2023.08.04.81
Files > Volume 8 > Vol 8 no 4 2023
Isolation and Identification of Fungal Species from the Insect Pest Callosobruchus maculatus (F.)
Noor Akmoosh1*, Ekhlas Al-Shareefi2, Kawther Mohammed Ali3
1 University of Babylon, Babylon, Iraq; [email protected]; +9647818198773.
2University of Babylon, Babylon/ Iraq
3University of Babylon/ Babylon/ Iraq; [email protected]; +9647800351337
* Correspondence: [email protected]; Tel.: (+9647822114389)
Available from: Available from. http://dx.doi.org/10.21931/RB/2023.08.04.81
ABSTRACT
Cowpea seed beetle Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) is one of the most common and economically important pests of stored cereal products worldwide. Furthermore, these beetles can act as vectors for several fungal post-harvest diseases. The current research aimed to isolate the fungi associated with adults of C. maculatus (F.) (Coleoptera: Bruchidae); the present study aimed to isolate and identify fungi associated with adult insects and evaluate their potential as biocontrol agents against the cowpea beetle, C. maculatus. In this study, we collected C. maculatus adults from the Babylon local market and five regions (Hilah, Mahaweel, Al-Mashrou’, Al-Haswa and Al-Musayyab) with no history of insecticide exposure. A potato dextrose agar medium was used to isolate the fungi attached to the surfaces of adult beetles. By Morphological and microscopic examination, Molecular identification and Sequencing analysis, Twenty-seven genera of fungi were isolated and identified from adult C. maculatus, including Aspergillus, Penicillium, Candida spp, white mycelium, Curvularia, Cladosporium, Chaetomium, Stachybotrys, Rhizopus, Drechslera, Scopulariopsis, Paecilomyces, Mucor, Geotrichum candidum, Streptomyces, Sarocladium, Beauveria bassiana, Talaromyces varians, Sporothrix flocculasa, Pseudozyma flocculasa, and Isaria fumosorosea. The molecular weights of the PCR products for the fungi isolates ranged from 650-700 bp, and the amplified ITS1-5.8S-ITS4 rDNA region of indigenous entomopathogenic fungal isolates showed a high sequence similarity (99-100%) with B. bassiana and M. anisopliae deposited in the NCBIGenebank. The phylogenetic tree analysis of the ITS region sequences showed a high degree of similarity between the isolates under study, ranging from 96.5-100. However, there were differences in the sequences among clades, indicating genetic variation possibly due to mating or mutations in different environments. Our study suggests that the storage pest, C. maculatus, would be essential in spreading fungal contaminants and consequently increasing mycotoxin contamination in stored cowpeas.
Keywords: Callosobruchus maculatus, Isolation, Fungi.
INTRODUCTION
Controlling pests in stored grains is as economically important as increasing the crop yield because, unlike crop damage during the growing season, post-harvest damage of stored grains is not financially compensated. Fungi and animal pests are the primary culprits for damage to stored grains, globally estimated to be responsible for 20% of food losses and up to 40-50% in some developing countries 23. Cowpea seed beetle C. maculatus (F.) (Coleoptera: Bruchidae) is one of the most destructive pests on cowpea and other legumes growing in tropical and sub-tropical countries, both in fresh green crusts in fields and in stored seeds 21. The adults are not harmful. But, the larvae of this pest feed on cowpea V. unguiculata (L.), chickpea, Cicer arietinum L., lentil, Lens culinaris (Medik.), soybean, Glycine max (Mer.), and haricot beans, Phaseolus vulgaris (L.) 17. The female adults of C. maculatus lay their eggs in the fresh cowpeas before reaping in the field. The larvae, hatched from these eggs, bore into the cowpea seeds, developed by feeding the embryo of the seeds, and matured in storage conditions in just about a month 8. Therefore, the larvae can lead to both quantitative, due to grain weight loss caused by larvae feeding, and qualitative, due to product alterations such as loss of nutritious and aesthetic values, which increases the loss level in the cowpea grain mass 1, 19. Therefore, this pest requires great care due to the potential for severe damage.
Various researchers have isolated fungal species from insects—Burnside 6 isolated A. flavus, A. niger and P. corylophilum from bees. Gillian and Prest 10 and Gillian et al. 11 also isolated various fungi from bees, identifying the species A. niger, A. flavus, P. corylophilum, C. cladosporoides and Alternaria sp.
Recently, Rehner & Buckley 20 used two DNA regions, rDNA ITS-5.8s and elongation factor EF1-α, and compared 86 isolates of Beauveria from around the world and from several hosts. In addition to the original isolate from Scottish soil, they found a Swiss isolate labeled B. bassiana (ARSEF 1567) from a scolytid beetle grouped in B. Caledonia. Similarly, an isolate classified as B. amorpha (ARSEF 2251), also grouped in B. caledonica, was originally from a coleopteran insect in Brazil. This suggests that B. caledonica has the potential to be an insect pathogen 12.
