2022.07.03.4
Files > Volume 7 > Vol 7 No 3 2022
Coffee’s Melanoidins. A critical review of contemporary scientific literature
Ostilio R. Portillo* 1 and Ana C. Arévalo 2
1 Faculty of Engineering, National Autonomous University of Honduras (UNAH), Tegucigalpa, Honduras.
2 Faculty of Chemistry & Pharmacy, National Autonomous University of Honduras (UNAH), Tegucigalpa, Honduras.
* Correspondence: [email protected]; Tel.: 504-9376-1660
Available from: http://dx.doi.org/10.21931/RB/2022.07.03.4
ABSTRACT
Melanoidins are brown pigments thermally generated during the non-enzymatic Maillard reaction and are present in a large number of baked and roasted food products (e.g., bakery products, dark beer, coffee, etc.), conferring their typical color and improving their appearance, which is usually considered, by the end-consumer, as an indicator of quality; After all, quality is in the eye of the beholder.
The amount of melanoidins varies depending on the precursors’ concentration and the type of processing to which a given food product is submitted (baking time + temperature). Additionally, melanoidins have been in our diets for millennia, not only improving the organoleptic qualities of food but also exerting a great array of physiological benefits directly linked to their chemical composition, molecular conformation, and structural size.
Aside from their prebiotic effects, melanoidins also display other beneficial properties, among which the most salient are their antioxidant capacity, antibacterial and chelating activities, and anticancer action. However, regardless of the plethora of in vitro experimental evidence that validates the properties mentioned above, there is still controversy about their significance for human health since many of these properties seem to be associated with high molecular weight melanoidins, which, because of their size, cannot cross the intestinal wall suggesting their action is relegated to the intestinal tract where after being fermented and fragmented are finally converted in a series of metabolic derivatives some of which manage to cross into the bloodstream while others are simply excreted through the feces.
The following is a synthesis collected from the available scientific literature which aims to elucidate several aspects of melanoidins (i.e., synthesis, determination, metabolism, & biological activity) to create awareness about their importance for human health and provide information about where to find them to improve our diets.
Keywords: Synthesis, fractionation, separation, antioxidant activity.
INTRODUCTION
Melanoidins are a complex mixture of different polymers (e.g., carbohydrates [polysaccharides], proteins, amino acids and chlorogenic acids),1,2 which not only make up the majority of the high molecular weight molecules present in roasted coffee but also contribute to the aroma 3,4 and typical coffee coloring thanks to its ability to absorb solar radiation in oscillating quantities between 405 to 420 nm 21-11 . In addition, they are also responsible for the brown coloring of unroasted and decaffeinated beans.
Melanoidins are nitrogen-containing compounds resulting from the last stage of the non-enzymatic Maillard reaction 2,7,8,10,12-16. Although coffee is considered its main source in the human diet,7 they are also produced during the processing of a series of foods such as bread, meat, malt, cocoa, and honey, among others.1,4,8,13
Their molecular structure is complex and unpredictable (Figure 1) 5,16,17 to the point that in some cases most of their molecular weight (≤ 90%) is constituted by unknown material 18; However, structurally speaking, they bear a resemblance to the cereals’ arabinoxylans 7, and although there is a substantial variation concerning their size, the consensus is that their molecular weight ranges between 2 to 22 kDa.2

Figure 1. Melanoidins’ molecular structures. Adapted from Kucera et al. 19
Melanoidins’ contribution to the dry weight of a substrate is calculated by subtraction after eliminating the percentual contribution of all other known compounds.1,2 Using this technique, it has been calculated that they constitute between 23 to 25% of the dry weight of roasted coffee 1,2,4,16,20-24 and 29 to 30% of the dry weight extracted from the coffee drink.1,7,20,21 Additionally, the observed variations within this range are linked to the species/variety, the degree and type of roast & the infusion preparation method (Figure 2).25
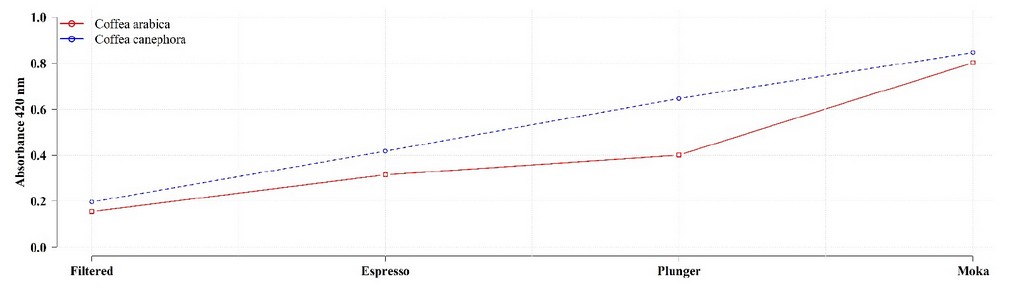
Figure 2. Melanoidin content in coffee drinks influenced by the species and the infusion preparation method. Adapted from Bravo et al. 26.
Regarding the type of roasting, there is evidence that roasted coffees produced by adding sugar during the roasting process contain a higher content of melanoidins (7-10%) than those conventionally roasted. 27
In addition, melanoidins concentration can also be calculated thanks to their ability to absorb ultraviolet light at 405 to 420 nm 2,3,10,28 and with the help of the extinction coefficient (ε) the concentration (c) of melanoidins present in a roasted coffee sample 3,9 can be calculated using the Beer-Lambert equation (A = εcl). 9
Melanoidins have different physicochemical properties that are not shared by all of them, such as their hydrophobicity or their metal ions chelating capacity (e.g., Fe+3, Mg+2, &, Cu+2), the latter being attributed to their anionic nature, which manifests more strongly in the high molecular weight molecules 1,2,9,13,23,29 and which seems to have its origin in the presence of chlorogenic acids coupled to its structure. 9
However, there is also experimental evidence that suggests that, in the absence of chlorogenic acids, the type of amino acid involved during its formation also plays an important role in defining its electrical charge. 9 In addition, they also can reduce hydroperoxides in non-radical compounds through the donation of a hydrogen atom.15,30
Synthesis
Although the mechanisms responsible for the formation of melanoidins in foods are not yet clearly elucidated,1,2,13 the evidence suggests that, in coffee, there is a positive relationship between their synthesis and the degree of grain roasting; In other words, the more roasted the grain is the greater the concentration of melanoidins will be.1,2,10,31
The high molecular weight carbohydrates (e.g., galactomannans & arabinogalactans) and proteins are considered melanoidins precursors. Currently, three hypotheses describe their synthesis: (a) they are formed due to the polymerization of unsaturated carbonyl compounds 5 or by the repetitive accumulation of low molecular weight compounds such as furans and pyrroles produced in the last stages of the non-enzymatic Maillard reaction, 13 (b) they are formed through the interaction of low molecular weight sugars with proteins (Figure 3) (c) or they are formed due to the interaction of sugar derivatives in the early stages of the Maillard reaction which are subsequently polymerized through condensations.1,7,17
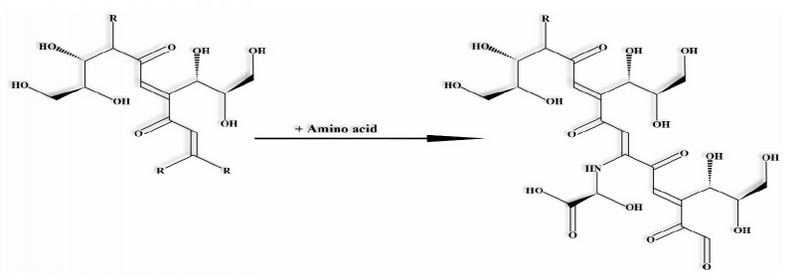
Figure 3. Synthesis of a melanoidin. R: H+, glucose or amino acid.
