2023.08.01.36
Files > Volume 8 > Vol 8 No 1 2023
Evaluating the clinical significance of RBP4, PAI-1, and some trace elements in women with Polycystic Ovary Syndrome
Adnan J. M. Al-Fartosy 1 , Nadhum Abdul Nabi Awad 2
, Nadhum Abdul Nabi Awad 2 and Amel Hussein Mohammed 3
and Amel Hussein Mohammed 3
1 Department of Chemistry, College of Sciences, University of Basrah, Basrah, Iraq ;
[email protected]. ORCID; https://orcid.org/0000-0002-0681-6452
2 Department of Chemistry, College of Sciences, University of Basrah, Basrah, Iraq ;
[email protected]. ORCID; https://orcid.org/ 0000-0001-5639-748x
3 Al-Kunooze University College, Basrah, Iraq ;
[email protected]. ORCID; https://orcid.org/ 0000-0002-0681-6452
*Correspondence: [email protected]; Tel.: 00964 771 258 2817
Available from: http://dx.doi.org/10.21931/RB/2023.08.01.36
ABSTRACT
To assess and compare clinical, hormonal, and metabolic factors with blood levels of RBP4, PAI-1, and trace elements in women with and without polycystic ovarian syndrome (PCOS). A cross-sectional clinical investigation was undertaken. From December 2020 until January 2022, samples were taken at the Basrah Hospital for Obstetrics and Children's infertility center. Significant changes (p< 0.05) were in HOMA-IR, E2 and Ts. Levels of PAI-1, RBP4, AMH, LH, LH/FSH, PRL and Cu were significantly (p<0.01) increased, and levels of Se, Zn, Mg and E2/T were significantly (p<0.01) decreased, between the patient (1o PCOS and 2o PCOS) and control groups, the QUICKI level did not differ significantly (p>0.05). Compared to the control group, FSH levels were especially (p<0.05) higher in non-obese PCOS patients and lower in obese PCOS patients. Area under the receiver operating characteristics (ROC) curve (AUC) results indicate RBP4 and PAI-1 may be more effective predictors biomarkers for PCOS in expectant women. While trace elements might be considered a protective factor in the emergence of PCOS, metabolic abnormalities and IR in PCOS-affected individuals are associated with the levels of RBP4 and PAI-1, which appear to be a more acceptable diagnostic marker in the early prediction of PCOS.
Keywords: Polycystic Ovary Syndrome, RBP4, PAI-1, Trace elements.
INTRODUCTION
A diverse hormonal and metabolic condition with only a partially understood pathophysiology, polycystic ovarian syndrome (PCOS) is the most prevalent endocrinopathy among women of reproductive age, with a frequency of up to 15%. In contrast, patients with PCOS who lost 5-7 % of their basal weight showed improvements in insulin resistance, hyperinsulinemia, and hyperandrogenism, which appear to be key factors in at least some causes of PCOS pathogenesis. Therefore, the pathogenesis of PCOS in women may still be largely unknown. Still, it has primarily been linked to increased anti-Mullerian hormone (AMH) levels brought on by oligo-ovulatory cycles or a folliculogenesis disorder, which results in increased preantral and small antral follicle counts. 1
Insulin resistance is a biological misunderstanding in which the body's insulin hormone receptors on cell membranes do not respond to insulin as intended, preventing blood glucose from entering cells and causing a hypoglycemic reaction. Reduced insulin hormone's capacity to control and signal changes in glucose levels in the blood may lead to insulin resistance due to the pancreas pumping out high insulin dosages to get the glucose out of circulation and into cells. 2
Whether PCOS or concomitant obesity is to blame for insulin resistance is still debatable. Any adipose tissue dysfunction may be the primary source of the observed IR and, as a result, the metabolic and cardiovascular effects of the illness. Additionally, some studies suggest that PCOS may cause changes in adipocyte function that affect adipokine release. 1
Retinol-binding protein 4 (RBP4) is an adipokine released by the liver and adipose tissue and is a member of the lipocalin protein family. Its primary function is transporting vitamin A (retinol) from the liver to the peripheral tissues. By attaching to cell surface receptors or acting through retinoic acid on retinoic acid receptors and retinoic acid-X receptors, RBP4 can affect peripheral tissues. 3 Human plasminogen activator inhibitor-1 (PAI-1) is an inhibitor of tissue-type and urokinase-type plasminogen activators (tPA and uPA), which turn plasminogen into plasmin. Because of its capacity to suppress the fibrinolytic activity of tissue-type plasminogen activator (tPA), which produces active plasmin from plasminogen and subsequently eliminates fibrin, PAI-1 is a key regulator of the endogen. Also, it is an essential member of the serine protease inhibitor superfamily, known as Serpin E-1; synthesized by many tissue and cell types, free PAI-1 is relatively inactive in its free form and readily converts into its latent state. 4
The present study examined and compared the association of insulin resistance with mentioned adipokines and trace elements as a clinical predictor for the development of POCS among obese and non-obese women in Basrah province (southern Iraq).
