2023.08.04.97
Files > Volume 8 > Vol 8 no 4 2023
Evaluation of the anti-inflammatory effect of plant extracts from Miconia pseudocentrophora, Brachyotum ledifolium, and Fuchsia loxensis in rats

Parra
Álvarez Paulina Fernanda1, Basantes
Vaca Carmen Viviana1,*, Mera
Cabezas Luis Alberto1, Benavides
Enríquez Celso Vladimir1
1
Facultad de Ciencias de la Educación
Humanas y Tecnologías, Universidad Nacional de Chimborazo (UNACH), 0601003,
Riobamba, Ecuador; [email protected],; [email protected],; [email protected],; [email protected],
Available from.
http://dx.doi.org/10.21931/RB/2023.08.04.97
ABSTRACT
Miconia pseudocentrophora, Brachyotum ledifolium,
and Fuchsia loxensis are some of the Ecuadorian ancestral medicines, a
heritage passed down through generations for treating various ailments,
including inflammation. This pioneering study delves into the ethnopharmacological
properties of extracts from these plants' leaves, stems, and fruits collected
in their native Ecuadorian habitats. The ethanolic and chloroform sub-extracts
underwent meticulous quality assessment, with the ethanolic extract efficiency
yielding between 78.6-98.5%. Phytochemical screening uncovered various
secondary metabolites, encompassing flavonoids, alkaloids, quinones,
triterpenes, and reducing sugars. In vivo evaluation at 1, 3, 5, 7, and 8 hours
of treatment, utilizing a rat paw-edema model, demonstrated a significant
reduction in inflammation volume comparable to naproxen sodium. The maximum
effect was observed after 3 hours of treatment. Miconia's chloroform
sub-extract exhibited superior performance, achieving a 54% inhibition of
inflammation, followed by Brachyotum and Fuchsia, both with 52%.
These findings support the traditional medicinal efficacy of these plants and
underscore the need for further exploration, holding considerable promise for
the pharmaceutical industry.
Keywords:
ethnopharmacology, anti-inflammation, percentage
inhibition, carrageenan-induced model, phytochemical screening.
INTRODUCTION
Throughout ancient times, plants have stood as the primary source of
medicinal preparations, having cultivated an ancestral medicine that endures to
the present day. This legacy remains a focal point in pharmaceutical research,
where exploration into conventional medicine relies on the empirical knowledge
transmitted across generations. The extensive diversity of plant species
worldwide still presents an untapped frontier. Numerous native species from the
Andean region of Ecuador captivate attention with their vibrant and intense
color palette1 . Beyond their allure to
pollinators, these colors imply the presence of flavonoids, secondary
metabolites recognized for their role in enhancing vivid colors and identification
in medicine as anti-inflammatory agents2 .
Inflammation is a body's physiological response aimed at protecting and
freeing the individual from the initial cause of injury and its consequences. Inflammation is characterized by persistent pain, swelling, and functional impairment
of the affected area to facilitate the destruction of pathogenic microorganisms.
Subsequently, it promotes the repair and healing of the injured tissue3 . Without the inflammatory
reaction, infections and toxins would spread throughout the body, causing
severe injuries and, ultimately, death4 . Inflammation is recognized to
consist primarily of three fundamental components. Firstly, there is a
modification in the caliber of vessels, resulting in an increased regional
blood flow to the affected area. Simultaneously, alterations in microvascular
permeability occur, facilitating the exit of cells and substances into the
interstitium. Finally, the migration of leukocytes is observed, leaving the
bloodstream to head toward the focus of the lesion, where the inflammatory
response is activated and organized4 . Chemical mediators involved in
this process include histamine, serotonin, lysosomal enzymes derived from
neutrophils, prostaglandins, leukotrienes, cytokines, nitric oxide, and
complement factors5 . Despite inflammation serving a
protective function, it can also be a tissue or systemic injury source. While
it eliminates the causal agent, it may simultaneously cause damage to the
affected organs and systems, leading to a complex interplay between protective
and detrimental effects. Inflammation can manifest acutely or be part of a
chronic disease that affects one or more organs in the body6 .
The metabolism of arachidonic acid, a component of the lipid portion of
the cell membrane, gives rise to inflammatory mediators of significant
biochemical and pharmacological importance. Different enzymes act on this
compound: cyclooxygenase produces prostaglandins and thromboxanes, while
lipoxygenase produces leukotrienes7 . Prostaglandins can modify the deformation and passage of leukocytes
through capillary walls, reduce gastric acid secretion, alter pituitary
functions, stimulate renin secretion, and, most importantly, are essential for maintaining
both macro and microvascular circulation7 .
Compounds
derived from plants, encompassing various chemical classes, have shown
established anti-inflammatory properties. The utilization of medicinal plants
and their secondary metabolites is increasingly prevalent in treating diseases
as a form of complementary medicine8 . Notably, alkaloids, terpenes, and phenolic
compounds like tannins, lignans, coumarins, and saponins, with a particular
emphasis on flavonoids, are prominent among them9–12 .
Flavonoids stand by exhibiting a high affinity for binding to proteins
and other biological macromolecules andetallic anions13,14 . Additionally, they
possess a significant ability to catalyze electron transport and capture free
radicals13 . Recently, there has been a
renewed interest in ethnopharmacology in Ecuador, addressing diversepecies15–20 . In the present study, we
have undertaken the characterization for the first time of the plants Miconia
pseudocentrophora, Brachyotum ledifolium, and Fuchsia loxensis, in
response to their significance in traditional medicine.