Based on the above-described mechanisms, three types of melanoidins can be identified:
a) Melanoproteins are partially brown color water-soluble polymers 3 formed from the reaction between deoxysones (sugar degradation products) and proteins.3,8,12,13,32 Deoxisones are α-dicarbonyl groups derived from reducing sugars (i.e., glucose and fructose) which act as reactive intermediates during the Maillard reaction; and their interaction with the amino groups (NH2) of amino acids and proteins results in the formation of low and high molecular weight molecules of up to 100 kDa.2,7,14
In other words, melanoidins molecular weight depends on the nitrogen-containing compounds (i.e., free amino acids, peptides, oligopeptides, polypeptides or proteins) involved in their synthesis during the Maillard reaction. 32
Melanoidins’ amino acid composition tends to be similar and the most abundant amino acids present in their molecular conformation are glutamic acid, glutamine, glycine, alanine, aspartic acid, isoleucine, leucine, and proline 2,13; In contrast, the least abundant are: methionine, lysine, tyrosine, histidine, and arginine.2,13 The predominance of certain amino acids in their molecular structure is attributed to the amino acid thermal degradation resistance during grain roasting.
According to Moreira et al. 1, about 50% of the high molecular weight melanoidins present in the coffee drink correspond to arabinogalactan-protein complexes (AGPs). AGPs are complexes formed by arabinogalactan covalently bound to proteins with a high content of hydroxyproline, an amino acid.
b) Conversely, some melanoidins are produced when dicarbonyl groups are condensed, during the Maillard reaction, with high molecular weight polysaccharides such as galactomannans and arabinogalactans 14,32 both constituents of the cell wall.
During grain roasting, the galactomannans and arabinogalactans are degraded (depolymerized) in addition to losing their lateral branches 1,11, and at the end of the process, these polysaccharides are responsible for 47% of the produced melanoidins. 2
c) However, there is also evidence of melanoidins interaction with phenolic compounds such as chlorogenic acids or their derivatives (e.g., caffeic, ferulic, coumaric, and quinic acids) through covalent and non-covalent bonds (e.g., hydrogen, hydrophobic, and ionic bonds) resulting in melano-chlorogenic complexes 1,4,13,15-17,21,33 which also have antioxidant properties.6,29,34
However, although the raw grain has up to 10% of chlorogenic acids, less than 1% of them are incorporated into the molecular structure of melanoidins. 2 This happens because chlorogenic acids are thermally degraded during grain roasting. In addition, the incorporation of chlorogenic acids in the melanoidin’s molecular structure is the cause of the development of dark pigments in other food matrices (e.g., sweet wine and grape syrup).2,9
However, although the raw grain has up to 10% of chlorogenic acids, less than 1% of them are incorporated into the molecular structure of melanoidins. 2 This happens because chlorogenic acids are thermally de-graded during grain roasting. In addition, the incorporation of chlorogenic acids in the melanoidin’s molecular structure is the cause of the development of dark pigments in other food matrices (e.g., sweet wine and grape syrup).2,9
Finally, there is evidence of coffee melanoidins’ interaction with a variety of low molecular weight volatile compounds suppressing or reducing their organoleptic attributes.12,23 For example, among the volatile compounds with sensory properties capable of forming complexes with melanoidins through covalent bonds, are the thiols (e.g., 2-furfurylthiol, 3-methyl-2-butentiol, 2-methyl-3-furantiol, & methanethiol),2,12,23,34 the 3-mercapto 3-methyl butyl format,2,12,23,34 the sulfides, pyrroles, pyrazines, pyridines, and the diketones 2,12 which causes the suppression of the typical sulfured and roasted odors of coffee. 14
Determination of melanoidins
Empirically, we can say that the greater the degree of roasting, the darker the appearance of the grain will be and the higher the concentration of high molecular weight melanoidins. In contrast, to a lesser degree of roasting, the grain coloration will be clearer, and low molecular weight melanoidins will prevail. 35
On the other hand, the main limitation in studying melanoidins structure is the lack of specific analytical methods for their isolation and separation into homogeneous fractions. One of the most common approaches is the isolation by molecular weights; however other high molecular weight molecules such as proteins and polysaccharides can be co-isolated with these methodologies, demonstrating the need for procedures to separate the different polymers. 13
Another limitation is that there are no quantitative methods for specific isolation, so their food content is only an estimate.13,16 However, several melanoidin fractions have been obtained by chromatographic separation or using separation protocols that allow a partial view of their structure and diversity. 13 The chemical analysis of these compounds can be divided into the steps described below:
The isolation (fractionation)
It is based on the estimation of molecular weights using different fractionation techniques that separate mela-noidins based on their molecular weight. 22 Among these techniques, we can mention ultrafiltration, diafiltration, and dialysis. The foundation of these techniques is the separation of particles based on their molecular weights using semipermeable membranes with specific pore sizes or hollow fiber with cut-off points ranging from 3 to 100 kDa. 22
Such techniques can remove low molecular weight molecules; However, modified polysaccharides and proteins with high molecular weight are retained together with melanoidins.13,14,16 The result of this fractionation is the recovery of the High Molecular Weight Material (HMWM). Next, the techniques’ basis used for the melanoidins isolation process is briefly described as follows:
Ultrafiltration: is the process through which substances are divided, separated and concentrated without them undergoing phase changes. In addition, a semipermeable membrane with defined pores is used to limit the particle size that will pass through it. Because of its semipermeable nature, applying pressure (4-8 atm) facilitates the flow of particles through the membrane with cut-offs ranging from 100, 50, 10 to 3 kDa. In other words, the size of molecules capable of passing through the membrane will depend on the membrane’s average pore diameter and applied pressure.