Various researchers have isolated fungal species from insects—Burnside 6 isolated A. flavus, A. niger and P. corylophilum from bees. Gillian and Prest 10 and Gillian et al. 11 also isolated various fungi from bees, identifying the species A. niger, A. flavus, P. corylophilum, C. cladosporoides and Alternaria sp.
Recently, Rehner & Buckley 20 used two DNA regions, rDNA ITS-5.8s and elongation factor EF1-α, and compared 86 isolates of Beauveria from around the world and from several hosts. In addition to the original isolate from Scottish soil, they found a Swiss isolate labeled B. bassiana (ARSEF 1567) from a scolytid beetle grouped in B. Caledonia. Similarly, an isolate classified as B. amorpha (ARSEF 2251), also grouped in B. caledonica, was originally from a coleopteran insect in Brazil. This suggests that B. caledonica has the potential to be an insect pathogen 12.
MATERIALS AND METHODS
Samples Locations
Cowpea samples infested with cowpea seed beetle C. maculatus (F.) were collected from the local market in Babylon and for five regions (Hilah, Mahaweel, Al-Mashrou’, Al-Haswa and Al-Musayyab), with no history of exposure to insecticides.
Ready-made dextrose potato medium
According to the manufacturer’s instruction, this medium is prepared by suspending 39 gm of the medium in 1000 ml of distilled water with added 250 mg of chloramphenicol that prevents the growth of bacteria and sterilized by autoclave at 121°C and 15 lbs pressure. Soak for 20 minutes; after the end of the sterilization period, leave the beaker until it cools down to 50 °C, then pour the nutrient media into Petri dishes with a diameter of 9 cm and put them in the refrigerator until they are used for isolation, cultivation and reproduction of the fungi used in study 24.
Isolation of fungi from adult insects
To isolate pathogenic fungi from the insects, the insects are sterilized with ethyl alcohol (70%) in order to get rid of the fungus on the external surface for one minute, and then they are washed with distilled water and then sterilized with a solution of sodium hypochlorite NaOCl (1%) for 30 seconds, then washed with distilled water and placed on filter papers and then transferred by sterile forceps to the PDA medium at the rate of three replications, where five insects were placed In each repetition. The dishes were incubated at a temperature of 27 ± 2 for a period of5-7 days; after that, the fungal isolates were purified on a new nutrient medium by taking a 0.3 cm diameter disc from the edge of the fungal colonies and transferred this disc by a sterilized needle to the center of a plastic petri dish containing 20 mm of PDA medium, and the dishes were incubated at a temperature of 27 ± 2 for 5-7 days. After incubation and identification, the percentages of frequency and appearance of isolated fungi were calculated according to the following equation: -
Percentages of frequency = Number of isolates per species/ Total Number of isolates of all species * 100
Percentages of appearance = Number of appearance in each species of all samples/ Total Number of samples * 100
Morphological and microscopic examination
After appearance growth, colonies of fungi concerning color, shape and texture (Powdery, Granular, Cottony) as recorded pigments are examined on the colony surface and appearance on the foundation. Fungi isolates are examined microscopically, and the fingerprint of the fungi in the colony is taken by adhesive tape. Transparent adhesive tape touches the surface of the fungal colonies, and then the tape is on a glass slide containing a drop of lactophenol cotton blue. Slides were examined under magnification 10X, 40X, and 100X as described 30.
Molecular identification
Fungal genomic DNA was extracted from mycelia by using a Favorgen kit. The internal transcribed spacer with 5.8 s rDNA was amplified using ITS5/ITS4 universal primer for fungal isolates. The PCR mixture was prepared according to Table (1) and augmented on the current cycler PCR system (Labnet, USA) by conditions in Table (2).0; (Pitt and Hocking, 2013; Rai, 2016). The PCR products were run on 1.5% agarose gel, and electrophoresis was made at 70 V for 30 min. The gel was pre-stained with 0.05% ethidium bromide. The PCR bands were noticed by using an ultraviolet transilluminator.
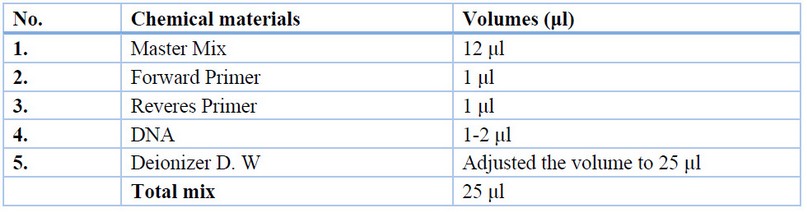
Table 1. PCR mixture.
