymal disease of the udder tissue is identified via various pathological alterations in the mammary cells and alterations in milk's physicochemical and biological composition 1. Mastitis falls into two main categories. The first is clinical mastitis, which presents as swelling, a rise in temperature, and pain in the udder or milk that indicates milk clotting being present, milk color change, and excessive counts of white blood cells in affected milk in animals. The other is sub-clinical mastitis, which manifests no apparent symptoms in the udder unless diagnostic tools are used. Mastitis is regarded as 1 of the more essential causes of loss of finances in milk production in animals globally because it affects both the quality and quantity of milk3. Lymphocytes, macrophages, polymorphonuclear cells, and some epithelial cells are examples of somatic cells, a component of the body's natural defense system (4). The California mastitis test) (CMT) allows for the quantitative measurement of (somatic cell count (SCC). A quick, easy, and Low-cost examination procedure is available for sub-clinical mastitis at dairy farms 5. old, milking stage, variance, time, fatigue, organization, daily difference, and primarily intramammary infection (IMI) are just a few factors that may influence SCC. Understanding the factors that may affect the number of somatic cells is necessary to accurately interpret somatic cell counts (6-8). Bacterial pathogens that can be divided into two groups typically cause inflammation of the udder9. Infectious microorganisms like Staph. Aureus, Mycoplasma bovis, Streptococcus agalactiae, and environmental pathogens like Streptococcus species (Streptococcus uberis and Streptococcus dysgalactiae). Additionally, there are environment-specific coliforms, which include gram-ve bacteria like Serratia, Pseudomonas, and Proteus, as well as Escherichia coli, Klebsiella species, Citrobacter species, Enterobacter species, Enterobacter faecalis & Enterobacter faecium 1.
MATERIALS AND METHODS
Area of Study
This research was done in Al-Khalis and Baquba district regions extending to the northeast of Baghdad. About 100 milk samples from 25 different crossbred dairy cows were collected for the study. These samples were divided into groups according to age (2.5 to 7 years), parity (one to four), and lactate phase (late and early).
Collection of samples:
After properly cleaning the teat surface with 70 percent ethanol, 100 milk samples were taken. 10 mL of milk were collected aseptically from each of the four quarters in separate, sterile polyethylene screw-capped containers 10. Specimen of milk was stored at four to ten C0 in a refrigerator for additional lab testing after being placed in an icebox and transported to the diagnostic lab of the veterinary hospital in Diyala Governorate.
Diagnostic tests
The following diagnostic tests were performed on milk samples from cows that appeared to be in good health:
Indirect Examination:
For the detection of milk pH, various tests, including the Arial mastitis test (AMT), Whiteside test (WST), chloride test (CT), and the Bovi-mastitis test (Card test), are used: It was prepared like the Surf field mastitis test (SFMT) according to (one) with a little alteration. Bromocresol purple was added to the three percent solution at a ratio of 1:10,000 after a basic washing ingredient weighing 3g (Arial) was added to a hundred ml of filtered regular water. One well is used to test each quarter of the cow on a four-well plastic paddle. Following that, the reaction was visually graded according to how much gel had formed:
Negative = no response trace = When the plate is rotating, streaks appear and can be seen as a trace.
1+ = remarkable thickening at rotating but no gel.
2+ = slight formation of gel to follow the rotating plate very slowly.
3+ = The solid gel that sticks to the plate base has formed.
The WST was carried out according to 11 by combining two drops of sodium hydroxide (4%) and five drops of milk on a dark.
Procedure
a- Mix the freshly collected milk samples thoroughly. Distribute ten μL of milk in a region (1 square cm2) on clear slides. Distribute milk equally. Do not heat the slide; dry it on a flat, horizontal surface. Using Newman- Lambert stain (Methylene-blue twelve g, ethyl alcohol ninety-five percent fifty-four ml, tetrachloroethane forty ml, and glacial acetic acid six ml). The benefit of the glass plate is to stir for 20 and display the degree of coagulation. The Chloride test was conducted according to 11.The yellow color was demonstrated in milk with an abnormally high chloride level (> 0.14 percent), showing a positive sample. As a result of the formation of silver chromate, the color was continuously red, meaning a negative sample. The Bovie Vet. Indicator paper was used to determine the pH of the milk sample (Figure 1).