MATERIALS AND METHODS
Study Design and Subjects Recruitment
This study is a clinical case-control trial. Samples were collected between December 2020 and the end of January 2022 from the "infertility center" at Basra Hospital for Obstetrics and Children in Basra Governorate, Iraq. Patient samples from a private clinic were also obtained. Women patients with polycystic ovarian syndrome totaled 124 individuals (PCOS) 60 patients with primary (couples with no prior pregnancies for at least a year after marriage; 28 were obese, and 32 were non-obese); 64 women with secondary (previously pregnant couples) but the pregnancy may not have been successful due to miscarriage, ectopic pregnancy, and other reasons; 31 obese and 33 non-obese); and 56 normal ovulatory women (fertile women presenting at the hospital with a genital prolapsed and history of at least. According to the American College of Obstetricians and Gynecologists criteria, the patients are already PCOS women. 5
Inclusion Criteria
The participants were all from Basrah (Souther of Iraq). The sample populations consist of married women who have been childless for one year and have not used contraception while living with their husbands. Volunteers who voluntarily volunteered to take part in the study served as the controls. It had regular menstrual cycles (26–30 days), had not taken oral contraceptives for at least the previous three months, and showed no symptoms of PCOS or other clinical indicators of hyperandrogenemia. Participants aged 18 to 45 for patients and 20 to 45 for healthy controls. Each participant signed a consent form after receiving complete information.
Exclusion Criteria
Diseases that affected the metabolic status and research eligibility were exclusion criteria. Women with endometriosis, uterine fibroids, breast cancer, epilepsy, migraines, and hormone-dependent cancers were not allowed to participate. Additionally, patients receiving estrogen replacement treatment and those with hyper- and hypothyroidism, diabetes, psychiatric illnesses, and significant diseases involving heart, liver, and kidney malfunction were eliminated.
Samples Preparation
After a 12-hour overnight fast and 30 minutes of rest in the supine position, the morning blood samples (10 ml) were taken. All samples were taken between days two and three of the menstrual cycle and separated into two parts. The first part (1 ml) was placed in polypropylene tubes containing EDTA and gently shaken to be utilized to determine Se concentration. The remaining material was transferred to an untreated plain tube, which could clot for 30 minutes at room temperature. The serum was extracted from the blood by spinning it at 402 x g for 10 minutes after it had clotted. The recovered serum was utilized right away to identify the study's variables, while the rest was kept in deep freezing at (-20 ºC) until it was used.
Biochemical Quantification
Blood samples from the controls and PCOS patients were examined for biochemical markers using the following protocols: Body mass index was calculated as the following formula [BMI (kg/m2) = Wt in kg / Ht in m2]. 6 The (Abnova-KA0831/Taiwan) kit was used to estimate serum glucose. The quantitative insulin sensitivity check index [QUICKI=1-(Log (insulin, μIU/mL) + Log (glucose, mg/dL)] and the homeostasis model assessment [HOMA-IR=insulin (μIU/mL) × glucose (mg/dL)/405] were used to estimate insulin resistance (IR). 7 The Insulin was estimated by kit (Abnova-KA0921/Taiwan), (BT-Lab, Shanghai- E1206Hu/China) kit was used to measure serum RBP4 level, and the (BT-Lab, Shanghai- E1159Hu/China) kit was used to measure serum PAI-1 level. The anti-Mullerian hormone was estimated by kit (E-EL-H0317/USA), Follicle stimulating hormone was estimated by kit (Abnova-KA0213/Taiwan), the Luteinizing hormone was estimated by kit (Abnova-KA0214/Taiwan), Testosterone was estimated by kit (Abnova- KA0236/Taiwan), and Estradiol was estimated by kit (Abnova-KA0234/Taiwan), Prolactin was estimated by kit (Abnova- KA0217/Taiwan). All of ELISAs kits were solid phase based on the sandwich principle. While serum copper (Cu) was measured using the 1TAA500-PG flameless AAS (PG Instruments, Leicestershire, England), serum magnesium zinc (Zn) and (Mg) concentrations were measured using the GBC 933 Plus flame atomic absorption spectrometry (AAS) (GBC, Braeside, Australia), and selenium (Se) in whole blood was measured using the hydride generation method AAS (PG Instruments, Leicestershire, England). 8,9
Statistical analysis
Statistical analysis was performed using SPSS version 26 (IBM Corporation, Armonk, NY, USA). The data were distributed normally, and the comparison between groups was analyzed using the analysis of variance followed by Dunnett's t-test to find the statistical significance. The ROC curve, which is formed by graphing sensitivity (y-axis) against 1- specificity (x-axis) and calculating the area under the curve (AUC), was used to calculate the sensitivities and specificities, as well as the 95% confidence interval. p< 0.05 was considered statistically significant, p< 0.01 highly substantial, and an AUC value near 0 (or 1) implies a strong diagnostic value; the values of one group are mainly greater (or lower) than the values of the comparison group in this circumstance.
RESULTS
Table 1 summarizes the overall demographics of all the women volunteers who participated in this work.