Regarding the Brachyotum genus, it has been reported that several
species are used as medicinal plants for treating chicken colds, dyeing, and construction,
as well as for firewood and brooms. In the specific case of Brachyotum
ledifolium, it is used medicinally by the Andean Kichwas21 . For Miconia
pseudocentrophora, there are no reports of its medicinal application;
however, species of the genus Miconia have been known to have
applications as a vermifuge, to stimulate dilation during childbirth, cure
throat and neck pain, treat tuberculosis, toothaches, oral infections, mycosis,
scabies, and to cure diarrhea in newborns. They can even be an antidote for ant
bites and abscesses 21 . Regarding Fuchsia loxensis,
there are no specific studies concerning its medicinal properties. However, it
has been observed that species from the Onagraceae family exhibits
pharmacological activities, including anti-inflammatory, antiarthritic,
analgesic, antioxidant, cytotoxic, antidiabetic, and antimicrobial effects22 .
Miconia pseudocentrophora (Melastomataceae), or "colca",
is a shrub or small tree with simple and opposite leaves. The leaves have
entire margins and a brownish pubescent underside, displaying main veins
extending from the base to the apex. The flowers are arranged in terminal
panicles, showcasing five white petals, white filaments, and purple anthers.
The fruits are green berries in their immature state, turning black upon
maturation, and contain numerous seeds23 .
Brachyotum ledifolium (Melastomataceae), commonly known
as "llumbre", "puka chaklla", or "inchichaklla"
from the Kichwa language, and is also known as "arete de inca" or
Inca earrings. This shrub has peeling bark, and ovate leaves covered with tiny
hairs. The hanging flowers have a red calyx with yellow hairs, and the
pale-yellow petals form a tube. The fruits are dry with tiny seeds24 . The
flowers are edible and sweet, and their stems serve multiple purposes, such as
brooms, beams and arches for building and decorations for festivities. The
juice is utilized for extracting indelible dyes and treating poultry colds.
Additionally, it finds application in agroforestry as a live fence24 .
Fuchsia loxensis (Onagraceae), also known as "pena
pena". Is a climber shrub with opposite leaves. The leaves are lanceolate
and simple, although they typically have serrated margins and are evergreen.
The flowers are pendulous, hanging from long peduncles that give them a
downward-facing appearance. The characteristic intense fuchsia calyx is
cylindrical, with four lobes, and the corolla consists of four petals,
resembling decorative earrings. The plant has four elongated, narrow sepals and
four short and wide petals. The fruit is a small berry, ranging from dark
red-green to intense red, it is edible and contains numerous small seeds. Some
indigenous groups use Fuchsia loxensis as medicine and hasigh potential as
an ornamental25,26 .
The present research aims to characterize the native plant species Miconia
pseudocentrophora, Brachyotum ledifolium, and Fuchsia loxensis and
evaluate their potential as anti-inflammatory agents. Our study started by
collecting plant material from the species for taxonomic identification.
Subsequently, the preparation of ethanolic extracts and chloroform sub-extracts
from this material occurred, followed by a comprehensive assessment of their
physical and chemical properties. The evaluation involved determining the
humidity content through the gravimetric method, ensuring avoidance of
enzymatic procedures. Additionally, total ashes were quantified for quality
assessment. Then, the anti-inflammatory effect was evaluated by using the
ethanolic extracts and chloroform sub-extracts from the mentioned plant
species, followed by in vivo tests conducted on Winstar rats. The
carrageenan-induced paw edema model was employed for screening the
anti-inflammatory efficacy in the acute phase of inflammation. Finally, for a
more rigorous analysis of the observed anti-inflammatory effect, a statistical
analysis was applied to ensure the validity and reliability of the results
obtained at each stage of the research.
MATERIALS
AND METHODS
Plant materials
The
leaves stems, and fruits of Miconia pseudocentrophora,
Brachyotum ledifolium, and Fuchsia loxensis
were collected in a rainy season (february) in the native forest of San
Francisco de Guayllabamba, Chambo, Chimborazo Province (Ecuador) and identified
in the herbarium of the Escuela Superior Politécnica de Chimborazo (ESPOCH) by
the curator Jorge Caranqui.
Quality
assessment was based on the determination of the humidity content (< 10%) to
avoid enzymatic procedures and it was performed through the gravimetric method.
Quality was also assessed employing the determination of total ashes.
Preparation
of plant extracts
The
preparation of the alcoholic extract involved the processing of plants by
cutting and triturating the leaves, stems, and fruits. Ethanol 96% is added,
and the mixture is left to macerate for 2 to 3 days at room temperature. After
maceration, the solution is decanted, and the filtrate is concentrated to
one-eighth of the initial volume. The resulting solution is then refrigerated
to eliminate chlorophyll, yielding the alcoholic extract alone.
To
prepare the alcoholic sub-extracts, chloroform was used to acquire the
secondary metabolites. An aliquot of 25 ml is taken from the alcoholic extract,
and placed in a separation funnel, and an equal volume of chloroform is added.
The mixture is stirred, and allowed to settle. Once two phases are formed, the
upper phase corresponding to the ethanolic extract is separated, and the lower
phase containing chloroform is collected in a previously tared container. This
process is repeated until the solution is transparent. Subsequently, all
chloroform phases are combined, the solvent is removed, and the sub-extract is
obtained. To reconstitute the extract and achieve the required volume for
application in rats, a liquid-liquid emulsion is prepared using water,
sub-extract, and alcohol, followed by alcohol evaporation.