Figure 1. Card-test 12
(VMED SUPPLY, INC). A Little drop of milk on the test sheet for the recording of spots' coloration. The company's recommendations are used to determine how pH and color change are related as follows: pH (6.6-6.7) pale green, (pH 6.8) moderates green, (pH 7.1) green, and (7.4) dark-blue green.
a-Direct examination of Somatic cell count (SCC).
This Staining will be discarding fat. Fix. Staining bacteria and WBCs.
b- Based on the stain's goodness, soak an air-dried smear for fifteen seconds to a minute.
c- Drying within the air.
d- Use the liquid to wash.
e- Dry within the air.
f- Check the field of immersion in oil for the appearance of bacteria and WBCs.
g- In 20–30 microscopic fields, count the WBCs (Figure 2).
No of WBCs/ ml milks=
A cell's numbers counted x microscopic agent (4 x 105). 10.
No. of fields examined
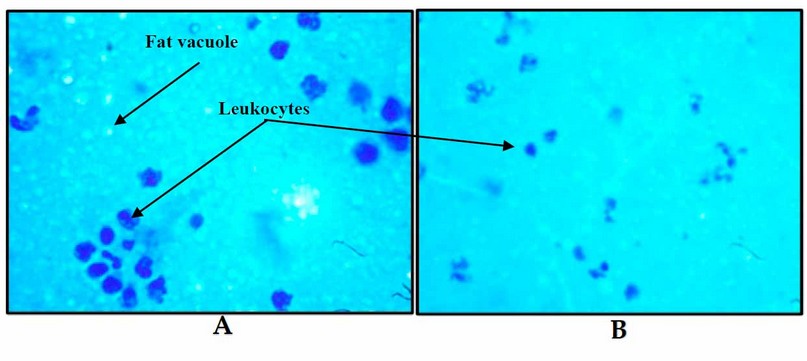
Figure 2. (A-B) Milk staining high somatic cells (WBCs) and vacuoles of fat (X 100)
H-Bacteriology:
Bacterial culture was performed according to 13. After being collected, milk specimens from both clinical and sub-clinical status streaked for 24 hours on (blood) and (Mac-Conkey) agars plates. They were examined after being cultured for twenty-four and forty-four hours at thirty-seven °C under anaerobic conditions. If there was no growth after 48 hours of incubation, the culture was regarded as negative. based on those sizes, form, pigment, and hemolytic properties, G-stain, and colony creation of catalase, bacteria on culture +ve plates were) recognized. Various biochemical tests were used for verification after subculturing and isolating different cultures 13.
RESULTS
All dairy cows had a clinical examination to determine whether or not they had reddish, suffering, swelling, a symmetrical or non-symmetrical udder, and abnormal in their (milk samples). Eight (32%) of 25 cows suffered abnormalities and inconsistency of milk in which clot, straw color, redness, and a non-symmetrical quarter of udder. When 68% of cows had sub-clinical mastitis, which doesn't manifest any symptoms in the udder unless diagnostic equipment is used. The test results revealed that indirect and direct examination shows a significant variation at (p < 0.05), as in Table 1.
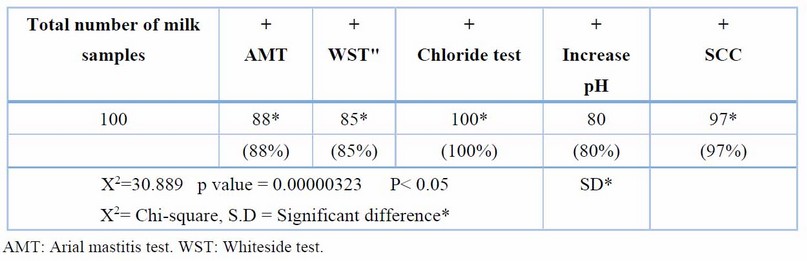
Table 1. Number and percentage of milk samples giving +ve reaction using the 4 tests
The present study shows that the age, breed, parties, and stage of lactation of cows affected SCC values, as in Table 2.