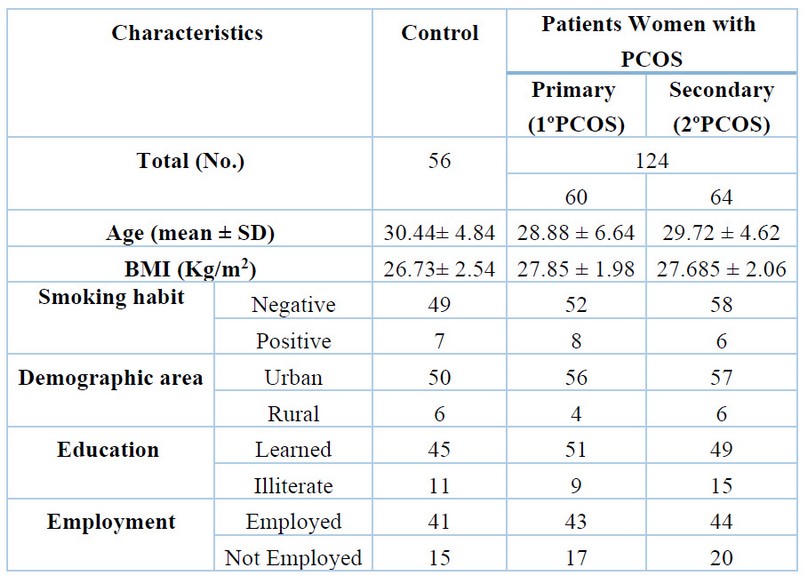
Table 1. The main demographic and clinical characteristics of the study population.
As indicated in Tables 2 to 4, the findings showed that patients with 1ºPCOS and 2ºPCOS (of both groups of obese and non-obese) HOMA-IR (p< 0.05), AMH (p< 0.01), LH (p< 0.01), LH/FSH ratio (p< 0.01), E2, Ts (p< 0.05), PRL (p< 0.01), PAI-1 (p< 0.01), RBP4 (p< 0.01), and Cu (p< 0.01) were all significantly higher than the control group. Furthermore, patients with PCOS had especially (p<0.01) reduced Se, Zn, Mg, and E2/T ratio levels. In contrast, QUICKI levels between the patients (1ºPCOS and 2ºPCOS) and control groups were not statistically different (p>0.05), as illustrated in Tables 2 to 4.
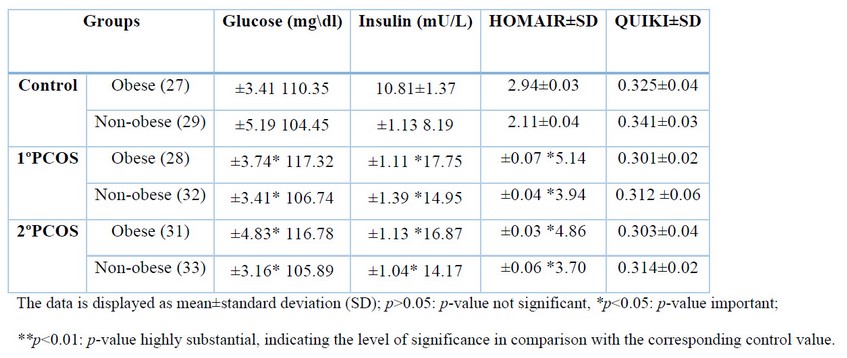
Table 2: Levels of Insulin Resistance Parameters.
As shown in Table 3, however, our findings revealed that FSH levels were significantly (p<0.05) higher in non-obese PCOS patients and lower in obese PCOS patients as compared to the control group.
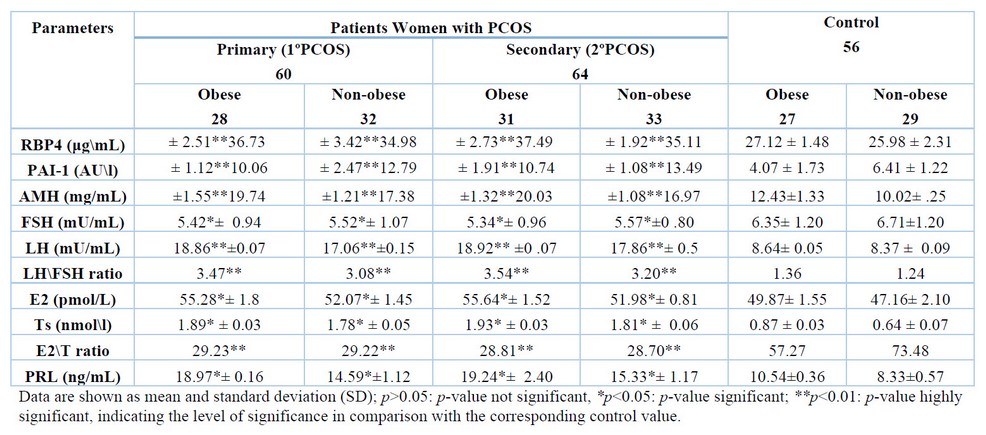
Table 3. Levels of RBP4, PAI-1, AMH and some endocrinological hormones in women of healthycontrol and PCOS patients.