Quality
control of plant extracts was performed to ensure purity, health, and interference-free
analysis. Organoleptic properties were tested by checking odor, color, taste,
and appearance. Properties like pH, refraction index, and relative density were
also determined.
Phytochemical
screening
Phytochemical
screening allowed us to qualitatively determine plants' main chemical group
constituents as secondary metabolites. Different approaches were used for the
phytochemical screening in this research: Dragendorff assay, Mayer assay,
Wagner assay, Baljet assay, Ferrum chloride assay, Foam assay, Shinoda assay,
and Bitter principles assay.
Animals
Twenty
adult (8 weeks-old) Wistar rats (180 -200 g) were obtained from the animal
facility of the Faculty of Sciences (ESPOCH), 13 females and 7 male rats. Three
repetitions were performed
per batch (alcoholic extract: L1, L2, L3); (chloroform
sub-extract: Ls1, Ls2, Ls3).
Animals were provided with water and diet ad
libitum.
Carrageenan-induced
rat paw edema model
Subcutaneous
administration of 0.5 ml of carrageenan solution at the rat's plantar
aponeurosis level was inoculated to induce an inflammatory reaction in the
posterior legs. The assays were
performed in batches of three rats. The normal volumes of the rats'
right hind legs were measured. The formulations were administered orally. The
control group received only the vehicle (distilled water), while the
experimental group received a Naproxen sodium 550mg (7.86 mg/kg). The experimental
group received 0.1 ml of Miconia pseudocentrophora, Brachyotum ledifolium, and
Fuchsia loxensis extracts and subtracted at concentrations of 100% via oral.
Thirty
minutes after administering the test substances, edema was induced by injecting
0.1 ml of a 0.5% aqueous carrageenan solution into the right plantar
aponeurosis of the rats. The volume of the inflamed right leg (length and
diameter) was measured. Inflammation was quantified by measuring the volume of
the legs at 0, 1, 3, 5, 7, and 8 hours after carrageenan injection. The
difference in volume between the inflamed right leg and the same normal right
leg before the carrageenan injection indicates the degree of inflammation.
Induced
inflammation volume was computed with the formula.
where
d represents the diameter of the leg and h is the length. The
results were determined as inflammation percentage applying the formula
Vpoxitive
control is the volume of the inflamed leg at
time X, and Vnormal is the length before the
application of the carrageenan.
The
inhibition percentage of the inflammatory reaction induced by carrageenan is
computed in acute phase at 1, 3, 5, 7, and 8 hours after the inoculation and is
calculated through:

Where Vproblem
control is the
volume of the inflamed leg without anti-inflammatory, Vpositive control represents the volume of the inflamed leg with
anti-inflammatory, and Vnormal is the volume before
the application of the carrageenan.
Statistical
analysis
Statistical
analysis was performed using R software. One-way analysis of variance (ANOVA) and
multiple Tukey comparison tests were carried out. A p-value < 0.05
was considered statistically significant.
Ethical
considerations
Experimental
procedures and protocols used in this study were approved by the animal
facility committee of the Escuela Superior Politécnica de Chimborazo (ESPOCH).
RESULTS
AND DISCUSSION
Quality assessment
of the Miconia
pseudocentrophora, Brachyotum ledifolium, and Fuchsia loxensis extracts.
To perform
quality control, leaves, stems, and fruits of Miconia pseudocentrophora, Brachyotum ledifolium, and Fuchsia loxensis were collected. The samples were
analyzed in triplicate for each test. The physical and chemical properties
obtained results are presented in Table 1. The percentage of humidity in the
fresh plant can be observed, yielding a value of 79.48% for Fuchsia loxensis, 49.49% for Brachyotum ledifolium, and 48.90% for Miconia pseudocentrophora. These values reflect the high
amount of free water present in the plant material. Newly harvested plants
contain significant water, varying in different organs. Therefore, the moisture
content is higher for Fuchsia loxensis, possibly due to the high humidity
in the area where the plant material was collected, characterized by fog27 . Another contributing factor could be that it
is a less woody shrub, retaining more water.
Additionally,
Table 1 shows the total ash content in the fresh plant, which is 4.07% for Brachyotum ledifolium, and 3.70% for Fuchsia loxensis, which may be attributed to its
thick and deep roots that absorb a higher number of organic compounds. For Miconia pseudocentrophora, the ash content is 2.91%, indicating
that this plant belongs to materials less submerged underground. These results
align with established limits, as traditional medicinal plants typically
exhibit ash contents up to 12%. Exceeding this threshold would warrant excluding
the plant material, indicating potential contamination with earthy materials
such as salts, sand, or heavy metals 28 .
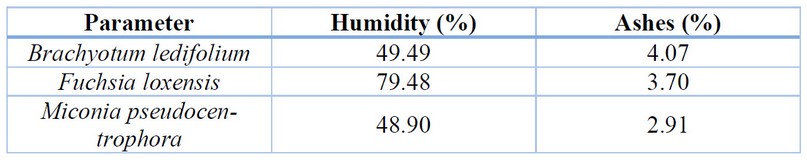
Table 1. Results of the
determination of the humidity and ashes content of the studied plants.