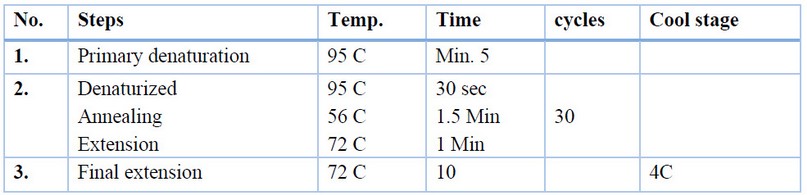
Table 2. PCR conditions
Sequencing analysis
Ten isolates of fungal species from this study PCR products are subjected to sequencing analysis. Direct sequencing analysis was performed on the 20 μl PCR product of AFU5S primer, sent to the Macrogen Laboratory in Korea. Afterward, the DNA sequencing data for different fungi isolates were compared with the gene bank using the NCBI Blast nucleotide database.
Phylogenetic tree
The phylogenetic tree is analyzed by using Mega version 6 software program with an unweighted pair group method with arithmetic mean (UPGMA) tree type based on sequences data of fungal species amplified by ITS5/ITS4 primer for 10 isolates.
RESULTS
Isolation of fungi from adult insects
The results indicate (table 3) that the highest occurrence of isolated fungi from C. maculatus was observed in the fungus A. niger, with a rate of 46.05%, followed by Penicillium sp. and A. flavus, which had occurrence rates of 12.91% and 12.33%, respectively. On the other hand, Aspergillus nidulans and Pseudozyma flocculate had the lowest occurrence rates, at 0.15% and 0.16%, respectively, among the other isolated fungi. The remaining fungi showed varying occurrence rates ranging from 0.22% to 5.16%.
The results also showed that the highest percentage of occurrence was found in the fungi isolated from C. maculatus males, specifically those isolated from the Al-Musayyib region (3.72%), followed by the Al-Hilla and Al-Mahawil regions, where the fungal presence was recorded at 3.70% in each. At the same time, the fungi isolated from C. maculatus female showed an occurrence rate of 3.69% among all insect samples collected from the study locations (Al-Musayyib, Al-Hassoah, Al-Mishrak, Al-Mahawil, and Al-Hilla). Among the fungi isolated from C. maculatus in this study, two fungi, namely B. bassiana and I. fumosorosea, were selected as biological agents for conducting further experiments.