The ROC (receiver operating characteristic) curve's area under (AUC) results obtained indicate that RBP4 and PAI-1 could potentially be used as greater predictive biomarkers in patients women with 1º PCOS (AUC; for obese= 0.909, 1.0 and for non-obese= 0.866, 0.990, respectively) and 2º PCOS (AUC; for obese= 0.905, 1.0 and for non-obese= 0.823, 1.0, respectively), as illustrated in Figure 1.
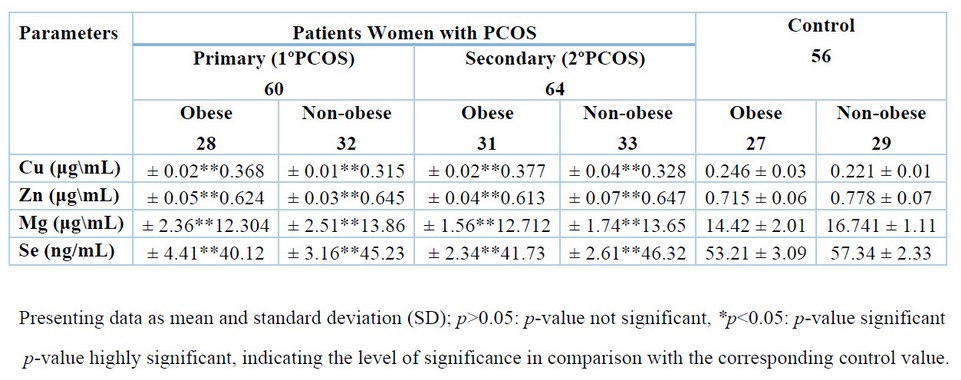
Table 4: Levels of essential trace elements in women of healthy control and PCOS patients.
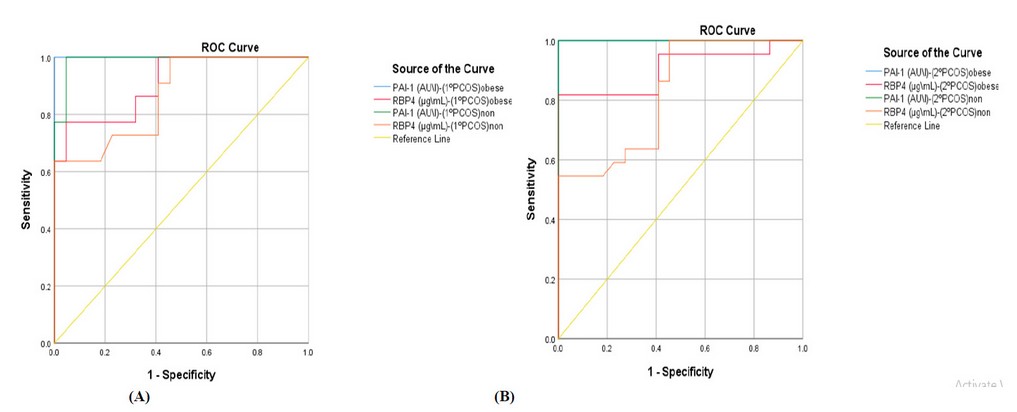
Figure 1. Receiver operating characteristic curve (ROC) for serum RBP4 and PAI-1 levels in obese and non-obese patients women with (A) primary and (B) secondary PCOS.
DISCUSSION
Infertility is the inability to become pregnant after 12 months or more of unprotected sexual activity. As a result, it might be initially assessed after a year of trying to conceive 8. The inhabitants of urban and rural areas differ significantly in several ways, including those related to food, genetics, social, psychological, environmental factors, pollution, and other environmental and environmental factors. On the other hand, workplace demands could impact women's psychological health. Additionally, tense household circumstances, including childrearing and marital relationships, aggravate the oxidant/antioxidant status issue. 10
A significantly elevated level of RBP4 in women with PCOS may imply a strong correlation between visceral obesity, high levels of circulating and adipose tissue RBP4, and insulin resistance. According to certain studies, the amounts of RBP4 in the blood may be directly linked to how sex hormones affect adiposity expression and secretion. Additionally, RBP4 levels have demonstrated a substantial correlation with serum levels of Testosterone, DHEA-S, FSH, and LH in patients with PCOS. 11 Hyperinsulinemia, insulin resistance, a higher risk of glucose intolerance, diabetes, particularly type 2, and an increased incidence of lipid-related abnormalities are all characteristics of PCOS women. Although the mechanisms generating PCOS in women are unknown, insulin resistance may play a crucial role in its development. In addition, the prevalence of hyperinsulinemia in women with PCOS patients was previously confirmed, independent of obesity. 1 Contrarily, obesity in PCOS-afflicted women may cause insulin resistance or a greater level of blood insulin, which directly contributes to increased serum RBP4. Previous research has suggested that circulating RBP4 levels may be correlated with the degree of insulin resistance in obese individuals and with the metabolic syndrome's symptoms, such as high body mass index, waist-to-hip ratio, serum triglyceride levels, systolic blood pressure, and decreased levels of HDL cholesterol. 12 The accumulation of Tg in the liver or ectopic fat, which results in hypertriglyceridemia and hyperinsulinemia, prompts RBP4 synthesis and secretion in the liver or ectopic fat and serves as a sign of infertility, according to a theory that suggests impaired free fatty acid metabolism may be a significant factor in controlling RBP4 levels. 13 Additionally, markedly high levels of RBP4 may impede insulin-stimulated glucose uptake in muscle and enhance hepatic glucose synthesis in PCOS women. They may also boost hepatic gluconeogenesis by stimulating phosphoenolpyruvate carboxykinase. 14
Insulin resistance may cause PCOS patients' significantly increased serum levels of (PAI-1) in obese and non-obese PCOS. According to several research, the relationship between PAI-1 and IR in PCOS-affected women patients is independent of body mass and likely reflects insulin-stimulated hepatic PAI-1 production. 4 The association between polycystic ovaries and recurrent miscarriage may be explained by one of the key processes that involves a markedly high level of PAI-1. Women with PCOS frequently exhibit hyperandrogenism, which can inhibit the formation of pores and prevent ovulation. This condition could be brought on by an imbalance in plasminogen levels, which causes theca (interstitium) enlargement and sets off a chain of events that perpetuates the production of the hormone testosterone in men. 15 The etiology of ovarian dysfunction, which eventually leads to disrupted and disordered endothelial, metabolic, and reproductive processes, may be explained by considerably higher levels of PAI-1 in patients with PCOS. The overproduction of PAI-1 brought on by the insulin-driven decrease in the amount of plasmin accessible for extracellular proteolysis may also contribute to folliculogenesis failure in PCOS patients and ovary patients. 16 Additionally, clinical strategies designed to block PAI-1 and lower insulin resistance may be essential to reestablish proteolytic balance in several tissues that have been damaged, such as the ovary, to result in correct ovulation, treatment for PCOS prevention to improve patient fertility, and prevention of its complications that lead to various cardiovascular disease, multiple menstrual irregularities, and infertility in PCOS. 17