Characterization of the extracts from Miconia
pseudocentrophora, Brachyotum ledifolium, and
Fuchsia loxensis
The
extraction of active principles is owed to the difference in osmotic pressure
within the plant cell. This process occurs as the solvent (ethanol) fills the
space between intracellular and extracellular fluids. Eventually, the cell
undergoes lysis, releasing its contents into the solvent. A portion of this
content constitutes the active principles responsible for the anti-inflammatory
activity.
Table 2 presents the physicochemical properties of the
plant extracts. Notably, for Brachyotum ledifolium,
the concentrated extract has a yield
percentage of 78.6%, implying its origin from a woody shrub with shallow roots,
resulting in reduced water absorption. The organoleptic characteristics of the
ethanolic extract include a liquid consistency, yellowish-green color, and a
slightly sweet taste, potentially attributed to the presence of sugars like
glucose in the plant, accompanied by a robust herbal aroma. The ethanolic
extract exhibits a pH of 4.18, indicating the presence of weakly acidic
chemical compounds. In comparison to the density of the solvent used (ethanol,
0.91 g/ml), this divergence implies the existence of dissolved substances
responsible for the observed activity. The refractive index, measuring 1.355,
further supports the presence of dissolved substances and hints at the
potentially lowest extract viscosity.
The concentrated extract from Fuchsia
loxensis shows a yield of 89.2% (Table 2),
considered optimal due to the woody nature of the plant, concentrating moisture
in leaves, flowers, and fruits. The ethanolic extract exhibits liquid
organoleptic characteristics, with a yellowish-brown hue, a possible sweet
taste attributed to glucose esters, and a distinctive odor. With a pH of 4.18,
it suggests the presence of weakly acidic compounds such as phenols, tannins,
and flavonoids. Comparing its density (0.91 g/ml) to that of the solvent
(ethanol) indicates the existence of dissolved substances. The refractive
index, 1.355, denotes the presence of dissolved substances, slightly higher
than that of water. It is essential to note that quality parameters lack
specific reference standards, as each extract species possesses its own values
and characteristics.
In the case of Miconia pseudocentrophora (Table
2), the extract yield percentage is 97.5%, with potential variability based on
the solvent recovery method, in this case, the rotavapor. The direct
distillation method was employed, and the plant-to-70% ethanol ratio increased
with maceration time, influencing the overall yield. The ethanolic extract
exhibited organoleptic characteristics of a liquid with a brown color and a
bitter taste typical of young plants. The pH of the ethanolic extract was 3.60,
indicating the presence of acidic compounds (phenols, tannins, flavonoids,
etc.) with OH characteristics. Notably, the extract's density was higher than
the solvent density (ethanol, 0.97 g/ml), suggesting the presence of dissolved
substances. The refractive index, at 1.363, further supported the presence of
dissolved substances, slightly surpassing the index of water (1.333). This
finding emphasizes the extract's purity relative to water density.
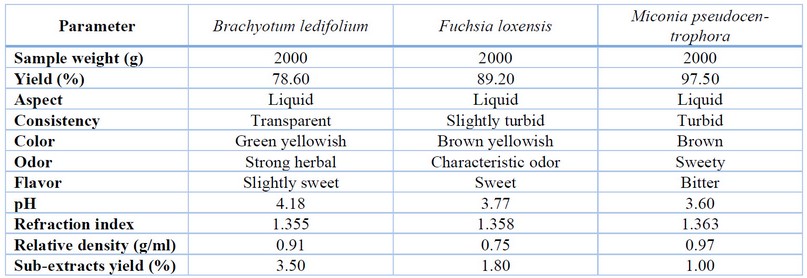
Table 2. Results of the determination of
the physicochemical properties of the plant extracts.
Phytochemical
screening
Qualitative assays, employing precipitation and coloration changes, were
conducted to determine the presence of secondary metabolites. The results, as
depicted in Table 3, encompass the screening outcomes of eight distinct
techniques aimed at identifying various compound types. Specifically, for Brachyotum
ledifolium and Miconia pseudocentrophora, both belonging to the
Melastomataceae family, the assays confirmed the presence of tannins,
flavonoids, alkaloids, quinones, triterpenes, steroids, and reducing sugars.
Meanwhile, the extract from Fuchsia loxensis, of the Onagraceae family,
not only exhibited positive results for the aforementioned secondary
metabolites shared with the Melastomataceae family but also tested positive for
coumarins.
Our findings from Miconia pseudocentrophora align
with those reported by Mencías Paredes29 conducted similar research in a comparable
region, supporting their screening outcomes through chromatographic assays and
the identification of purified terpenoids and flavonoids as secondary
metabolites. Regarding Fuchsia loxensis
and Brachyotum ledifolium, no prior characterizations exist, although
other members of the Melastomataceae30,31 and Onagraceae32 families have undergone similar studies and
align with our results. Hydroalcoholic extracts from the Onagraceae family
demonstrated anti-inflammatory, antimicrobial, antiproliferative, and
anti-angiogenic properties attributed to polyphenols and flavonoids (gallic
acid, caffeic acid, epicatechin, coumaric acid, ferulic acid, rutin, and
rosmarinic acid), as determined by Shimadzu Chromatograph32 . In the case of the Melastomataceae family,
antioxidant properties have been observed, linked to the presence of phenolic
compounds, flavonoids, and fatty acids alongside topical anti-inflammatory
activity with low toxicity30 .