The results also showed that the highest percentage of occurrence was found in the fungi isolated from C. maculatus males, specifically those isolated from the Al-Musayyib region (3.72%), followed by the Al-Hilla and Al-Mahawil regions, where the fungal presence was recorded at 3.70% in each. At the same time, the fungi isolated from C. maculatus female showed an occurrence rate of 3.69% among all insect samples collected from the study locations (Al-Musayyib, Al-Hassoah, Al-Mishrak, Al-Mahawil, and Al-Hilla). Among the fungi isolated from C. maculatus in this study, two fungi, namely B. bassiana and I. fumosorosea, were selected as biological agents for conducting further experiments.
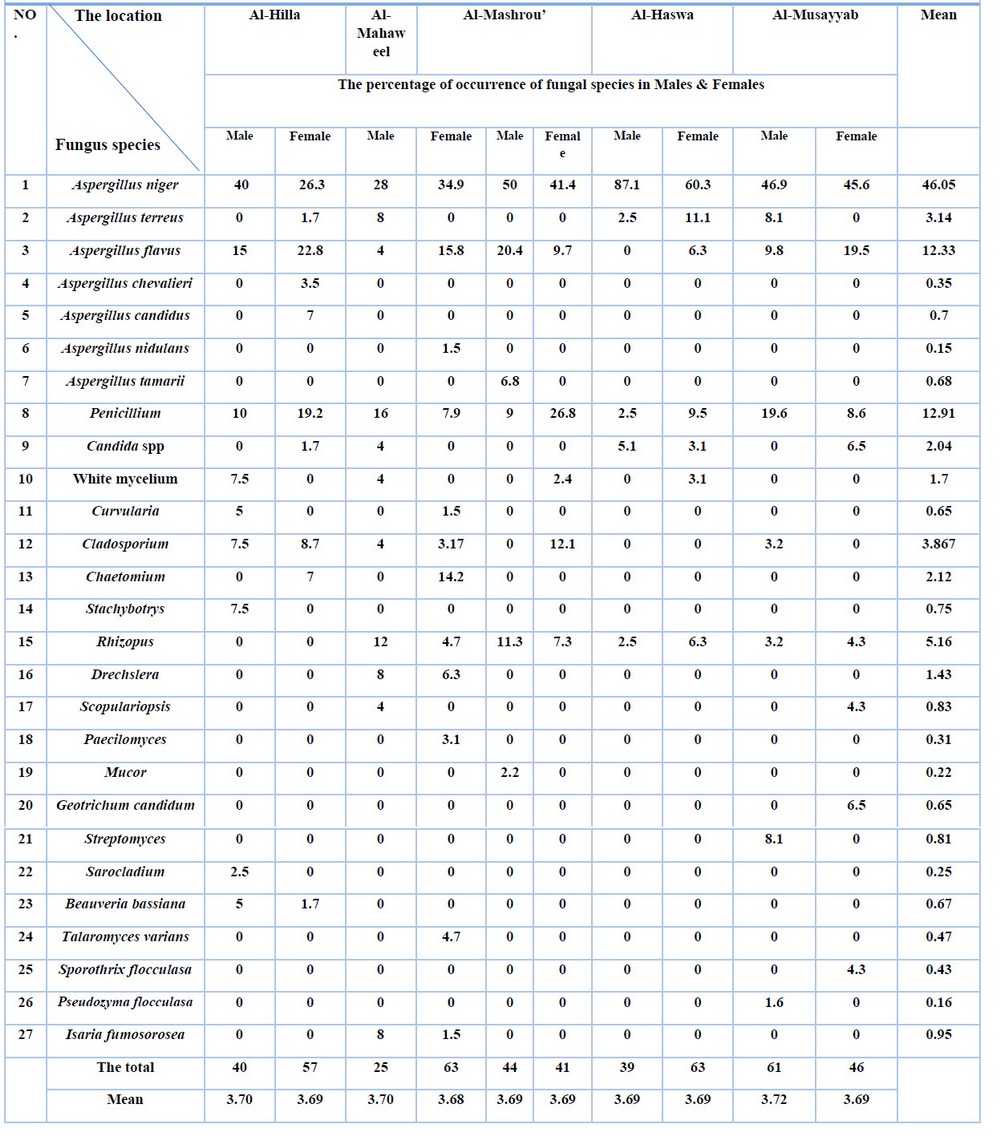
Table 3. Percentages for the emergence of fungal species in males and females of C. maculatus adults.
Molecular diagnosis of Fungi
DNA extraction and PCR assay
Ten isolates of fungi species in this study were subjected to DNA extraction. The ITS5 and ITS4 are universal primer pairs that targeted the sequences place of the ITS1-5.8S-ITS2 gene of the fungi isolates, which was used to discriminate fungi to the species level. The molecular weights of the PCR products for the fungi isolates under study ranged from 650-700 bp., as there were clear differences in the molecular weight of fungi species when ITS5 and ITS4 primers were used. The figure shows agarose gel electrophoresis of PCR products for fungi species.
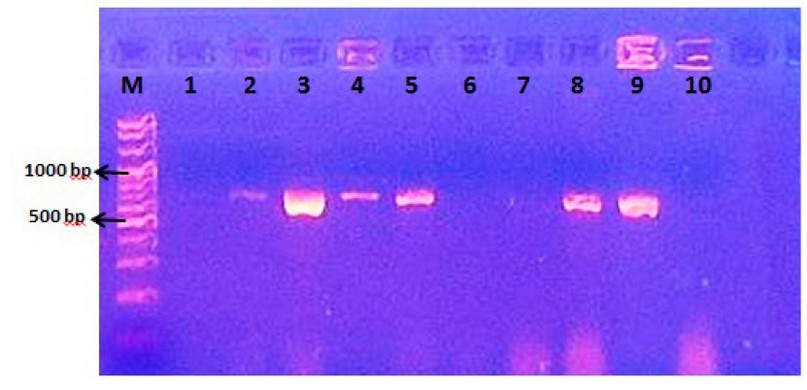
Figure 1. Agarose gel electrophoresis of PCR products for ITS1-5.8S-ITS2 gene of fungi species. Lane M= molecular marker 1500 bp.
Sequencing analysis
PCR was done for ten fungi isolates for the ITS region; direct sequencing analysis was performed on the 20 µl PCR product of the ITS region and was sent to the Macrogen Laboratory in Korea. After obtaining the sequence of the nitrogenous bases of the sent isolates, they are matched with the sequence of reference samples in the gene bank using the NCBI Blast Nucleotide Database to confirm the highest proportion of the genus and species name for each isolate.
Table (4) shows the results of molecular diagnosis for fungi isolates under study comparison with reference strains in NCBT by Telomorphe name, and the Anamorphe name offsets it. The Table showed that the molecular diagnosis using the nitrogenous bases sequence of some samples was identical to the phenotypic diagnosis using traditional laboratory methods except for several isolates, some of which have not been diagnosed with traditional methods to species level, which are diagnosed by molecular methods.