Insulin resistance is a biological misunderstanding when the cell membrane insulin hormone receptors do not respond to insulin as expected. As a result, blood glucose cannot enter cells, resulting in a hypoglycemic response. To move the glucose out of the blood and into cells, the pancreas must produce high insulin dosages, which reduces the hormone's capacity to control and communicate variations in blood glucose levels and may lead to insulin resistance. 18 The mechanisms for dramatically increased insulin resistance in patients with PCOS seem too complex; thus, before starting treatment, it is crucial to understand how insulin functions. The insulin hormone has a cell surface receptor with a similar structure to the insulin-like growth factor-1 (IGF-1) receptor that it may bind to. It increases the translocation of the insulin-responsive glucose transporter 4 (GLUT4) from intracellular vesicles to the cell surface in tissues, stimulating the tissue cells to take up glucose. Phosphatidylinositol 3-kinase may be necessary for sharing and mediating this pathway for it to exist (PI3-K). However, because it can activate a cascade of enzymes, including serine/threonine, Raf, MAPK, and MAPK-ERK1/2 of the non-classical models, the MAPK-ERK pathway is involved in the growth and differentiation of cells. 19 The quantitative insulin sensitivity check index (QUICKI) was created to improve fasting measures' sensitivity and precision. QUICKI and clamp measures show a strong correlation in patients with a greater BMI and leanness. A substantial connection with HOMA-IR may also be seen because the euglycemic insulin clamp method is not feasible globally. In clinical settings and population-based research investigations, the HOMA-IR technique is regarded as the standard gold test for assessing insulin resistance. 20
Women with PCOS may have lower whole blood Se levels due to being exposed to oxidative stress, which is known to have a role in the pathophysiology of all known illnesses. The decreased selenium in the blood may indicate increased free radical generation and highly active selenium or glutathione peroxidase enzyme scavengers. According to several scientific studies, selenium is crucial for the reproductive system to function normally, supporting the link between selenium intake and fertility and procreation-related problems. 9 On the other hand, the pathophysiology of PCOS may be heavily influenced by oxidative stress and insulin resistance. In was reported that found a negative correlation between the blood levels of selenium and the hormones LH and total Testosterone in women with PCOS. This finding may suggest that selenium plays a role in the pathogenesis of PCOS, which is linked to hyperandrogenism. So, through enhanced markers of insulin metabolism and decreased oxidative stress, Se supplementation may lead to reduced levels of serum DHEA and hirsutism. Numerous earlier investigations have demonstrated an association between high androgen levels brought on by PCOS in women and hyperandrogenism, hyperinsulinemia, and insulin resistance. 21 Additionally, insulin resistance and hyperandrogenism, which contribute to endocrine and biochemical changes in women with functional ovarian hyperandrogenism, may be directly connected with oxidative stress (FOH). Se may play a crucial protective role in the active region of enzyme-based antioxidants, including glutathione peroxidase (GPx) and thioredoxin reductase (TrxRs). Therefore, decreased Se level may induce an increased risk factor of cardiovascular disorder, gestational complications, miscarriages, neurodegenerative diseases and increased risk of cancer. Women with PCOS are susceptible to infertility, hirsutism, increased inflammatory factors and increased biomarkers of oxidative stress. 22 Tumor necrosis factor-a (TNF-a), nuclear factor-kappa b (NF-jb), and interleukin-1 (IL-1) are a few examples of inflammatory cytokines that are prevented from being produced and active by selenium, according to numerous research (IL-1 and IL-18). Additionally, the role of reactive oxygen species (ROS) in insulin signaling may impact the imbalance of oxidants and antioxidants. Selenium may be able to lessen ROS and improve insulin resistance due to its significant role as a potent antioxidant. 9 In contrast to a study by Hosseinzadeh et al., who discovered that selenium supplementation might exacerbate insulin resistance in PCOS women, earlier research suggested that selenium intake is linked to lower insulin resistance in PCOS women. 21