Diafiltration: is a type and specialized form of ultrafiltration where the retained fraction is diluted in water and re-circulated so that the concentration of soluble components is reduced, thereby increasing the concentration of insoluble compounds in the retained fraction.
Dialysis: is a form of molecular filtration through which molecules are separated according to their size, by using semipermeable membranes with pores of dimensions smaller than macromolecular. These pores block the passage of larger molecules but allow small molecules (e.g., solvents, salts, and small metabolites) with cut-off (molecular weights) ranging from 2, 12 or 14 kDa to diffuse through the membrane.
Separation (isolation or purification)
Once the HMWM has been isolated, the separation of the melanoidins from it is performed based on their Physico-chemical properties:
Solubility
The solubility of coffee melanoidins depends on the polymer size and its chemical nature. 22 Once HMWM is obtained, polysaccharides-derived melanoidins can be separated based on their solubility. 9
The fractionation is performed using EtOH: H2O in different proportions. The galactomannan-rich fraction is precipitated in 40 to 50% EtOH whereas the arabinogalactan-rich fraction is precipitated in 75 to 80% EtOH. In contrast, the low carbohydrate fraction remains soluble in 75 to 80% EtOH.36,37
The Yariv phenyl glycoside reagent is used to induce the precipitation of melanoidins containing water-soluble arabinogalactan-protein (AGP) complexes. 13
Electrical charge
The type of amino acid present during their synthesis determines the anionic property of melanoidins. 9 According to their anionic behavior, coffee melanoidins can be isolated by anion exchange chromatography. When precipitation with EtOH does not allow the recovery of homogeneous fractions, combined methodologies can be applied. For instance, ion-exchange chromatography followed by purification through metal ion affinity chromatography (e.g., Cu+2) can be used in the first stage. 13
Chelating capacity
It is based on separating fractions with chelating capacity from the non-chelating ones using techniques such as Cu+2 affinity chromatography.
Hydrophobicity
The separation mechanism is based on hydrophobic interactions induced by salt formation between the melanoidins’ non-polar functional groups and the stationary phase ligands. 16
The coffee melanoidins have a hydrophobic behavior due to their average molecular weight. 22 Based on this property, melanoidins can be separated from the coffee infusion or the HMWM using gel filtration chromatography (Sephadex-LH20) followed by hydrophobic interaction chromatography (Octylsepharose) that separates them based on their surface hydrophobicity. 13 A general scheme of melanoidin purification is shown in Figure 4.
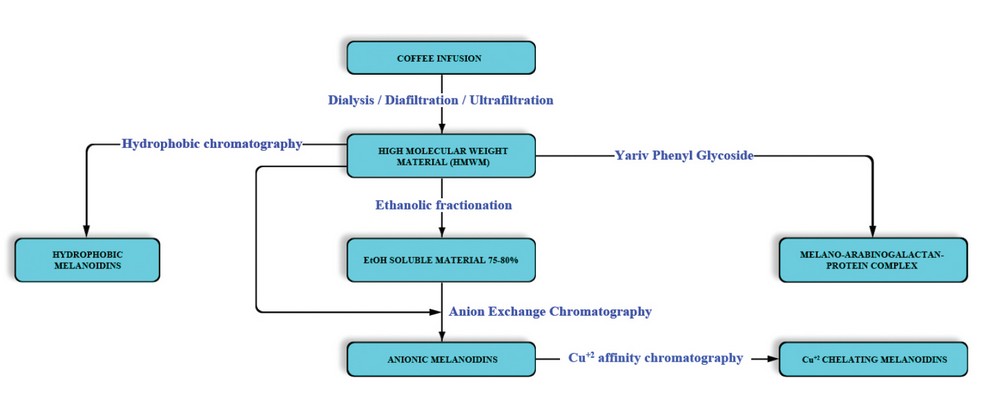
Figure 4. General scheme for the purification of melanoidins in coffee. Adapted from Nunes et al. 13.
Quantification
The content of melanoidins, in a food sample, can be measured at an absorbance ranging from 405 to 420 nm using a specific extinction coefficient 3,14,38 to spectrophotometrically estimate the level of browning (absorbance values) about the concentration of melanoidins as a means to characterize them in coffee. 9
According to the Beer-Lambert equation A = εcl, there is a direct linear relationship between absorbance (A), and concentration (c). To quantify the melanoidins using this equation, the specific extinction coefficient (ε) for each substance must be known.
These coefficients have been calculated through reaction models of different sugars and amino acids, thus producing various types of melanoidins. After their evaluation, the specific extinction coefficients for each type have been reported. 9
Structural characterization
The characterization of the different recovered fractions is done through two-dimensional Nuclear Magnetic Resonance (NMR) as in the studies by SQHC (Simple Quantum Hetero-Nuclear Correlation).
The analysis is done by grouping the compounds according to their carbon-hydrogen correlation and the chemical displacement of H+ and C+4. This technique allows the correlation between the H+ and C+4 of different compound groups.
These experiments have shown the correlation between H+ ions coupled to C+4 atoms linked to hetero atoms such as oxygen and nitrogen (e.g., HC-O & HC-N) which differ from the typical bonds of proteins and carbohydrates.13,16 This allows inferring the presence of chromophores substances in the complex structure of melanoidins.13,16
The above-described stages were applied in a study developed by Gniechwitz et al. 16, in which hydrophobic interaction chromatography was implemented in the analysis of the fraction poor in carbohydrates and amino acids but possibly rich in chromophores and antioxidant compounds present in arabica coffee.
For this, fractionation was performed by ultrafiltration, recovering fractions with molecular weights ranging between 3 to 10, 10 to 50, 50 to 100, & > 100 kDa. However, the predominant fractions were those with molecular weights ranging between 3 to 10 & > 100 kDa which represent about 37 & 38% of the high molecular weight material, respectively. 16
The predominant fractions were subsequently isolated with Sephadex LH-20 using aqueous NaCl (0.5 mol l-1) as the mobile phase until fraction A (SepA) was eluted. Subsequently, fraction B (SepB), characterized by intense brown color, was also eluted with water. 16 In addition, fraction B constituted 4 & 13% of fractions with a molecular weight ranging from 3 to 10 & > 100 kDa respectively. 16
Monitoring was performed using a UV detector at 405 nm, and the fractions were collected at 32 min intervals. Subsequently, the recovered fractions (ie, SepA & SepB) were washed with water by ultrafiltration to remove the salt. The fractions were dried and weighed for further analysis.
Fraction B was subjected to another fractionation via hydrophobic interaction chromatography with Octyl Sepharose using aqueous NaCl as solvent (Figure 5).16 The detection was performed at 405 nm, and the fractions were collected at 20 min intervals 16; However, the results indicated that a significant additional fractionation was not possible when applying this procedure (Figure 6).