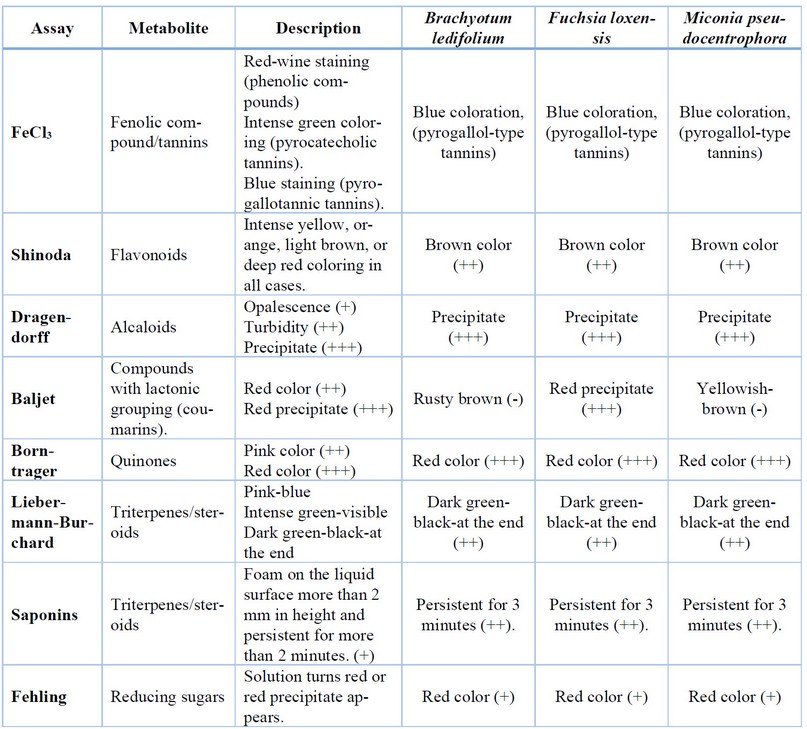
+++ strong evidence, ++ evidence, + low evidence, - negative
Table
3. Results of the different phytochemical screening assays of the plant
extracts to determine the presence of secondary metabolites with potential
anti-inflammatory activity.
Inflammation
volume in carrageenan-induced rat paw edema model in Rattus norvegicus
Carrageenan-induced paw edema in rats served as the in
vivo model for analyzing the anti-inflammatory effects of plant extracts
from Miconia pseudocentrophora, Brachyotum ledifolium, and Fuchsia
loxensis. The paw volume was measured after inducing inflammation and
administering each plant extract to achieve this. The corresponding values are
detailed in Table 4 and Figure 1. Both compounds, ethanolic and chloroform
sub-extracts, show performance comparable to naproxen sodium. The most
efficient appears to be chloroform sub-extract and Melastomataceae extracts
after quickly reducing the volume of the inflamed paw. Besides, the peak of
inflammation was seen at the third hour of the experiment and then started to
decline.
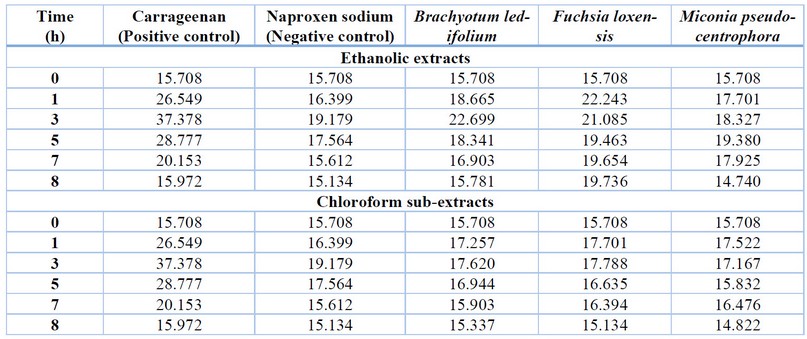
Table 4.
Inflammation volume (cm3) after treatment with the studied plant
extracts.
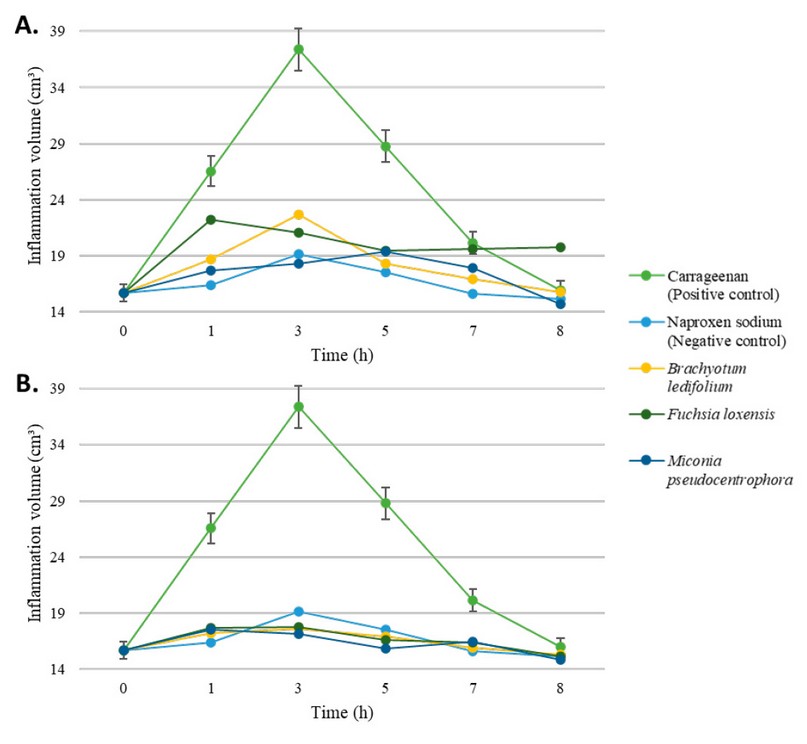
Figure 1. Effect through time of ethanolic (A) and
chloroform-based (B) plant extracts over inflammation on rats' paw. Control
positive is carrageenan alone (green), while negative control is given by the
administration of Naproxen sodium (light blue). Error percentage bars are
included.