The trace elements Zn and Cu play crucial and essential roles as stabilizers, cofactors in several enzymes, and components for healthy and effective hormonal activities. Zn can operate as an antioxidant by shielding the sulfhydryl groups of various proteins and enzymes from free radicals and reducing oxidative stress. In contrast, copper is regarded as a pro-oxidant and oxidant metal in physio-chemistry. 23 One of the trace elements necessary for adequate insulin hormone response is zinc. It is also needed for normal insulin hormone synthesis, storage, and release. In addition, when zinc binds to the insulin receptor, it may have an insulin-like effect. 8 Therefore, acute zinc shortage brought on by oxidative stress may result in severe abnormalities during the per-ovulatory phase that affects egg maturation, cumulus expansion, and ovulation, as well as explain why PCOS patients have higher insulin resistance. 21 On the other hand, higher Cu concentrations in PCOS-affected women's serum may directly impact infertility rates by lowering progesterone levels, which may lead to anovulation, implantation failure, or luteal phase deficit. Furthermore, it is unclear from this research whether elevated copper levels are linked to undiagnosed inflammatory diseases. Ceruloplasmin, regarded as a protein of the acute phase, may also raise serum copper levels while lowering zinc levels. 9 This may suggest that a deficiency in serum copper and zinc may be the primary factor causing infertility. Elevated levels of this element may harm fertility in a variety of ways. For instance, excessive copper levels may disrupt neuronal signaling in the central nervous system (CNS), essential for neuroendocrine fertility regulation. 23 Second, the high serum Cu concentration emphasizes how vital zinc is for controlling ovulation. Therefore, a markedly higher serum Cu level and a significantly lower serum Zn level in pregnant women may hurt fetal brain development and result in minor birth abnormalities. In addition, zinc must be consumed because it is a metal cofactor for many different enzymes (amine oxidase, copper-dependent superoxide dismutase, cytochrome oxidase, and tyrosinase). The enzyme Cu / Zn superoxide dismutase, which reduces superoxide's toxicity by converting it to hydrogen peroxide and oxygen, contains Cu as well. 21 Therefore, a stable balance between the copper-zinc components is responsible for the effectiveness of enzyme function (SOD). Because of enzyme failure, the imbalance in the copper-to-zinc ratio may raise the ratio of dangerous free radicals in the body, which could cause serious harm to cell membranes and walls. 22 In our study, the PCOS group's copper/zinc ratio was noticeably higher than that of the control group. Furthermore, this ratio imbalance may be a significant factor in the disruption of ovarian function, as well as in the lack of follicle maturation persistence, growth of the formation of dominant follicles, and opposition to follicle decay, which may also result in anovulation in polycystic ovarian syndrome. 9 The impact of obesity is another explanation for our findings, higher Cu levels in PCOS. Kurdoglu et al. showed a negative association between copper levels and BMI, but this finding is only valid for PCOS-positive non-obese women. The BMI of PCOS-afflicted women was 28.4 kg/m2, substantially higher than the BMI of the control group (25.6 kg/m2). However, Li et al. showed no appreciable difference in copper content if BMI was greater than 25 kg/m2. 23 Compared to the healthy control group in the current study, the level of Cu in obese and non-obese PCOS patients was significantly significant. Additionally, the obesity shown in some cases may be a symptom of the metabolic syndrome seen in PCOS patients. 8 More research is needed to further understand how zinc or copper contribute to PCOS pathogenicity. This research must consider matched cases and controls for BMI, measure oxidative stress levels, and assess dietary behavior. 21
Women with PCOS demonstrated a statistically significant decline in serum magnesium levels as compared to healthy participants in the current investigation. There have been several research on the physiologic processes, pathological conditions, and therapeutic uses of this necessary metal, including the following: To maintain the structure of ribosomes, nucleic acids, and some proteins-all of which are essential for good health-magnesium, a trace element that is eaten with food or water, is also a crucial cofactor for numerous enzymes involved in glucose metabolism. Second, Mg is thought to have two key functions in biological systems: (i) it can compete with calcium for binding sites on proteins and membranes; and (ii) it can form chelates with significant intracellular anionic ligands, including adenosine triphosphate (ATP). 8 (iii), the intracellular Mg plays a crucial role as a cofactor for several enzymes involved in the metabolism of carbohydrates, particularly those involved in phosphorylation events like tyrosine kinase. Magnesium contributes significantly to the creation of metal-phosphate complexes, which may play a crucially important role by triggering the biocatalysts involved in converting triglycerides and glucose. 