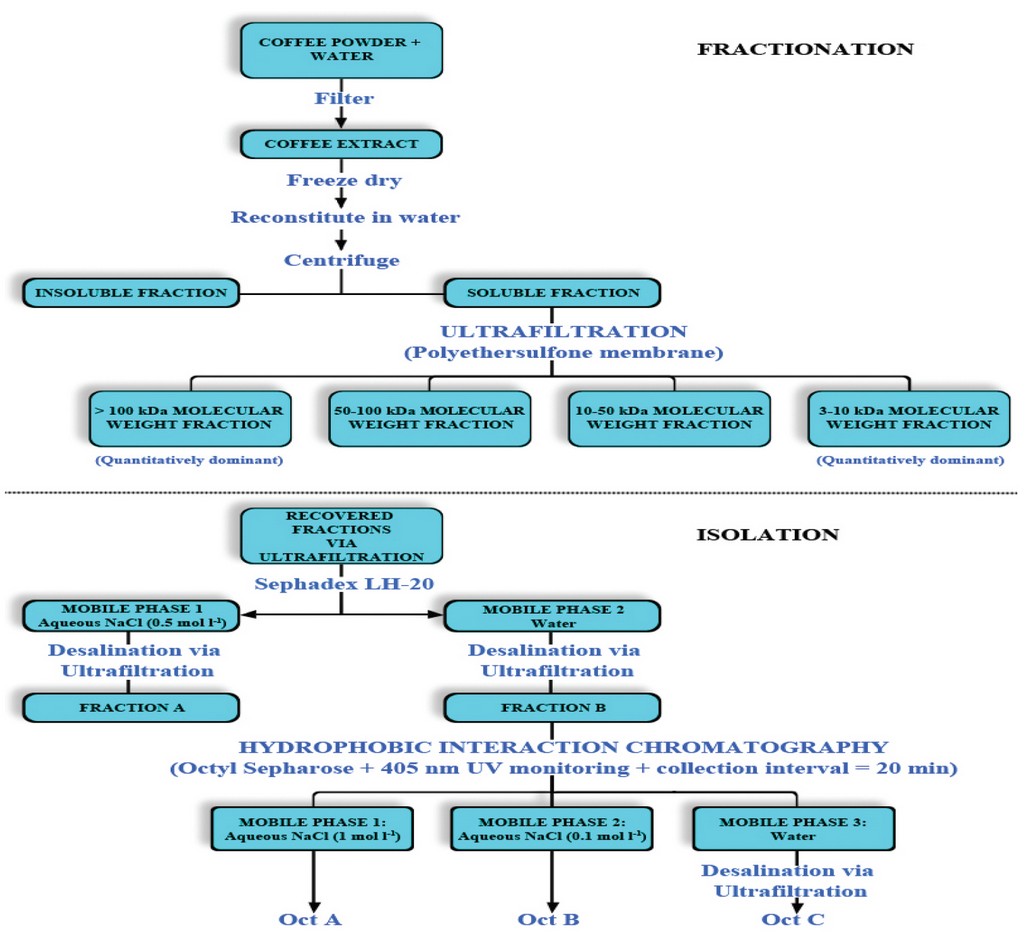
Figure 5. Melanoidins’ fractionation and isolation in arabica coffee samples with a medium roasting degree.
In figure 6 the result obtained during the chromatographic separation, with a UV detector, of the fractions re-covered by ultrafiltration using Sephadex LH-20 is shown graphically. The molecular masses of the predominant fractions ranged from 3 to 10 kDa & > 100 kDa.

A. Separation of fraction obtained via ultrafiltration with molecular weights ranging from 3-10 kDa.
B. Separation of fraction obtained via ultrafiltration with molecular weights > 100 kDa.
Adapted from Gniechwitz et al. 16.
Figure 6. Chromatographic separation of fractions obtained by ultrafiltration using Sephadex LH-20.
The results show the importance of the membrane’s pore size. It can be seen in figure 6.A how fraction B (SepB) tends to increase as the membrane used in the ultrafiltration process facilitates the circulation of particles with low molecular weight. Subsequently, fraction B (SepB) was analyzed through SQHC and the compounds (identified or not) were grouped according to their signal intensity (Figure 7). For instance, the phenolic/aromatic/olefinic compounds showed high-intensity signals suggesting a high concentration thereof.
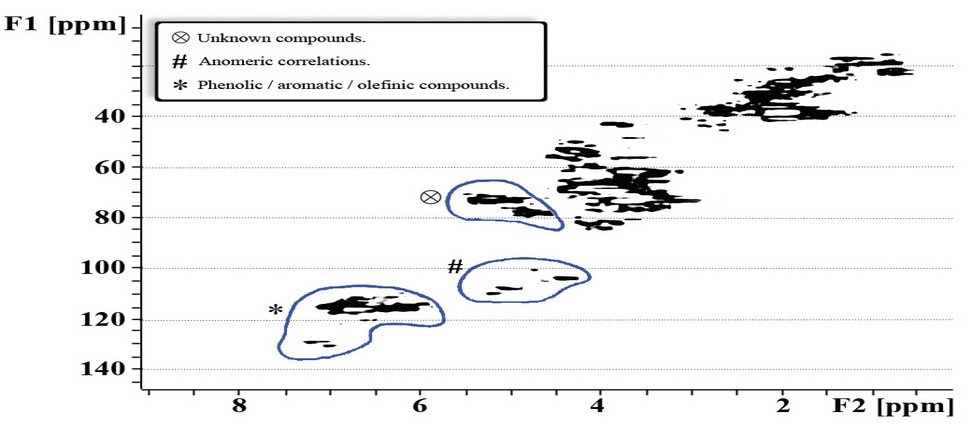
Adapted from Gniechwitz et al. 16
Figure 7. SQHC NMR Spectrum of fraction B isolated via ultrafiltration with a 3-10 kDa cut-off membrane. Adapted from Gniechwitz et al. 16
On the other hand, the anomeric 1H/13C correlations were characterized by their low intensity and could correspond to carbohydrates present in the analyzed fraction. Finally, there were correlations of unknown compounds which do not correspond to carbohydrates or proteins.
The HSCQ-NMR analysis shows no evidence of ferulic or caffeic acid presence, so it is inferred that phenolic compounds bound to melanoidin structures were linked through a phenolic condensation reaction.