Inhibition
percentage produced by the ethanolic extracts and chloroform sub-extracts
To conduct a comprehensive statistical analysis of
the anti-inflammatory activity of the different obtained extracts, assays were
performed in batches of three rats, using a negative control group and a
positive control group as reference. These groups were analyzed at six different
time points (0, 1, 3, 5, 7, and 8 hours) during the inflammation process, as
shown in Table 5.
After one hour of conducting
the study, it was observed that all groups exhibited a similar percentage of
inflammation inhibition, around 33%. The only extract showing a relatively
lower performance is the alcoholic extract of Fuchsia loxensis with an
inhibition percentage of 16%. Later on, the trend is that at 3 hours, the
percentage of inflammation inhibition increases, reaching a maximum of 54%, and
from that point onward, it begins to decline. At 7 hours, the average
inflammation is 15%, and by 8 hours, it drops to 1%. Notably, the chloroform
sub-extract of Miconia pseudocentrophora performs the best, while the
ethanolic extract of Fuchsia loxensis shows the lowest performance.
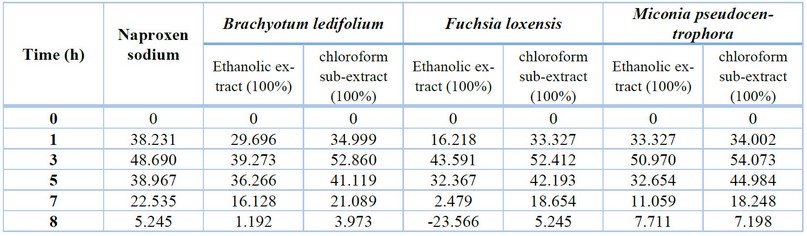
Table
5. The anti-inflammatory activity of ethanolic extracts and chloroform
sub-extracts is represented as the average percentage of inflammation
inhibition (%).
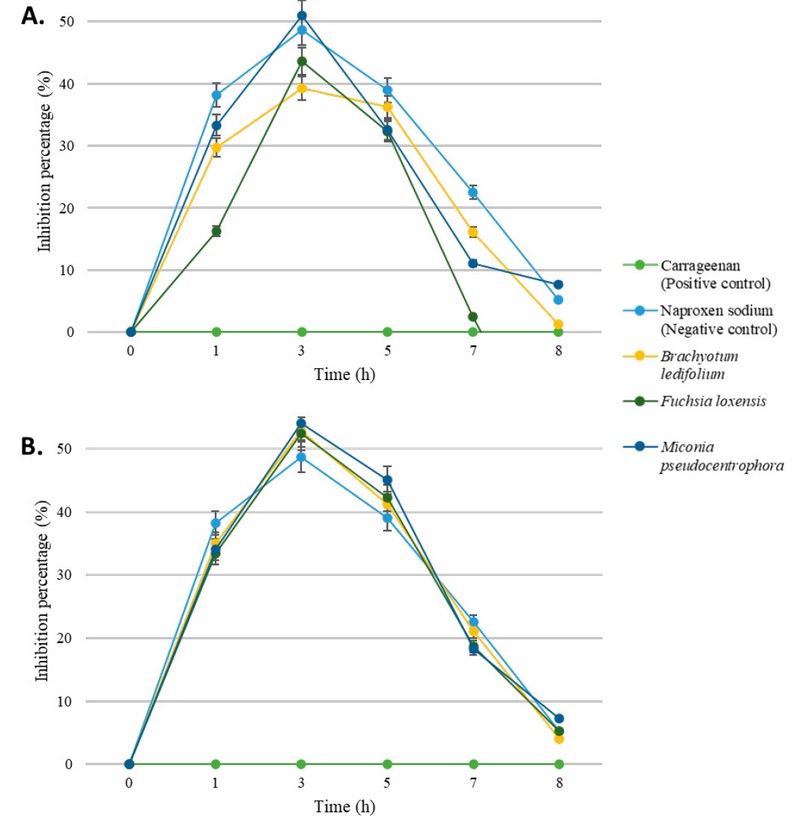
Figure 2. Inflammation
inhibition percentage through time of ethanolic (A) and chloroform-based (B)
plant extracts over carrageenan-induced model. Error percentage bars are
included.
The ANOVA statistical test was employed to conduct a
comprehensive analysis and ascertain the efficacy of each tested extract,
followed by post hoc multiple comparisons using the Tukey HSD test at a 95%
confidence level. The null hypothesis (H0) postulates that there is
no significant difference among the study groups concerning their
anti-inflammatory activity, while the alternative hypothesis asserts that at
least one of the study groups displays a statistically significant difference
in anti-inflammatory activity. The decision to accept or reject the null
hypothesis hinges on the p-value; if it falls below 0.05, the null hypothesis
is rejected, and the alternative hypothesis is accepted. In terms of the
p-values, after 1 hour of experimentation is 0.0002×10-5, at 3 hours
it is 0.0008×10-2, at 5 hours it is 0.0006×10-5, at 7
hours it is 0.0006, and at 8 hours it is 0.0003×10-1. Consequently,
at every point in time, we reject H0 and accept the alternative
hypothesis, affirming the existence of statistical differences between groups.