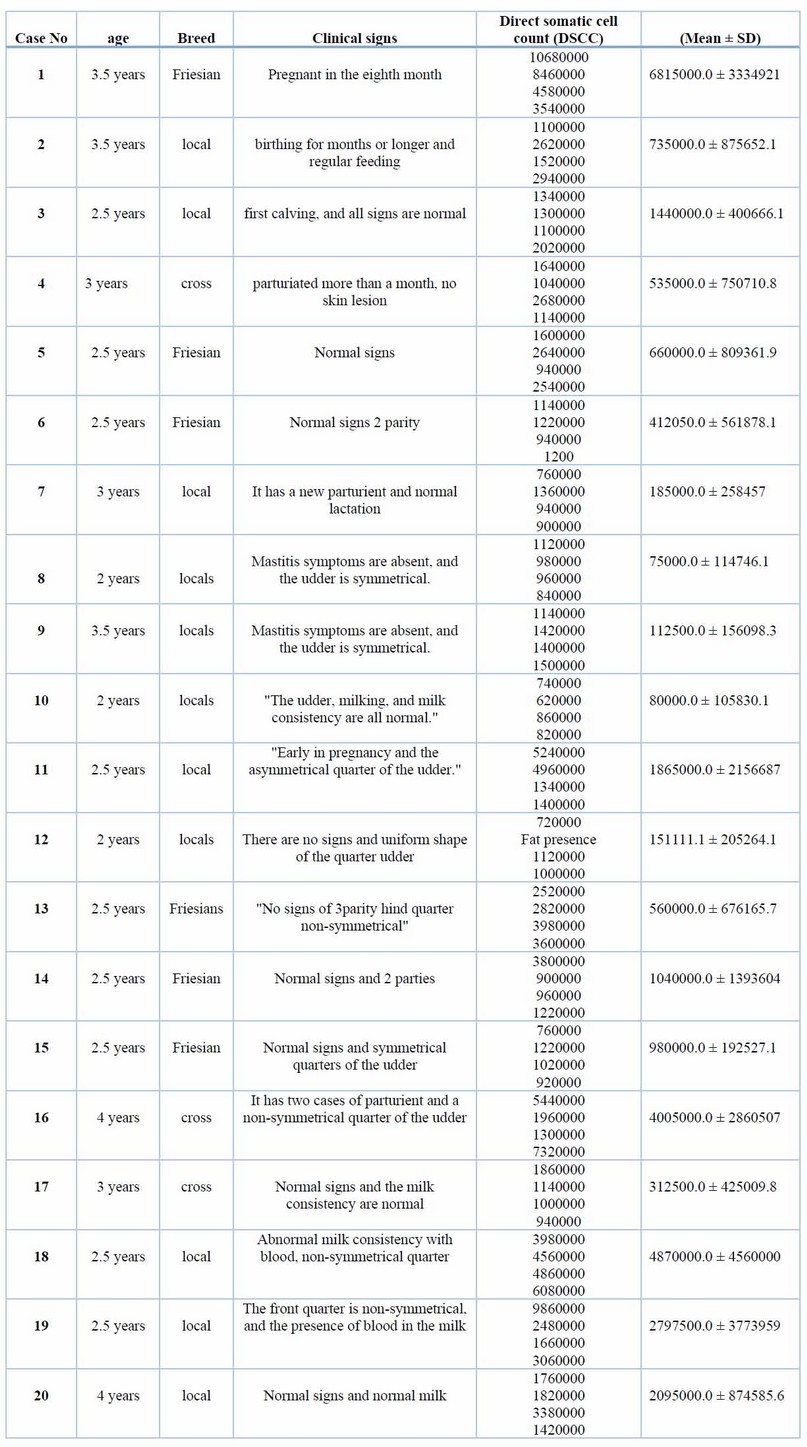
Table 2. Case history, ages, breed of cattle, and (DSCC) of milk specimens
Bacteriology
Milk samples from the clinical and sub-clinical areas were gathered for bacteriological culture. Table 3 shows 40 isolates were gram +ve and 19 gram-ve isolates.