Metabolism
The average daily melanoidins consumption is approximately 1 to 2 g 4,39 (others have reported 0.2-2.6 g per day 18), but for those who consume ≥ 6 cups of coffee daily, the intake can reach up to 5 g reason why it is estimated that coffee’s melanoidins can contribute to meet the daily requirement of dietary fiber (~ 10 g day-1) 31,39; However, only the soluble fraction should be taken into account as insoluble melanoidins are retained in the paper filters used in many coffee makers. 31
However, although several authors qualify them as dietary fiber, the truth is, that they are not coffee bean polysaccharides; in consequence, they are not part of the dietary fiber per se.7,31 The most accepted dietary fiber definition includes all plant polysaccharides (i.e., hemicelluloses, cellulose, lignin, oligosaccharides, polysaccharides, pectins, gums, and waxes) present in foods that are not degraded by the human’s small intestine enzymes. 40
In addition, dietary fiber is divided into two fractions: soluble and insoluble. The soluble fraction has a greater water retention capacity, reduces the lipids and carbohydrates absorption in the small intestine and promotes bacterial multiplication. 41 In contrast, the insoluble fraction has limited water retention capacity but promotes fecal motility through the digestive tract 41 so it effectively mitigates the effects of constipation.
Furthermore, the high molecular weight carbohydrates extracted from food in a chemical, physical or enzymatic way are also considered as dietary fiber as well as those of synthetic origin that have proven health benefits.
However, the presence of a protein fraction and phenolic compounds not only increases the complexity of melanoidins structures (which are not clearly elucidated) but also means that they do not conform to the above-described definition despite the fact they show many dietary fiber characteristics. 31
High molecular weight melanoidins are not digested in the small intestine and due to their alleged inability to cross the intestinal wall, they end up being fermented by microorganisms in the large intestine (colon) being partially fragmented, and in the process, producing high concentrations of acetate and propionate.4,18,39 These can reach concentrations of 0.5 to 2.0 g day in the case of moderate and constant drinkers respectively 7,14,31 to then be excreted through feces.
In contrast, the evidence suggests that low molecular weight melanoidins partially cross the intestinal wall (~ 30%) 9 and, after being metabolized, end up being excreted in the urine. According to Finot and Magnenat, experiences with rodents indicated the urinary excretion of low molecular weight melanoidins was 27% whereas 61 and 87% of the low and high molecular weight fractions respectively were excreted through feces. 4
Biological activity
Although there is in vitro and in vivo evidence of a series of biological activities attributed to melanoidins, there is also doubt about the relevance that these could have on human health; since being molecules of variable molecular weight there is no confirmation of their ability to cross through the intestinal wall (duodenum).1,4,7,15,18,21
Among the biological activities attributed to melanoidins we can cite its anti-caries effect by preventing Streptococcus mutans (causative agent of caries) from adhering to the surface of the teeth, thus preventing plaque formation 4,7,9; In contrast, some authors argue that this effect is due to the presence of chlorogenic acids or trigonelline in the coffee drink. 9
In any case, their biological activity is not limited to the above; on the contrary, it varies due to the size fluctuation, electrical charge as well as structure heterogeneity. Among the documented bioactive properties, we can mention its antibacterial effect, antioxidant capacity,8,10,14,15,18,29 its anti-inflammatory action, antihypertensive action,29,42 chelating capacity, and ability to regulate the activity of certain enzymes 10,14,18 and protection against the development of cancers 29,39 in the intestinal tract.
Chelating activity
Due to their negative charge, melanoidins are considered chelating agents due to their ability to couple with other compounds with opposite charges (cations) such as acrylamide and phenolic acids as well as transition metal ions (eg, Ca+2, Fe+3, Cu+2, Mg+2, Zn+2) forming chemically inactive complexes with varying degrees of digestibility.4,8,9
The ability of melanoidins to chelate Cu+2 is of particular importance since this transition metal can alter homeostasis and possibly indirectly induce intracellular oxidative stress thereby damaging proteins, lipids, and DNA.35,43 Additionally, their ability to form complexes with cations such as Fe+3 and Mg+2 gives melanoidins an antimicrobial effect. 27
However, excessive coffee consumption should be taken with caution due to anti-nutritional effects such as Ca+2 and Fe+3 deficiencies caused by their precipitation thus rendering them indigestible.
One important physiological effect associated with melanoidins chelating properties is their antihypertensive capacity through the inhibition of the angiotensin-converting enzyme (ACE) which is a blood pressure regulatory factor.4,8 The ACE is a zinc (Zn+2) dependent dipeptidyl carboxypeptidase which once activated its function is to turn angiotensin I into angiotensin II, a potent vasoconstrictor,8,44-46 thus causing hypertension.
Although the molecular mechanisms through which melanoidins inhibit in vitro the action of ACE have not been elucidated, the prevailing hypothesis is based on their ability to sequester Zn+2 thus preventing its activation. 8
Antibacterial activity
Coffee’s bioactive properties are many. For example, experiences reported by Nakayama et al. 47 suggest that in rodents it’s habitual consumption helps to regulate the intestinal microflora due to its antibacterial effect (e.g., bacteriostatic or bactericidal) that contributed to reducing Escherichia coli and Clostridium spp. populations; while concomitantly increasing the population of beneficial bacteria such as Bifidobacterium spp.40,47
Food compounds capable of promoting the multiplication of beneficial bacteria (e.g., Bifidobacterium spp., Lactobacilli) in the intestinal tract and simultaneously reducing populations of pathogenic bacteria are known as prebiotics. 18
This result could be attributed to coffee melanoidins ability to regulate the colon’s microflora 4,7,13,42 presumably because of its high molecular weight polysaccharide fraction which is similar to that observed in cereals. 4This effect has been corroborated in human trials which demonstrate that a melanoidins-rich diet increases the concentration of bifidobacteria in the participants’ feces. 4
The evidence suggests that at low concentrations, in vitro melanoidins exert a bacteriostatic effect on the digestive tract bacteria such as Gram-negative (i.e., Escherichia coli, Proteus mirabilis, Pseudomonas aeruginosa, and Salmonella typhymurium) and Gram-positive (Staphylococcus aureus & Bacillus cereus) at ≥ 4 mg ml-1 and 2 to 3 mg ml-1 respectively, probably due to its chelating capacity.1,4 However, Gram-positive bacteria are more susceptible to melanoidins’ antimicrobial activity presumably as a result of the absence of an external cell membrane. 9 On the other hand, the evidence suggests the existence of a positive correlation between the bacteriostatic effect and the coffee roasting degree, that is, the more roasted the coffee the greater its bacteriostatic capacity 4,9 Also, the bacteriostatic effect is greater with high molecular weight molecules and decreases as their size are reduced.1,4,48
The ability to transfer H+ ions thus turning free radicals (eg, NO+, NO-, ONOO-, H2O2, O2-, & OH-) into stable non-reactive molecules is one of the mechanisms through which melanoidins inhibit lipids oxidation which otherwise contributes to the development of atherosclerosis in humans 24,25,35 and in the case of processed foods they contribute by extending their shelf life by preventing oxidative rancidity of mono and polyunsaturated lipids 4,8,9 and by preventing the proliferation of microorganisms due to their bacteriostatic and bactericidal effect.8,9 For this reason, they can be used as additives in the food industry.