Table (4) shows the results of molecular diagnosis for fungi isolates under study comparison with reference strains in NCBT by Telomorphe name, and the Anamorphe name offsets it. The Table showed that the molecular diagnosis using the nitrogenous bases sequence of some samples was identical to the phenotypic diagnosis using traditional laboratory methods except for several isolates, some of which have not been diagnosed with traditional methods to species level, which are diagnosed by molecular methods.
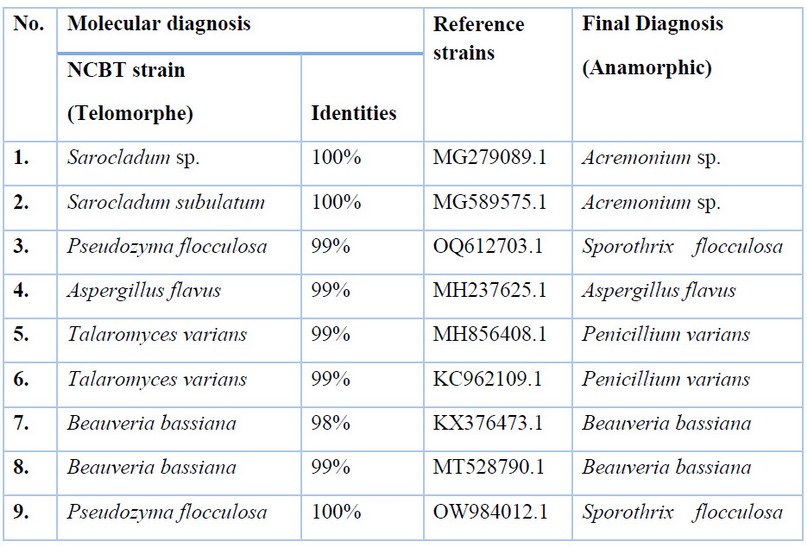
Table 4. Results of molecular diagnosis for fungi isolates under study comparison with reference strains in NCBT.
Phylogenetic tree
The phylogenetic tree was analyzed using Mega 6 software program with an unweighted pair group method with arithmetic mean (UPGMA) tree type based on sequences data of ITS region amplified by ITS5/ITS4 primers pair for 9 isolates. The results of phylogenetic tree analysis for entomopathogenic fungi were observed in five groups. T. varians, B. bassiana, P. flocculosa, A. flavus and Sarocladum sp. (Figure 2).
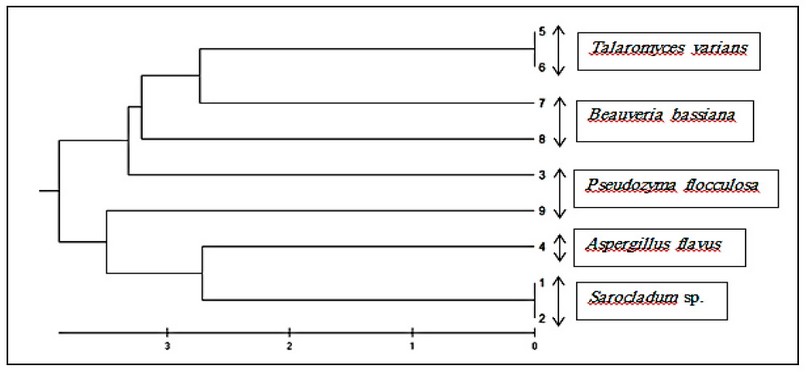
Figure 2. Phylogenetic tree based on ITS region sequences for Fungi isolates understudies.
DrawingDrawing the phylogenetic tree of the ITS region sequences for the isolates under study shows a high degree of similarity between species, ranging from 96.5-100 %. The difference in sequence among any clades shows approximately 0.0-3.5 %. The weighted pair group method with arithmetic mean (UPGMA) tree type was a common type of phylogenetic tree to determine the diversity of pathogenic fungi. Therefore, many studies have used this type of tree.
DISCUSSION
Isolation of fungi from adult insects
Twenty-seven genera of fungi were isolated and identified from adult C. maculatus, including Aspergillus, Penicillium, Candida spp, white mycelium, Curvularia, Cladosporium, Chaetomium, Stachybotrys, Rhizopus, Drechslera, Scopulariopsis, Paecilomyces, Mucor, Geotrichum candidum, Streptomyces, Sarocladium, Beauveria bassiana, Talaromyces variants, Sporothrix flocculasa, Pseudozyma flocculasa, and Isaria fumosorosea.