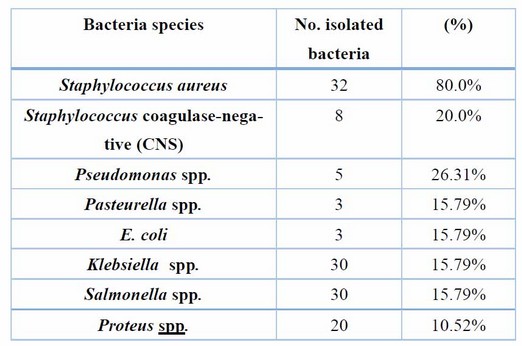
Table 3. Show the number and percentage of isolated bacteria
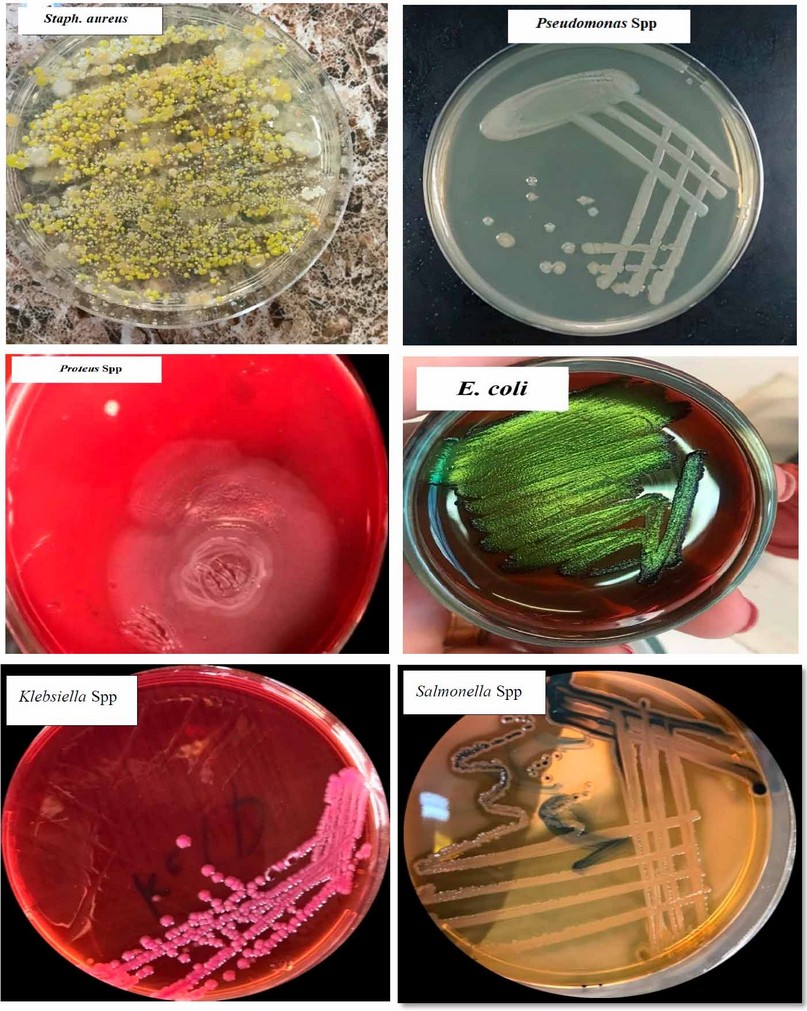
Figure 3. Morphological Characters of Isolated Colonies
Clinically, clinical mastitis cases recorded in the present study agree with another result (14). Sub-clinical mastitis is the most common in cattle raised in various Diyala Governorate regions. AMT and WST are recognized to be utilized as indirect processes for numeration or (count) WBCs into milk specimens on the farm and in the lab. Table 1 shows a strong relationship between the total affirmative responses to these two tests. This result was compatible with (15); they found that the Surf-mastitis tests (SMT), WST, and chloride -tests had the same sensitivity. The chloride test revealed that more chloride ions were present in all samples. There is variation in the standard level of chloride ions found in common milk specimens. Elango, Doraisamy, and Rajarajan Kumaresan 16) showed that healthy animal milk typically had a chloride concentration between 0.08 and 0.14 percent. While Sharma, SinghBhadwal 17 believed the usual milk sample's chloride concentration was 0.91 percent. The results of the chloride examination were in agreement with those obtained by (18), and they regarded them as an invaluable tool for identifying sub-clinical mastitis in buffaloes. Comparing multiple diagnostic tests for identifying subclinical mastitis revealed a 97 percent sensitivity for SCC, an 88 percent sensitivity for AMT, and an 85 percent compatibility with the result for WST reaction(19,20). The current research demonstrated that the SCC was the most accurate and closely matched the bacteriological studies. Direct microscopic testing of the somatic cell count was a more reliable technique to diagnose subclinical inflammation of the udder in milk production. The modifying California-mastitis tests (MCMT), the modifying White-side tests (MWST), and SCC were the three tests most effective at identifying sub-clinical mastitis (21). According to(22), CMT had more excellent dependability (85.69%) than MWST (79.74 percent). Dependent on the analysis data of SCC values in Table (2), the study shows SCC was affected by age and breed and increased as parity increased. These results are compatible with earlier studies (23-25) and revealed increased SCC for subsequent equivalencies. At younger and older ages, Haas 23) suggested that there was a different defense mechanism