CONCLUSIONS
This
study is presented as the first-time evaluation of the ethnopharmacological
properties of the extracts from Miconia pseudocentrophora, Brachyotum
ledifolium, and Fuchsia loxensis collected in situ. The extracts,
characterized with attention to quality through humidity and ash content
assays, exhibited ethanolic extract yields of Miconia pseudocentrophora:
97.5%, Brachyotum ledifolium: 78.6%, and Fuchsia loxensis: 89.2%,
along with chloroform sub-extract yields of Miconia pseudocentrophora:
1%, Brachyotum ledifolium: 3.5%, and Fuchsia loxensis: 1.8%.
The
comprehensive characterization of these extracts provided insights into their
purity based on various physicochemical properties. A phytochemical screening
employing multiple qualitative assays unveiled the presence of diverse
secondary metabolites, notably flavonoids, alkaloids, quinones, triterpenes,
and reducing sugars. Given flavonoids' well-known anti-inflammatory properties,
our observed inflammation reduction in animal models is noteworthy. Although
attributing the effect to a specific secondary metabolite remains challenging,
further studies are imperative to explore this aspect further. The remarkable
reduction in paw inflammation volume in our rat models, comparable to naproxen
sodium, supports the potential anti-inflammatory activity of Miconia
pseudocentrophora, Brachyotum ledifolium, and Fuchsia loxensis
across leaves, stems, and fruits. These findings validate the traditional
medicinal properties of these plants, warranting additional research into
toxicology, determination of minimum effective doses, and stability of the
phytopharmaceuticals, with potential implications for the pharmaceutical
industry.
Author Contributions: P.F.P.A. conducted the experiments,
writing—original draft preparation; C.V.B.E. supervision; C.V.B.V., L.A.M.C.,
and C.V.B.E. writing—review and editing. All authors have read and agreed to
the published version of the manuscript.
Conflicts of Interest: The authors declare no
conflict of interest.
REFERENCES
1. Coyago-Cruz
E, Guachamin A, Vera E, Moya M, Heredia-Moya J, Beltrán E. Physicochemical
characteristics and antioxidant capacity of Ecuadorian paramo flowers. Vol. 8,
Bionatura. Centro de Biotecnologia y Biomedicina, Clinical Biotec. Universidad
Católica del Oriente (UCO), Univesidad Yachay Tech; 2023.
2. Abou
Baker DH. An ethnopharmacological review on the therapeutical properties of
flavonoids and their mechanisms of actions: A comprehensive review based on up
to date knowledge. Vol. 9, Toxicology Reports. Elsevier Inc.; 2022. p. 445–69.
3. Chen
L, Deng H, Cui H, Fang J, Zuo Z, Deng J, et al. Inflammatory responses and
inflammation-associated diseases in organs. Oncotarget [Internet].
2018;9(6):7204–18. Available from: www.impactjournals.com/oncotarget/
4. García
Barreno P. INFLAMACIÓN. Vol. 102, Cienc.Exact.Fís.Nat. (Esp). 2008.
5. Abdulkhaleq
LA, Assi MA, Abdullah R, Zamri-Saad M, Taufiq-Yap YH, Hezmee MNM. The crucial
roles of inflammatory mediators in inflammation: A review. Vol. 11, Veterinary
World. Veterinary World; 2018. p. 627–35.
6. Lintermans
LL, Stegeman CA, Heeringa P, Abdulahad WH. T Cells in Vascular Inflammatory
Diseases. Front Immunol. 2014 Oct 14;5.
7. Ricciotti
E, Fitzgerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc
Biol. 2011 May;31(5):986–1000.
8. Ghasemian
M, Owlia S, Owlia MB. Review of Anti-Inflammatory Herbal Medicines. Adv
Pharmacol Sci. 2016;2016:9130979.
9. A.
Hussein R, A. El-Anssary A. Plants Secondary Metabolites: The Key Drivers of
the Pharmacological Actions of Medicinal Plants. In: Herbal Medicine.
IntechOpen; 2019.
10. Manzano - Santana PI, Peñarreta Tivillin JP, Chóez-Guaranda IA,
Barragán Lucas AD, Orellana - Manzano AK, Rastrelli L. Potential bioactive
compounds of medicinal plants against new Coronavirus (SARS-CoV-2): A review.
Bionatura. 2021 Feb 15;6(1):1653–8.
11. Ullah A, Munir S, Badshah SL, Khan N, Ghani L, Poulson BG, et
al. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules.
2020 Nov 11;25(22):5243.
12. Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A.
Flavonoids and Other Phenolic Compounds from Medicinal Plants for
Pharmaceutical and Medical Aspects: An Overview. Medicines. 2018 Aug
25;5(3):93.
13. Panche AN, Diwan AD, Chandra SR. Flavonoids: An overview. Vol.
5, Journal of Nutritional Science. Cambridge University Press; 2016.
14. Sosa del Castillo D, Quintero Mesa JJ, Rojas Alvear YJ,
Rodríguez M, Rea Suárez RA, Miranda Martínez M. Chemical evaluation and
anti-radical activity of varieties of Morus alba l. (morera, moraceae) from
Venezuela. Bionatura. 2021 Feb 15;6(1):1579–85.