Most of them belonged to Aspergillus, from which seven species were isolated. The reason for this is that Aspergillus possesses small reproductive units in large numbers, allowing for long-distance dispersal and the ability to form specific structures to resist unfavorable environmental conditions for its growth 25. These findings are consistent with 26, who isolated several fungal genera from the mosquito Gx. quinquefasciatus, including Aspergillus, Fusarium, Penicillium, and Trichoderma. These results also agreed with 23, who isolated several fungal genera from the Tribolium castaneum, including Aspergillus spp., Cladosporium spp., Hyphopichia burtonii, Penicillium spp., Mucor spp., Rhizopus spp., Cephaliophora spp., Alternaria alternate, Monascus sp., Fusarium, Nigrospora, Beauveria, Chaetomium, Coprinellus, Irpex, Lichtheimia, Trichoderma, Byssochlamys, Cochliobolus, Cunninghamella, Mortierella, Polyporales, Rhizomucor and Talaromyces.
Among the fungi isolated from C. maculatus insects, two fungi, Beauveria bassiana and Isaria fumosorosea, were found. These fungi were utilized as biological agents in this study for insect control, as they have been used to combat various insects.
The selection of B. bassiana in the current study agreed with Ozdemir et al. 27, who used B. bassiana in combating the insect C. maculatus. Additionally, I. fumosorosea was chosen to control the Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae), which is consistent with the findings of the current study on biological control.
Molecular diagnosis of Fungi
The molecular weights of the PCR products for the fungi isolates under study ranged from 650-700 bp. As there are clear differences in the molecular weight of fungi species by ITS5 and ITS4 primer were used, this agrees with most references that used ITS1-5.8S-ITS2 region of fungal rDNA by ITS5 and ITS4 primer 22.
While there are several references were used for ITS1 and ITS4 primer for ITS1-5.8S-ITS2 region of fungal rDNA to the identification of entomopathogenic fungi [5,18,4] were isolated twenty-one entomopathogenic fungi, the resulting from the electrophoresis of the PCR product using the universal primer for the amplification of ITS rRNA gene of bands with a size of 650 bp. Also, Gebremariam et al. 9 used ITS1 and ITS4 primers to identify Beauveria bassiana and Metarhizium anisopliae with a band size of 545 bp. for two species. Another study 15, for the first time, used the primer binding site (PBS) marker system to discriminate among the entomopathogenic fungi species successfully.
The PCR sequencing of the ITS region of rRNA is currently regarded as the standard method for phylogenetic analyses and identification of fungal species. It provides an excellent tool for identifying fungi species that do not present typical morphological characteristics 28,14.
Some studies used sequence analysis of ITS region by using ITS1 and ITS4 primers or ITS5 and ITS4 primers for identification of entomopathogenic fungi such as Imoulan et al.,13 were isolated and identified several species of Beauveria, Species identification using only ITS region of rDNA as a DNA barcode reached its resolution limit within Beauveria. While Lu et al.,16 four entomopathogenic fungi strains were isolated and identified as Lecanicillium attenuatum, Beauveria bassiana, Lecanicillium longisporum, and Akanthmyces lecanii based on rDNA-ITS sequence analysis. Also, Yang et al.,22 isolated four genes of entomopathogenic fungi from soil samples collected from different localities of southern China as Beauveria bassiana, Cordyceps fumosorosea, Aspergillus nomius and Akanthmyces attenuatus.
Results of the phylogenetic tree coincided with several recent studies that show a high degree of similarity and homogeneity in the ITS region among entomopathogenic fungi. Al-Shindah et al.,29 isolated twenty-one different fungal isolates from samples of infected insects from different areas in Salah El-Din Governorate in Iraq and showed the percentage of similarity between the isolates of entomopathogenic fungi and their conformity with the globally registered fungal species in NCBI. The similarity ratio reached 97.51 - 99.80% with the globally registered strains, confirming the diagnosis’s accuracy. While Gebremariam et al.,9 used sequences of ITS1-5.8S-ITS4 rDNA region of all indigenous entomopathogenic fungal isolates showed 99–100% sequence similarity with B. bassiana and M. anisopliae deposited in NCBI/Genebank.
Although there is a high similarity to the fungi isolates in this study in matching the nucleotide sequences, they are genetically different isolates because the match was not 100%. This may be due to matings or mutations that occur in fungi due to their presence in different environments 2,3.