against udder infection. It does not matter if the cattle have an illness or if there is no SCC increases as lactation progresses and delayed feeding (8). The neutrophil ratio appears to rise in earlier and delayed breastfeeding, whereas the ratio of lymphocytes appears to be low (17). SCC often exceeds 1 million cells per ml before parturition and falls to 100,000 cells/ ml 7 to 10 days after delivery. In cases of disease or mammary infection, the SCC increased over the usual range. The complexity of sub-clinical mastitis and variations in farm management practices, lactation stage, parity, and breed may contribute to the variable results (26). Differences in farm management practices, phase of lactating, valence, generation, and old and intramammary diseases may all contribute to the elevation of SCC values shown in the current research of diseased cattle with sub-clinical mastitis; this result is compatible with other authors (25-28). The most common bacterium was S. aureus, as evidenced by the fact that it was isolated from 32 (57.14 percent) of the mammary glands with sub-clinical inflammation of the udder, whereas Staphylococcus. In comparison, coagulase-negative (CNS) were identified in eight (14,28 percent) specimens. The finding was in line with the majority of projects in cow inflammation of the udder, in which numerous researchers showed S. aureus was a more prevalent pathogen that separated a cow from the epidermis of the teat and teat-end, milk and the typical pathogens were separated (4,29,30). The result of (Koivula, Pitkälä, PyöräläMäntysaari 31) showed that 10.5 percent of cow milk specimens were contaminated with CNS and that Staph. Aureus was present in 36.8 percent of them, supporting the predominance of Staphylococcus spp. it indicated that (36.8%) of bovine milk samples had been infected with S.aureus and 10.5% with CNS, supporting the notion that Staphylococcus spp. Predominate. Predominate. In Baghdad, Staphylococcus epidermidis was the more prevalent pathogen in subclinical inflammation of the udder (53.84%), while Staph. Aureus was the more common pathogen in clinical inflammation of the udder (44.44%) (27). This research also showed Enterobacteriaceae in many cases of bovine mastitis, including E. coli 3 (5.20%), and Klebsiella spp. 3 (5.3%), Salmonella spp. 3 (5.3%) & Proteus spp. 1 (1.78%). These findings were consistent with those of other studies conducted in Iraq and other regions of the world (31-33).