Antioxidant activity
The ability to transfer H+ ions thus turning free radicals (eg, NO+, NO-, ONOO-, H2O2, O2-, & OH-) into stable non-reactive molecules is one of the mechanisms through which melanoidins inhibit lipids oxidation which otherwise contributes to the development of atherosclerosis in humans 24,25,35 and in the case of processed foods they contribute by extending their shelf life by preventing oxidative rancidity of mono and polyunsaturated lipids 4,8,9 and by preventing the proliferation of microorganisms due to their bacteriostatic and bactericidal effect.8,9 For this reason, they can be used as additives in the food industry.
Its antioxidant capacity is, in part, attributed to chlorogenic acids (CAs) bound to their molecular structure through undefined bonds and which is presumably manifested by its metallo-chelator and free radical cleaning activities.1,4,7,9,24,25,49
Melanoidins’ antioxidant capacity could also be attributed to the arabinoxyloxanes of the polysaccharide fraction, which also have the antioxidant capacity, as has been demonstrated through the study of these in cereals. 4 Unfortunately, analyzing arabinoxyloxanes’ antioxidant capacity in coffee is expensive and difficult to measure due to the simultaneous presence of chlorogenic acids and other phenolic compounds in the same matrix.
In addition, melanoidins antioxidant activity is not limited to the lower intestinal tract (colon) or blood plasma since there is experimental evidence that shows a reduction in free radicals’ synthesis (hydroperoxides) during a simulated turkey meat gastric digestion assay. High molecular weight melanoidins (> 10 kDa) isolated from an instant coffee sample were incubated (37 °C) with a turkey meat sample during an in vitro digestion test, in which they were able to reduce hydroperoxides levels when melanoidins concentration reached 1.5 mg ml-1. Still, its suppressive effect was even more significant when reaching 3 mg ml-1. 18
From previous experience, it can be deduced that melanoidins can inhibit the production of free radicals at the stomach level, and the intensity of the suppressive effect is linked to their concentration.
Of particular importance is the effect of the degree of roasting on the coffee drink’s antioxidant capacity. It has been shown that at a higher degree of roasting, the concentration of CAs is reduced (due to its degradation), and consequently, the coffee drink’s antioxidant capacity is lower. In contrast, the evidence shows that the higher the degree of roasting, the higher the concentration of high molecular weight melanoidins.4,9
However, low molecular weight melanoidins have a higher antioxidant capacity than those of high molecular weight because they form complexes with a greater number of phenolic compounds 26,35,36,50 while those of higher molecular weight are affected by steric hindrance that decreases their ability to react with other compounds.
Consequently, the synergistic effect of the loss of CAs and a higher concentration of high molecular weight melanoidins would cause a drastic decrease in the antioxidant capacity of black or roasted coffees compared to what is observed in moderately roasted ones.
In contrast, Torrefacto coffees that normally show an intense black color are an exception to the rule because due to their higher content of melanoidins they also have a greater antioxidant capacity compared to what is observed in conventionally roasted coffees. 27
In summary, melanoidins are responsible for approximately 26 to 38% of the antioxidant capacity of coffee drinks 4,14,18 through different mechanisms such as electron transfer and scanning of oxygen (O2) molecules or hydroxyl radicals (HO-).8,9,22
Anticancer activity
Melanoidins can inhibit, in vitro, the metalloprotease matrices (MMP-1, MM-P2, and MM-P3) which are a class of endopeptidases associated with the development of inflammatory and degenerative diseases as well as playing an important role in tumor growth (especially at the level of the colon) and metastasis 1,4,18,25,42 which results in possible protection against colon cancer in humans, especially when their concentrations range between 0.25 to 1.0 mg ml-1. 4
On the other hand, the evidence suggests that the daily consumption of ~ 600 ml of coffee for five consecutive days can protect the human lymphocytes’ DNA from damage caused by free radicals. 4
In summary, melanoidins are responsible for approximately 26 to 38% of the antioxidant capacity of coffee drinks 4,14,18 through different mechanisms such as electron transfer and scanning of oxygen (O2) molecules or hydroxyl radicals (HO-).8,9,22
CONCLUSION
From a nutritional standpoint, lightly or medium roasted coffees are more convenient, for the end consumer, due to a greater concentration of melano-chlorogenic complexes with a stronger antioxidant effect attributed to the presence of chlorogenic acids or their monomeric constituents. However, the general public’s preference for black or well-roasted coffees demonstrate that the end consumer choice is not driven by nutritional facts, but by the product’s organoleptic properties.
This, of course, does not imply the public is well-informed about nutritional and other important issues (e.g., antioxidant properties, species/varieties, blends, roasting degree, origin) to be able to discriminate between the quality of roasted coffees since such information, in the majority of cases, is not even display in the package label.
Nevertheless, regardless of their lower antioxidant capacity, black or well-roasted coffees tend to have a higher concentration of high molecular weight melanoidins which even though are not considered dietary fiber per se partially cover its daily requirement promoting gastrointestinal motility which in turn lessens constipation.
Additionally, due to their inability to cross the intestinal membrane (at the duodenum), the high molecular weight melanoidins are forced into the lower intestinal tract (colon) where they exert their prebiotic, antibacterial, bacteriostatic, and anticancer effect during their transition.
Melanoidins are found in many common products where their precursors undergo the non-enzymatic Maillard reaction when submitted into thermal processing. Unfortunately, due to the high incidence of metabolic syndrome, which is a collection of physiological disorders that occur concomitantly and are typically associated with impaired glucose metabolism, some of these products (e.g., bakery products, dark beer among others) can not be consumed in sufficient quantities to fulfill the daily dietary fiber requirements as in the case of individuals diagnosed with obesity-related pathologies (e.g., diabetes mellitus type II, non-alcoholic fatty liver disease, abnormal cholesterol or triglyceride levels, and hypertension).
In conclusion, coffee as a source of melanoidins seems to be a good alternative for patients suffering from the above-mentioned maladies since the infusion has a glycemic index equal to zero (http://www.montignac.com), provided the beverage is not consumed with added sugar. Nevertheless, it is worth mentioning that coffee melanoidins’ properties presented herein should be interpreted only as educational or informational material and never as a substitution for your physician’s advice since all health issues required medical supervision.
Acknowledgments
Funding: The authors thank the Office for the Scientific, Humanistic and Technological Research (DICIHT) of the National Autonomous University of Honduras for their sponsorship during the publication of this article.
Conflicts of Interest: The authors declare no conflict of interest.