21 Infertility, spontaneous miscarriages, congenital abnormalities, preeclampsia, placental abruption, preterm rupture of membranes, stillbirths, and low birth weight have all been linked to decreased levels of trace elements like Mg. 22 In addition, lower Mg levels can paradoxically lower the risk of oncogenesis or protect against it. Mg is said to be essential for the cell cycle, and its lack plays a vital role in the development of precancerous cells. On the other hand, too much magnesium can harm the growing fetus, the liver, the kidneys, and the brain. 23 In this study, reduced blood Mg levels in PCOS patients may be related to the prevalence of different metabolic syndrome symptoms or reproductive phenotypes in PCOS-afflicted women of reproductive age, which may increase our understanding of the pathophysiology of PCOS. Therefore, doing dietary research and interventional trials using magnesium supplements becomes sensitive. Despite prior reports of lower serum Mg levels in obese, healthy individuals, we could not detect any differences in serum Mg concentrations between obese and non-obese patients and women with PCOS. The variations may explain this mismatch in eating habits among these various populations. Reduced magnesium absorption as a result of a larger intake of fat and less fiber may be one of the most significant and likely causes of decreased serum Mg in obese PCOS women. 24
CONCLUSIONS
From this study, it can be concluded that blood levels of RBP4, PAI-1, and trace elements (Se, Zn, Cu, and Mg) are linked to BMI, metabolic abnormalities and IR, and physical activity in women with PCOS living in Basrah, Iraq. Considering the multivariate regression analysis, RBP4 and PAI-1 seem to be better diagnostic indicators for PCOS early detection, while trace elements may operate as a preventive factor, particularly in people with central obesity. However, more research involving larger sample sizes should be carried out to determine the diagnostic relevance of other biomarkers for the early-stage detection of PCOS and the efficacy of treatments for female infertility issues.
Funding: Self-funding.
Acknowledgments: The authors thank all the staff of "the infertility center" at Basra hospital for Obstetrics and children in Basrah Province-Iraq.
Conflicts of Interest: The authors declared no conflicts of interest. No funding was received for this study.
REFERENCES
1. Al-Fartosy A.J.M., Awad N.A. and Mohammed A.H. Intelectin-1 and Endocrinological Parameters in Women with Polycystic Ovary Syndrome: Effect of Insulin Resistance. Ewha Med J 2020; 43(1): 1-11. https://doi.org/10.12771/emj.2020.43.1.1.
2. Al-Fartosy A.J.M., Awad N.A. and Abdalemam D.J. Biochemical study of the effect of insulin resistance on adiponectin, lipid profile and some antioxidants elements with relation to obesity in type 2 diabetic patients/Basrah-Iraq. Amer J Biochem 2017; (7): 73-82. doi: 10.5923/j.ajb.20170704.03.
3. El-Barbary R.H.A., El-Said E.E., Ahmad F. and Al-Wakeel M.E.S. Retinol-binding protein 4, leptin, and insulin resistance in idiopathic hirsutism and hirsute women with polycystic ovary syndrome. Journal of the Egyptian Women's Dermatologic Society 2013, 10: 94–100. doi: 10.1097/01.EWX.0000424171.02538.56.
4. Sahay S., Jain M., Dash D., Choubey L. and Jain S.T.B. Singh Role of plasminogen activator inhibitor type 1 (PAI-1) in PCOS patient. Int J Reprod Contracept Obstet Gynecol 2017; 6(9): 4052-4058. doi: http://dx.doi.org/10.18203/2320-1770.ijrcog20174061.
5. Infertility workup for the women's health specialist. ACOG Committee Opinion No. 781. American College of Obstetricians and Gynecologists. Obstet Gynecol 2019; 133: e377-84.
6. Kumar A.N., Naidu J.N., Satyanarayana U., Anitha M. and Ramalingam K. Association of Insulin Resistance and Serum 25–OH Vitamin-D in Indian Women with Polycystic Ovary Syndrome. Int J Clin Bioch Res 2015; 2(1): 22-26.
7. Gutch M., Kumar S., Razi S.M., Gupta K.K. and Gupta A. Assessment of insulin sensitivity/resistance. Ind J Endoc Metab 2015; 19(1): 160-164. doi: 10.4103/2230-8210.146874.
8. Al-Fartosy A.J.M., Awad N.A. and Mahmood R.A. A Comparative Study of Leptin, Oxidant/Antioxidant Status and Some Trace Elements in Women of Healthy Control and Unexplained Infertility in Basrah-Iraq. Indones Biomed J 2019; 11(3): 327-337. doi: 10.18585/inabj. v11i3.915.
9. Al-Fartosy A.J.M., Shanan S.K. and Awad N.A. Biochemical study of the effects of some heavy metals on oxidant/antioxidant status in gasoline station workers/Basra-Iraq. Int J Sci Res Public 2017; 7: 83-94. www.ijsrp.org.
10. Al-Fartosy A.J.M., Al-Sawaad H.Z. and Al-khazali I.H.A. Seminal Biochemical Markers and Serum Fertility Hormones in Men with or without Infertility/Basrah-Iraq. Inter Med J 2020; 25(4): 2129-2140.