15. Malagón O, Ramírez J, Andradea JM, Morochoa V, Armijosa C,
Gilardoni G. Phytochemistry and Ethnopharmacology of the Ecuadorian Flora. A
Review. Nat Prod Commun. 2016 Mar;11(3):297–314.
16. Armijos C, Ramírez J, Vidari G. Poorly Investigated Ecuadorian
Medicinal Plants. Plants. 2022 Jun 16;11(12):1590.
17. Chong Aguirre P, Martínez MM, Santana PM. Medicinal Plants of
Ecuador. 1st ed. Vol. 1. Boca Raton: CRC Press; 2023.
18. Tinitana F, Rios M, Romero-Benavides JC, de la Cruz Rot M,
Pardo-de-Santayana M. Medicinal plants sold at traditional markets in southern
Ecuador. J Ethnobiol Ethnomed. 2016 Dec 5;12(1):29.
19. Mencias F, Salazar T, Cerna M. Phytochemical screening and
antioxidant activity of Epidendrum nocturnum. Bionatura. 2021 Feb 15;6(1):1486–9.
20. Flores Fiallos LM, Flores Fiallos JJ,
Rodríguez Basantes AI, Guadalupe Alcoser MA, Godoy Ponce SC. Actividad
hipoglucémica de las hojas de Yaca (Artocarpus heterophyllus Lam). Bionatura.
2023 Sep 15;8(3):1–7.
21. Freire Fierro A, Fernández D, Quintana C.
USOS DE MELASTOMATACEAE EN EL ECUADOR. SIDA, Contributions to Botany. 2002;20(1):233–60.
22. Shawky EM, Elgindi MR, Ibrahim HA, Baky MH. The potential and
outgoing trends in traditional, phytochemical, economical, and
ethnopharmacological importance of family Onagraceae: A comprehensive review.
J Ethnopharmacol. 2021 Dec;281:114450.
23. Missouri Botanical Garden. Miconia pseudocentrophora Cogn.
[Internet]. Tropicos.org. [cited 2023 Aug 16]. Available from:
https://tropicos.org/name/20302446
24. Aguilar M. Zornitza, Ulloa Ulloa C,
Hidalgo V. Pamela. Guía de plantas útiles de los páramos de Zuleta,
Ecuador. EcoCiencia, Proyecto Páramo Andino; 2009.
25. León-Yánez S. Fuchsia loxensis. In: Libro
Rojo de Plantas Endémicas del Ecuador Publicaciones del Herbario QCA,
Pontificia Universidad Católica del Ecuador, Quito [Internet]. 2017 [cited 2023 Aug 7]. Available
from: https://bioweb.bio/floraweb/librorojo/FichaEspecie/Fuchsia%20loxensis
26. Missouri Botanical Garden. Fuchsia loxensis Kunth [Internet].
Tropicos.org. [cited 2023 Aug 4]. Available from:
https://tropicos.org/collection/4848474
27. Renvoize S, Luteyn JL, Churchill SP, III DG, Gradstein SR,
Sipman HJM, et al. Paramos: A Checklist of Plant Diversity, Geographical
Distribution, and Botanical Literature (Memoirs of the New York Botanical
Garden Volume 84). Kew Bull. 2000;55(4):1017.
28. Sarma H, Deka S, Deka H, Saikia RR. Accumulation of Heavy
Metals in Selected Medicinal Plants. In 2012. p. 63–86.
29. Mencías Paredes JM. Separación,
Purificación e Identificación de Metabolitos Secundarios de Extracto Etanolico
de Colca (Miconia pseudocentrophora) [Internet]. [Riobamba]: Escuela Superior
Politécnica de Chimborazo; 2015 [cited 2023 Aug 7]. Available from:
http://dspace.espoch.edu.ec/handle/123456789/4023
30. Corrêa JG de S, Bianchin M, Lopes AP, Silva E, Ames FQ, Pomini
AM, et al. Chemical profile, antioxidant and anti-inflammatory properties of
Miconia albicans (Sw.) Triana (Melastomataceae) fruits extract. J
Ethnopharmacol. 2021 Jun 12;273.
31. Bomfim EMS, Coelho AAOP, Silva MC, Marques EJ, Vale VLC.
Phytochemical composition and biological activities of extracts from ten
species of the family Melastomataceae Juss. Brazilian Journal of Biology.
2022;82.
32. Fecker R, Buda V, Alexa E, Avram S, Pavel IZ, Muntean D, et al.
Phytochemical and biological screening of Oenothera biennis L. Hydroalcoholic
extract. Biomolecules. 2020 Jun 1;10(6).
Received: 26
September 2023 / Accepted: 15 April 2023 / Published:15 December 2023
Citation: Parra Álvarez P F, Basantes Vaca C V, Mera
Cabezas L A, Benavides Enríquez C V.. Evaluation of the anti-inflammatory effect of plant extracts from Miconia
pseudocentrophora, Brachyotum ledifolium, and Fuchsia loxensis in rats. Revis Bionatura 2023;8 (4) 97. http://dx.doi.org/10.21931/RB/2023.08.04.97
Publisher's Note: Bionatura stays neutral concerning jurisdictional claims in
published maps and institutional affiliations.
Copyright: ©
2023 by the authors. Submitted for possible open-access publication under the
terms and conditions of the Creative Commons Attribution (CC BY) license
(https://creativecommons.org/licenses/by/4.0/).