Declaration of Interest: The authors declare no conflicts of interest related to the material in the manuscript.
REFERENCES
1. Moreira A.S.; Nunes F.M.; Domingues M.R.; Coimbra M.A. Coffee melanoidins: structures, mechanisms of formation and potential health impacts. Food Funct. 2012;3(9):903-15. doi: 10.1039/c2fo30048f.
2. Rufián-Henares J.A.; Pastoriza S. Melanoidins in coffee. In: Coffee in health and disease prevention. Academic Press: San Diego; 2015. p. 183-88. doi: 10.1016/b978-0-12-409517-5.00020-6.
3. Chu Y.F.; Hu K.; Hatzold T.; Black R.M.; Chen D. Flaking process increases the NF-kappaB inhibition activity and melanoidin extractability of coffee. Food Sci Nutr. 2013;1(5):363-68. doi: 10.1002/fsn3.19.
4.Rufián-Henares J.A.; Pastoriza S. Biological effects of coffee melanoidins. In: Coffee in health and disease prevention. Academic Press: San Diego; 2015. p. 853-58. doi: 10.1016/b978-0-12-409517-5.00094-2.
5. Ciampa A.; Renzi G.; Taglienti A.; Sequi P.; Valentini M. Studies on coffee roasting process by means of nuclear magnetic resonance spectroscopy. J Food Qual. 2010;33(2):199-211. doi: 10.1111/j.1745-4557.2010.00306. x.
6. Farah A. Coffee constituents. In: Coffee. 1 ed. Chu Y-F, editor. Wiley-Blackwell: Oxford, UK; 2012. p. 21-58. doi: 10.1002/9781119949893.ch2.
7. Ludwig I.A.; Clifford M.N.; Lean M.E.; Ashihara H.; Crozier A. Coffee: biochemistry and potential impact on health. Food Funct. 2014;5(8):1695-717. doi: 10.1039/c4fo00042k.
8. Rufián-Henares J.A.; Morales F.J. Functional properties of melanoidins: in vitro antioxidant, antimicrobial and antihypertensive activities. Food Res Int. 2007;40(8):995-1002. doi: 10.1016/j.foodres.2007.05.002.
9.Wang H.-Y.; Qian H.; Yao W.-R. Melanoidins produced by the Maillard reaction: structure and biological activity. Food Chem. 2011;128(3):573-84. doi: 10.1016/j.foodchem.2011.03.075.
10. Dasgupta A.; Klein K. Tea, coffee, and chocolate. In: Antioxidants in food, vitamins and supplements. Elsevier: San Diego; 2014. p. 237-57. doi: 10.1016/b978-0-12-405872-9.00013-6.
11. Poisson L.; Blank I.; Dunkel A.; Hofmann T. The chemistry of roasting - Decoding flavor formation. In: The craft and science of coffee. Folmer B, editor. Academic Press: 2017. p. 273-309. doi: 10.1016/b978-0-12-803520-7.00012-8.
12. López-Galilea I.; Andriot I.; de Pena M.P.; Cid C.; Guichard E. How does roasting process influence the retention of coffee aroma compounds by lyophilized coffee extract? J Food Sci. 2008;73(3):S165–S71. doi: 10.1111/j.1750-3841.2008.00672.x.
13. Nunes F.M.; Coimbra M.A. Role of hydroxycinnamates in coffee melanoidin formation. Phytochem Rev. 2009;9(1):171-85. doi: 10.1007/s11101-009-9151-7.
14. Bartel C.; Mesias M.; Morales F.J. Investigation on the extractability of melanoidins in portioned espresso coffee. Food Res Int. 2015; 67:356-65. doi: 10.1016/j.foodres.2014.11.053.
15. Ludwig I.A.; Sanchez L.; Caemmerer B.; Kroh L.W.; De Peña M.P.; Cid C. Extraction of coffee antioxidants: impact of brewing time and method. Food Res Int. 2012;48(1):57-64. doi: 10.1016/j.foodres.2012.02.023.
16. Gniechwitz D.; Reichardt N.; Ralph J.; Blaut M.; Steinhart H.; Bunzel M. Isolation and characterisation of a coffee melanoidin fraction. J Sci Food Agric. 2008;88(12):2153-60. doi: 10.1002/jsfa.3327.
17. Esquivel P.; Jiménez V.M. Functional properties of coffee and coffee by-products. Food Res Int. 2012;46(2):488-95. doi: 10.1016/j.foodres.2011.05.028.
18.Tagliazucchi D. Melanoidins from coffee and lipid peroxidation. In: Coffee in health and disease prevention. Academic Press: San Diego; 2015. p. 859-67. doi: 10.1016/b978-0-12-409517-5.00095-4.
19. Kucera L.; Papousek R.; Kurka O.; Bartak P.; Bednar P. Study of composition of espresso coffee prepared from various roast degrees of Coffea arabica L. coffee beans. Food Chem. 2016;199:727-35. doi: 10.1016/j.foodchem.2015.12.080.
20. Simoes J.; Madureira P.; Nunes F.M.; Domingues Mdo R.; Vilanova M.; Coimbra M.A. Immunostimulatory properties of coffee mannans. Mol Nutr Food Res. 2009;53(8):1036-43. doi: 10.1002/mnfr.200800385.
21. Esposito F.; Morisco F.; Verde V.; Ritieni A.; Alezio A.; Caporaso N., et al. Moderate coffee consumption increases plasma glutathione but not homocysteine in healthy subjects. Aliment Pharmacol Ther. 2003;17(4):595-601. doi: 10.1046/j.1365-2036.2003.01429.x.
22. Cämmerer B.; Kroh L.W. Antioxidant activity of coffee brews. Eur Food Res Technol. 2006;223(4):469-74. doi: 10.1007/s00217-005-0226-4.
23.Wang X.; Lim L.-T. Physicochemical characteristics of roasted coffee. In: Coffee in health and disease prevention. Academic Press: San Diego; 2015. p. 247-54. doi: 10.1016/b978-0-12-409517-5.00027-9.
24.Vignoli J.A.; Viegas M.C.; Bassoli D.G.; Benassi M.d.T. Roasting process affects differently the bioactive compounds and the antioxidant activity of arabica and robusta coffees. Food Res Int. 2014;61:279-85. doi: 10.1016/j.foodres.2013.06.006.
25. Argirova M.D.; Stefanova I.D.; Krustev A.D. New biological properties of coffee melanoidins. Food Funct. 2013;4(8):1204-08. doi: 10.1039/c3fo60025d.
26. Bravo J.; Juaniz I.; Monente C.; Caemmerer B.; Kroh L.W.; De Pena M.P., et al. Evaluation of spent coffee obtained from the most common coffeemakers as a source of hydrophilic bioactive compounds. J Agric Food Chem. 2012;60(51):12565-73. doi: 10.1021/jf3040594.