11. Guducu N., Gormus U., Kavak Z.N., Isci H., Yigiter A.B. and Dunder I. Retinol-binding protein 4 is elevated and is associated with free Testosterone and TSH in postmenopausal women. J Endocrinol Invest 2013; 36: 831-834. doi: 10.3275/8948.
12. Yavuz IH, Yavuz G.O., Çokluk E., Kurtoğlu Z. and Bilgili S.G. Investigation of galectin-3, lipocalin 2, retinol binding protein (RBP), small dense low-density lipoprotein (sdLDL) in patients with hirsutism. Advances in Dermatology and Allergology 2019; 36(2):177-183. doi: 10.5114/ada.2019.84593.
13. Zhou Z., Chen H., Ju H. and Sun M. Circulating retinol binding protein 4 levels in nonalcoholic fatty liver disease: a systematic review and meta-analysis. Lipids in Health and Disease 2017; 16:180. doi 10.1186/s12944-017-0566-7.
14. Lingaiah S., Papunen L.M., Piltonen T., Poromaa I.S., Victorin ES and Tapanainen J.S. Serum retinol-binding protein 4 levels in polycystic ovary syndrome. Endocrine Connections 2019; 8, 709–717. doi: 10.1530/EC-19-0116.
15. Ye Y., Vattai A., Zhang X., ZHU J., Thaler C.J., Mahner S., Jeschke U. and Von Schonfeldt V. Role of Plasminogen Activator Inhibitor Type 1 in Pathologies of Female Reproductive Diseases. Int J Mol Sci. 2017; 18(8): 1-17. doi: 10.3390/ijms18081651.
16. Burchall G.F., Pouniotis D.S., Teede H.J., Ranasinha S., Walters K.A. and Piva T.J. Expression of the plasminogen system in the physiological mouse ovary and in the pathological polycystic ovary syndrome (PCOS) state. Reproductive Biology and Endocrinology 2019; 17(33): 2-14. doi: 10.1186/s12958-019-0472-0.
17. Liu Y., Sun M.G., Jiang R., Ding R., Che Z., Chen Y.Y., Yao C.J., Zhu X.X. and Cao J.Y. Plasminogen Activator Inhibitor-1 -675 4G/5G Polymorphism and Polycystic Ovary Syndrome Risk: A meta-analysis. J Assist Reprod Genet 2014; 1-8. doi 10.1007/s10815-013-0171-2
18. Al-Fartosy A.J.M. and Mohammed I.M. Comparison of insulin resistance, prolactin and HbA1c with relation to obesity in men and women of healthy control and diabetic patients/Meisan-Iraq. Int J Cur Res 2017; 9(8): 55643-55648. http://www.journalcra.com.
19. Cakir E., Topaloğlu O., Bozkurt N.O., Bayraktar B.K., Gunguneş A., Arslan M.S., Unsal I.O., Tutal E., Ucan B. and Delibaşi T. Insulin-like growth factor 1, liver enzymes, and insulin resistance in patients with PCOS and hirsutism. Turk J Med Sci 2014; 44: 781-786. doi: 10.3906/sag-1303-80.
20. Tam L.M., Sa L.V.N., Duong L.D., Quoc Huy N.V.Q, Chen C. and Thanh C.N. Exploration of the role of Anti-Mullerian Hormone and LH/FSH ratio in diagnosis of polycystic ovary Syndrome. Clin Endoc 2019; 90(4): 579-585. doi: 10.1111/cen.13934.
21. Al-Fartosy A.J.M. and Mohammed I.M. Biochemical Study of the Effects of Insulin Resistance on Sex Hormones in Men and Women Type-2 Diabetic Patients / Meisan-Iraq. Advances in Biochemistry 2017; 5(5): 79-88. doi:10.11648/j.ab.20170505.11.
22. Mohmmed A.H., Awad N.A. and Al-Fartosy A.J.M. Study of Trace Elements Selenium, Copper, Zinc and Manganese Level in Polycystic Ovary Syndrome (PCOS). Int Res Appl Sci Biotec 2019; 6(6): 16-22. https://doi.org/10.31033/ijrasb.6.6.4.
23. Al-Fartosy A.J.M., Awad N.A. and Alsalimi S.A. Osteoprotegerin and Some Trace Elements in Type 2 Diabetic Patients with or without Nephropathy: Effect of Insulin Resistance. Inter Med J 2020; 25(4): 1771- 1784.
24. Al-Fartosy A.J.M., Awad N.A. and Alsalimi S.A. Clinical markers and some trace elements in patients with type-2 diabetic nephropathy: Impact of insulin resistance. J Med Invest. 2021; 68(1): 76-8.
Received: December 23, 2022 / Accepted: January 30, 2023 / Published:15 February 2023
Citation: Al-Fartosy A J M, Nabi Awad N A, Mohammed A H. Evaluating the clinical significance of RBP4, PAI-1, and some trace elements in women with Polycystic Ovary Syndrome. Revis Bionatura 2023;8 (1)36. http://dx.doi.org/10.21931/RB/2023.08.01.36