CONCLUSIONS
Twenty-seven genera of fungi were isolated and identified from adult C. maculatus, including Aspergillus, Penicillium, Candida spp, white mycelium, Curvularia, Cladosporium, Chaetomium, Stachybotrys, Rhizopus, Drechslera, Scopulariopsis, Paecilomyces, Mucor, Geotrichum candidum, Streptomyces, Sarocladium, Beauveria bassiana, Talaromyces variants, Sporothrix flocculate, Pseudozyma flocculate, and Isaria fumosorosea
The molecular weights of the PCR products for the fungi isolates ranged from 650-700 bp, and the amplified ITS1-5.8S-ITS4 rDNA region of indigenous entomopathogenic fungal isolates showed a high sequence similarity (99-100%) with B. bassiana and M. anisopliae deposited in the NCBIGenebank. The phylogenetic tree analysis of the ITS region sequences showed a high degree of similarity between the isolates under study, ranging from 96.5-100. However, there were differences in the sequences among clades, indicating genetic variation possibly due to matings or mutations in different environments
Two fungi, Beauveria bassiana and Isaria fumosorosea, were selected as biological agents based on their occurrence rates in male and female Callosobruchus maculatus adults.
Funding: This research received no external funding
Data Availability Statement: Data Availability Statements in the “Bionatura Research Data Policies” section at https://www.revistabionatura.com/policies.html.
Acknowledgments: The authors would like to thank all the Advanced Mycology Laboratory at the College of Science for Women / University of Babylon for their collaboration.
Conflicts of Interest: The authors declare no conflict of interest.
REFERENCES
1. Al Shareefi, E., & Cotter, S. C. (2019). The nutritional ecology of maturation in a carnivorous insect. Behavioral Ecology, 30(1), 256-266.
2. Al-Abedy A.N.; Abu Dakka A.B.; Al-Ghazali N.A. and Ali U.A. (2018). Molecular diagnosis of isolates belonging to the fungi Rhizoctonia, Fusarium Pencillium tardochrysogenum, Fusarium solani and Verticilliodes isolated from the roots of some tomato plants lycopersicum solanum. Karbala Journal of Agricultural Sciences). 5(2).
3. Al-Jawer Z.W.; Ahmed K.D. and Al-Shukurji M.A. (2018). Molecular identification of local isolates of the fungus Aureobasidium pullulans. The Journal of Mesopotamian Sciences, 27 (4) / Issue of the Third Conference on Life Sciences from 126-115.
4. Al-Shindah1R.S.D.; Hassan A.A. and Mansour M.S. (2022). Isolation and Identification of Entomopathogenic Fungi from Green Peach Aphid Myzus Persicae and Evaluation of Their Activity for Insect Control. IOP Conference Series: Earth and Environmental Science.
5. Bich G.A.; Castrillo M.L.; Kramer F.L.; Villalba L.L. and Zapata P.D. (2021). Morphological and Molecular Identification of Entomopathogenic Fungi from Agricultural and Forestry Crops. Floresta e Ambiente; 28(2): 1-11.
6. Al-Abedy A.N.; Kadhim J.H; Abdalmoohsin R.G; Al-Taey DK. Genetic diversity of Tomato yellow leaf curl virus isolates and the effect of the virus on the hormone content of tomato (Solanum lycopersicum) plants. Research on Crops. 2021;22(2):347-55.
8. Fox, C. W., & Tatar, M. (1994). Oviposition substrate affects adult mortality, independent of reproduction, in the seed beetle Callosobruchus maculatus. Ecological Entomology, 19(2), 108-110.
9. Gebremariam A.; Chekol Y., and Assefa F. (2021). Phenotypic, molecular, and virulence characterization of entomopathogenic fungi, Beauveria bassiana (Balsam) Vuillemin, and Metarhizium anisopliae (Metschn.) Sorokin from soil samples of Ethiopia for the development of mycoinsecticide. Heliyon, 7.
10. Gilliam, M., & Prest, D. B. (1972). Fungi isolated from the intestinal contents of foraging worker honey bees, Apis mellifera. Journal of Invertebrate Pathology, 20(1), 101-103.
11. Gilliam, M., Prest, D. B., & Morton, H. L. (1974). Fungi were isolated from honey bees, Apis mellifera, fed 2, 4-D and antibiotics. Journal of invertebrate pathology, 24(2), 213-217.
12. Glare, T. R., Reay, S. D., Nelson, T. L., & Moore, R. (2008). Beauveria caledonica is a naturally occurring pathogen of forest beetles. Mycological Research, 112(3), 352-360.
13. Imoulan A.; Hussain M.; Kirk P.M.; El Meziane A. and Yao Y. (2017). Entomopathogenic fungus Beauveria: Host specificity, ecology and significance of morpho-molecular characterization in accurate taxonomic classification. Journal of Asia-Pacific Entomology, 20 (4): 1204-1212.
14. Kawasaki M (2011). Verification of a taxonomy of dermatophytes based on mating results and phylogenetic analyses. Medical Mycology 52:291–295.