According to our findings, subclinical and clinical mastitis is significant in Diyala Province, and both communicable and environmental pathogens are involved. Following cultural isolation, Somatic cell count was the most accurate test, followed by the Arial mastitis and Whiteside tests. Despite minor variations in the recorded scores, we concluded that using the locally manufactured Arial mastitis test, Whiteside test, and the imported Bovi card test is appropriate for the early diagnosis of subclinical mastitis in cattle. Staph. Aureus was more prevalent than other intramammary infections and ambient pathogens in cases of bovine mastitis.
Author Contributions:
AKA developed the research concepts, design, methodology, and data analysis and wrote the manuscript.
Funding:
This research received no external funding
Acknowledgments:
The author sincerely thanked Ibrahim Kadhim Ibrahim for their insightful comments and suggestions that helped significantly improve this research work.
Conflicts of Interest:
The authors declare no conflict of interest.
1. Radostits O, Gay C, Blood D, Hinchcliff K. Mastitis In: Veterinary Medicine, A Textbook of the Diseases of Cattle, Sheep, Pigs, Goats and Horses. Edn 9th publ Book power with Saunders, London 2000:611-613.
2. Cobirka M, Tancin V, Slama P. Epidemiology and classification of mastitis. Animals 2020;10:2212.
3. Donovan DM, Kerr DE, Wall RJ. Engineering disease resistant cattle. Transgenic research 2005;14:563-567.
4. Pillai S, Kunze E, Sordillo L, Jayarao B. Application of differential inflammatory cell count as a tool to monitor udder health—Journal of Dairy Science 2001;84:1413-1420.
5. Dingwell RT, Leslie KE, Schukken YH, Sargeant JM, Timms LL. Evaluation of the California mastitis test to detect an intramammary infection with a major pathogen in early lactation dairy cows. The Canadian Veterinary Journal 2003;44:413.
6. Sargeant J, Leslie K, Shirley J, Pulkrabek B, Lim G. Sensitivity and specificity of somatic cell count and California Mastitis Test for identifying intramammary infection in early lactation. Journal of dairy science 2001;84:2018-2024.
7. Pyörälä S. Indicators of inflammation in the diagnosis of mastitis. Veterinary research 2003;34:565-578.
8. Berglund I, Pettersson G, Östensson K, Svennersten‐Sjaunja K. Quarter milking for improved detection of increased SCC. Reproduction in Domestic Animals 2007;42:427-432.
9. Hettinga K, Van Valkenburg H, Lam T, Van Hooijdonk A. Detection of mastitis pathogens by analysis of volatile bacterial metabolites. Journal of Dairy Science 2008;91:3834-3839.
10. Buswell J. Simple mastitis bacteriology for the practice. In Practice 1995;17:426-432.
11. Coles E. Veterinary clinical Pathology 4th ed WB Saunders company Philadelphia. London, Toronto, Mexico, Riodejenario, Sydney, Tokyo & Hong Kong 1986:136-170.
12. Matei ST, Groza I, Bogdan L, Ciupe S, Petrean A, Andrei S. Researches regarding implementation of a computerized surveillance program of mammary gland in a dairy cow farm. Lucrări Științifice-Medicină Veterinară, Universitatea de Științe Agricole și Medicină Veterinară" Ion Ionescu de la Brad" Iași 2010;53:1066-1071.
13. Quinn P.J. MEaCGR. Clinical veterinary microbiology. London: Wolf Publishing, 1994.
14. Hashemi M, Kafi M, Safdarian M. The prevalence of clinical and subclinical mastitis in dairy cows in the central region of Fars province, south of Iran. 2011.
15. Harini H, Sumathi B. Screening of bovine milk samples for sub-clinical mastitis and antibiogram of bacterial isolates. Veterinary World 2011;4:358.
16. Elango A, Doraisamy K, Rajarajan G, Kumaresan G. Bacteriology of sub-clinical mastitis and antibiogram of isolates recovered from crossbred cows. Indian Journal of Animal Research 2010;44:280-284.