27. Jiménez-Zamora A.; Pastoriza S.; Rufián-Henares J.A. Revalorization of coffee by-products. prebiotic, antimicrobial and antioxidant properties. LWT - Food Science and Technology. 2015;61(1):12-18. doi: 10.1016/j.lwt.2014.11.031.
28. Rodrigues N.P.; Bragagnolo N. Identification and quantification of bioactive compounds in coffee brews by HPLC–DAD–MSn. J Food Compost Anal. 2013;32(2):105-15. doi: 10.1016/j.jfca.2013.09.002.
29. Martinez-Saez N.; Ullate M.; Martin-Cabrejas M.A.; Martorell P.; Genoves S.; Ramon D., et al. A novel antioxidant beverage for body weight control based on coffee silverskin. Food Chem. 2014;150:227-34. doi: 10.1016/j.foodchem.2013.10.100.
30. Komes D.; Bušić A. Antioxidants in coffee. In: Processing and impact on antioxidants in beverages. Preedy V, editor. Academic Press: San Diego; 2014. p. 25-32. doi: 10.1016/b978-0-12-404738-9.00003-9.
31. Vitaglione P.; Fogliano V.; Pellegrini N. Coffee, colon function and colorectal cancer. Food Funct. 2012;3(9):916-22. doi: 10.1039/c2fo30037k.
32. Kroh L.W.; Fiedler T.; Wagner J. α-Dicarbonyl compounds--key intermediates for the formation of carbohydrate-based melanoidins. Ann N Y Acad Sci. 2008;1126:210-15. doi: 10.1196/annals.1433.058.
33. Hwang C.-F.; Chen C.-C.; Ho C.-T. Contribution of coffee proteins to roasted coffee volatiles in a model system. Int J Food Sci Technol. 2012;47(10):2117-26. doi: 10.1111/j.1365-2621.2012.03078.x.
34. Oestreich-Janzen S. Chemistry of coffee. In: Comprehensive natural products II. Elsevier: Oxford; 2010. p. 1085-117. doi: 10.1016/b978-008045382-8.00708-5.
35.Liu Y.; Kitts D.D. Confirmation that the Maillard reaction is the principle contributor to the antioxidant capacity of coffee brews. Food Res Int. 2011;44(8):2418-24. doi: 10.1016/j.foodres.2010.12.037.
36. Bravo J.; Monente C.; Juániz I.; De Peña M.P.; Cid C. Influence of extraction process on antioxidant capacity of spent coffee. Food Res Int. 2013;50(2):610-16. doi: 10.1016/j.foodres.2011.04.026.
37. Campos-Vega R.; Loarca-Piña G.; Vergara-Castañeda H.A.; Oomah B.D. Spent coffee grounds: a review on current research and future prospects. Trends Food Sci Technol. 2015;45(1):24-36. doi: 10.1016/j.tifs.2015.04.012.
38. Kitzberger C.S.G.; Scholz M.B.d.S.; Benassi M.d.T. Bioactive compounds content in roasted coffee from traditional and modern Coffea arabica cultivars grown under the same edapho-climatic conditions. Food Res Int. 2014;61:61-6. doi: 10.1016/j.foodres.2014.04.031.
39. Folmer B.; Farah A.; Jones L.; Fogliano V. Human Wellbeing - Sociability, performance, and health. In: The craft and science of coffee. Folmer B, editor. Academic Press: 2017. p. 493-520. doi: 10.1016/b978-0-12-803520-7.00020-7.
40. Moreira A.S.P.; Nunes F.M.; Domingues M.R.M.; Coimbra M.A. Galactomannans in coffee. In: Coffee in health and disease prevention. Academic Press: San Diego; 2015. p. 173-82. doi: 10.1016/b978-0-12-409517-5.00019-x.
41.Ballesteros L.F.; Teixeira J.A.; Mussatto S.I. Chemical, functional, and structural properties of spent coffee grounds and coffee silverskin. Food Bioproc Tech. 2014;7(12):3493-503. doi: 10.1007/s11947-014-1349-z.
42. Kovalcik A.; Obruca S.; Marova I. Valorization of spent coffee grounds: a review. Food Bioprod Process. 2018; 110:104-19. doi: 10.1016/j.fbp.2018.05.002.
43. Jeszka-Skowron M.; Stanisz E.; De Peña M.P. Relationship between antioxidant capacity, chlorogenic acids and elemental composition of green coffee. Lwt. 2016;73:243-50. doi: 10.1016/j.lwt.2016.06.018.
44. Grassi D.; Desideri G.; Ferri C. Blood pressure and cardiovascular risk: what about cocoa and chocolate? Arch Biochem Biophys. 2010;501(1):112-15. doi: 10.1016/j.abb.2010.05.020.
45. Sarmadi B.; Ismail A.; Hamid M. Antioxidant and angiotensin converting enzyme (ACE) inhibitory activities of cocoa (Theobroma cacao L.) autolysates. Food Res Int. 2011;44(1):290-96. doi: 10.1016/j.foodres.2010.10.017.
46. Grassi D.; Ferri C. Cocoa, flavonoids and cardiovascular protection. In: Polyphenols in human health and disease. Academic Press: San Diego; 2014. p. 1009-23. doi: 10.1016/b978-0-12-398456-2.00078-5.
47. Nakayama T.; Oishi K. Influence of coffee (Coffea arabica) and galacto-oligosaccharide consumption on intestinal microbiota and the host responses. FEMS Microbiol Lett. 2013;343(2):161-68. doi: 10.1111/1574-6968.12142.
48. Friedman M. Biological effects of Maillard browning products that may affect acrylamide safety in food. In: Chemistry and safety of acrylamide in food. Advances in experimental medicine and biology. 561. Friedman M, Mottram D, editors. Springer US: 2005. p. 135-56. doi: 10.1007/0-387-24980-X_12.
49. Parras P.; Martineztome M.; Jimenez A.; Murcia M. Antioxidant capacity of coffees of several origins brewed following three different procedures. Food Chem. 2007;102(3):582-92. doi: 10.1016/j.foodchem.2006.05.037.
50. Castillo M.D.d.; Gordon M.H.; Ames J.M. Peroxyl radical-scavenging activity of coffee brews. Eur Food Res Technol. 2005;221(3-4):471-77. doi: 10.1007/s00217-005-1209-1.
Received: 25 March 2022 / Accepted: 15 july 2022 / Published:15 Agoust 2022
Citation: Portillo, O.R..; Arévalo, A.C. Coffee’s Melanoidins. A critical review of contemporary scientific literature. Revis Bionatura 2022;7(3) 4. http://dx.doi.org/10.21931/RB/2022.07.03.4