15. Kushiyev R.; Tunçer C.; Özdemir I.O.; Erper I.; Kalendar R.; Alkan M. and Özer GT. (2022). Molecular Characterization of Native Entomopathogenic Fungi from Ambrosia Beetles in Hazelnut Orchards of Turkey and Evaluation of Them in Vitro Efficacy. Insects 13:1-17.
16. Lu Q.; Wang P.; Ali A. and Zang L.S. (2022). Molecular Identification and Virulence of Four Strains of Entomopathogenic Fungi Against the Whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). Journal of Economic Entomology, 115, (3): 731–738.
17. Mahfuz, I., & Khalequzzaman, M. (2007). Contact and fumigant toxicity of essential oils against Callosobruchus maculatus. University Journal of Zoology, Rajshahi University, 26, 63-66.
18. Mohammed A.A.; Ahmed F.A.; Younus A.S.; Kareem A.A. and Salman A.M. (2022). Molecular identification of two entomopathogenic fungus Clonostachys rosea strains and their efficacy against two aphid species in Iraq. Journal of Genetic Engineering and Biotechnology, 20 (67): 1-8.
19. Khudai M Y, Abdulateef S M, Mouhammed T Th, Alamili H S. Use of modern geometric design of fish ponds to increase welfare and blood parameters. Revis Bionatura 2023;8 (2) 82. http://dx.doi.org/10.21931/RB/2023.08.02.82
20. Rehner, S. A., & Buckley, E. (2005). A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia, 97(1), 84-98.
21. Singh, S. R., & Emden, H. V. (1979). Insect pests of grain legumes. Annual Review of Entomology, 24(1), 255-278.
22. Suwaid, A. H. .; Rashid, M. A. .; Taha, M. M. . Genetic Analysis For Combining Ability And Estimation Of Some Genetic Parameters Of Yield And Its Components In Maize Using Half Diallel Cross. Journal of Life Science and Applied Research. 2020, 1, 60-64.
23. Yun, T. S., Park, S. Y., Yu, J., Hwang, Y., & Hong, K. J. (2018). Isolation and identification of fungal species from the insect pest Tribolium castaneum in rice processing complexes in Korea. The plant pathology journal, 34(5), 356.
24. Griffith, G. W., Easton, G. L., Detheridge, A., Roderick, K., Edwards, A., Worgan, H. J., ... & Perkins, W. T. (2007). Copper deficiency in potato dextrose agar causes reduced pigmentation in cultures of various fungi. FEMS microbiology letters, 276(2), 165-171.
25. Samson, R. A., Evans, H. C., & Latge, J. P. (1988). Atlas of entomopathogenic fungi Springer. Verlag., Berlin, Heidelberg, New York, 1-187.
26. Govindarajan, M., Jebanesan, A., & Reetha, D. (2005). Larvicidal effect of extracellular secondary metabolites of different fungi against the mosquito, Culex quinquefasciatus Say. Tropical biomedicine, 22(1), 1-3.
27. Ozdemir, I. O., Tuncer, C., Erper, I., & Kushiyev, R. (2020). Efficacy of the entomopathogenic fungi; Beauveria bassiana and Metarhizium anisopliae against the cowpea weevil, Callosobruchus maculatus F. (Coleoptera: Chrysomelidae: Bruchinae). Egyptian Journal of Biological Pest Control, 30(1), 1-5.
28. Cafarchia, C., Iatta, R., Latrofa, M. S., Gräser, Y., & Otranto, D. (2013). Molecular epidemiology, phylogeny and evolution of dermatophytes. Infection, Genetics and Evolution, 20, 336-351.
29. Al-Shindah, R. S., Hassan, A. A., & Mansour, M. S. (2022, July). Isolation and Identification of Entomopathogenic Fungi from of Green Peach Aphid Myzus Persicae and Evaluation of Their Activity for Insect Control. In IOP Conference Series: Earth and Environmental Science (Vol. 1060, No. 1, p. 012093). IOP Publishing.
30. Frisvad, J. C., Thrane, U., Samson, R. A., & Pitt, J. I. (2006). Important mycotoxins and the fungi which produce them. Advances in food mycology, 3-31.
Received: 26 September 2023 / Accepted: 15 April 2023 / Published: 15 December 2023
Citation: Akmoosh N., Al-Shareefi E., Kawther Mohammed Ali. Isolation and Identification of Fungal Species from the Insect Pest Callosobruchus maculatus (F.). Revis Bionatura 2023;8 (4) 81. http://dx.doi.org/10.21931/RB/2023.08.04.81
Publisher’s Note: Bionatura stays neutral concerning jurisdictional claims in published maps and institutional affiliations.
Copyright: © 2023 by the authors. Submitted for possible open-access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