17. Sharma N, Singh N, Bhadwal M. Relationship of somatic cell count and mastitis: An overview. Asian-Australasian Journal of Animal Sciences 2011;24:429-438.
18. Guha A, Gera S, Sharma A. Evaluation of milk trace elements, lactate dehydrogenase, alkaline phosphatase and aspartate aminotransferase activity of subclinical mastitis as an indicator of subclinical mastitis in riverine buffalo (Bubalus bubalis). Asian-Australasian journal of animal sciences 2012;25:353.
19. Sudhan N, Singh R, Singh M, Soodan J. Studies on prevalence, etiology and diagnosis of subclinical mastitis among crossbred cows. Indian Journal of Animal Research 2005;39:127-130.
20. Goswami S, Roy A, Kalyani I. A comparative study on various indirect tests to direct cultural isolation for detection of subclinical mastitis (SCM). In: Proceedings of XXI Indian Society for Veterinary Medicine (ISVM) Conference 2003.
21. Neelesh S, Maiti S, Vijay P. Sensitivity of indirect tests in the detection of sub-clinical mastitis in buffaloes. Veterinary Practitioner 2008;9:29-31.
22. Patel P, Raval S, Rao N, Mandali G, Jani R. Status of mastitis in Gujarat State. In: Proceedings of Round Table Conference of the Indian Association for the Advancement of Veterinary Research (IAAVR) on Mastitis 2000;45-52.
23. Haas D. Somatic cell count pattern improvement of udder health by genetic and managementPhD thesis, University Wageningen, 2003.
24. Göncü S, Özkütük K. Factors effective at somatic cell count (SCC) in the milk of Black and White cows kept in intensive dairy farms at Adana province and their relationship with mastitis. J Anim Prod 2002;43:44-53.
25. Sederevičius A, Balsytė J, Lukauskas K, Kazlauskaitė J, Biziulevičius GA. An enzymatic cow immunity-targeted approach to reducing milk somatic cell count: 3. A comparative field trial. Food and agricultural immunology 2006;17:1-7.
26. Almaw G, Zerihun A, Asfaw Y. Bovine mastitis and its association with selected risk factors in smallholder dairy farms in and around Bahir Dar, Ethiopia. Tropical animal health and production 2008;40:427-432.
27. Eyduran E. Determination of milk somatic cell counts in dairy cattle. In: MSc. Thesis, Ankara University, Turkey, 2002.
28. Haltia L, Honkanen-Buzalski T, Spiridonova I, Olkonen A, Myllys V. A study of bovine mastitis, milking procedures and management practices on 25 Estonian dairy herds. Acta Veterinaria Scandinavica 2006;48:1-6.
29. Al-Kubaisi S. Clinical and subclinical mycotic mastitis and the sensitivity and specificity of California Mastitis Test for diagnosis of subclinical mastitis in ewes in Al–Fallouja city. 2008.
30. Al-Obaidy H. Comparative usage of local propolis formula (single and synergistic with antibiotic) with antibiotic group for treatment of clinical mastitis in ewes. In: M. Sc. Thesis, College of Veterinary Medicine, University of Baghdad, Iraq, 2010.
31. Koivula M, Pitkälä A, Pyörälä S, Mäntysaari EA. Distribution of bacteria and seasonal and regional effects in a new database for mastitis pathogens in Finland. Acta Agriculturae Scand Section A 2007;57:89-96.
32. Watts JL. Etiological agents of bovine mastitis. Veterinary microbiology 1988;16:41-66.
33. Beheshti R, Shaieghi J, Eshratkhah B, Ghalehkandi JG, Maheri-Sis N. Prevalence and etiology of subclinical mastitis in ewes of the Tabriz region, Iran. Global veterinaria 2010;4:299-302.
Received: 28 May 2023/ Accepted: 15 July 2023 / Published:15 September 2023
Citation: Kamil Awad A, The direct microscopic examination of somatic cell count to detect sub-clinical mastitis in cows of Diyala province. Revis Bionatura 2023;8 (3) 29. http://dx.doi.org/10.21931/RB/2023.08.03.29
