2021.06.02.20
Files > Volume 6 > Vol 6 No 4 2021 > Vol 6 No 2 2021
Protective potential of Cynara scolymus extract in thioacetamide model of hepatic injury in rats.

Deena El-Deberky1, Manar Rizk1, Faten Elsayd1, Aziza Amin2, Abubakr El-Mahmoudy1*
Available from: http://dx.doi.org/10.21931/RB/2021.06.02.20
ABSTRACT
Hepatic injury is a worldwide health problem. This study aimed to evaluate the possible hepatoprotective potential of Artichoke (Cynara scolymus) extract (CSE) in albino rats using the thioacetamide (TAA) a model of liver injury. Acclimatized 42 rats were divided randomly into seven groups, each consists of six rats, and subjected to different treatments. Hepatic injury model was induced by administration of TAA at a dose of 100 mg per kg, intraperitoneally, twice weekly for 8 weeks (+ve control); test groups rats received CSE at doses of 100 or 200 mg/kg BW, orally, daily for 8 weeks adjunct with TAA; standard group rats received Silymarin at a dose of 100 mg per kg, orally, daily for 8 weeks adjunct with TAA; other 2 groups of rats received only CSE at the same dose levels; while -ve control rats received only the vehicles. Blood and liver tissue samples were collected at the end of the experimental course for different assessments. Results revealed that CSE exhibited dose-dependent hepatoprotection indicated by nearly normalized parameters, including enzymatic liver function parameters (AST, ALT, GGT & ALP with potential % of 94.06, 86.96, 85.93, 64.85, respectively, after large dose when standardized by Silymarin); non-enzymatic parameters (total protein, albumin, globulins, total bilirubin, conjugated bilirubin, unconjugated bilirubin, TAGs, Cholesterol, HDL, LDL & VLDL with potential % of 83.42, 85.9, 83.44, 98.1, 77.41, 91.5, 97.51, 97.46, 81.41, 88.52 & 89.4, respectively, after large dose when standardized by Silymarin). The underlying mechanism of the observed hepatoprotection of CSE was attributed to impeding the oxidative stress-mediated by TAA, indicated by reduced hepatocyte lipid peroxidation product MDA (95.96 % of Silymarin), and improved antioxidative enzymes in liver homogenate, namely, GPx, Catalase & SOD with potentials of 95.44, 87.02 & 81.48 % of Silymarin, respectively. Macroscopic and microscopic pathological pictures were supportive to the biochemical findings, where the pathological lesions caused by TAA as congestion and dilatation of central and portal veins with perivascular fibrous connective tissue proliferation admixed with few mononuclear leukocytes plus necrotic hepatocytes and hyperplastic biliary epithelium, were ameliorated dose-dependently when CSE was administered together with TAA. The present study's data may suggest CSE as a natural source for promising hepatoprotective and antioxidant drug preparations.
Keywords: Cynara scolymus, Artichoke, Liver injury, Antioxidant, Phytomedicine.
INTRODUCTION
The liver is considered one of the large body organs that play significant roles in metabolizing absorbed food and dealing with toxic substances easily excreted from the body.
The liver's health problem (hepatic) disease has a high rate of incidence all over the world. Various factors may cause it as viruses, alcohol, obesity, drugs, or genetic. Upon the prolonged exposure to one or more of these factors, liver damage occurs in the form of fibrosis or scarring, or cirrhosis, which finally may result in hepatic failure, which is severe and life-threatening 1.
Drugs, in particular, are a significant cause of liver disease with different mechanistic pathways. Some drugs directly affect liver cells, while others are non-injurious by themselves; however, after being transformed by the liver into metabolites, the latter may cause injury to hepatic cells either with direct or indirect mechanisms. From another aspect, the liver cells' toxicity may be dose-dependent or idiosyncratic, or allergic 2.
The Incidence of hepatic adverse drug reactions (ADRs) remains unknown in the general population. 3 reported that the main drugs implicated in ADRs were anti‐infectious, psychotropic, hypolipidemic agents, and nonsteroidal anti‐inflammatory drugs (NSAIDs).
Health professionals as physicians, veterinarians, dentists, and pharmacists use chemical drugs for controlling and treating disease conditions, including liver diseases. However, although beneficial, these chemicals may have harmful side effects on the body organs, including the liver, especially in chronic states. Therefore, many researchers are looking for safe source derived from natural products as medicinal plants in the treatment of liver diseases and drug-induced liver injury.
Among medicinal plants, artichoke is a good source of natural antioxidants such as vitamin C, hydroxycinnamic acids, and flavones. It produces a concentration-dependent inhibition of oxidative stress when cells are stimulated with agents that generate reactive oxygen species (ROS): hydrogen peroxide, phorbol-12-myristate-13-acetate N-formyl-methionyl-leucyl-phenylalanine 4.
However, there is relatively little research on artichoke as a medicine. Studies on animals revealed that administration of root and leaf liquid extracts of artichoke exhibited good hepatoprotective results and helped hepatocytes regenerate. Although research is not yet conclusive, scientists were optimistic that its long-standing use in humans for digestive and bowel problems was justified. The plant also may have a hypocholesterolemic effect and thus may help protect against heart disease. Boiled wild artichoke reduced postprandial glycemic and insulinemic responses in normal subjects but did not affect metabolic syndrome patients 5.
Therefore, the present study aims to evaluate the possible hepatoprotective potential of artichoke and its antioxidant properties in thioacetamide-induced liver injury. To achieve this aim, the following assessments have been performed: liver function biomarkers, oxidative stress markers, and pathological examination in diseased and artichoke-treated rats as experimental models for humans.
METHODS
Plant part and its identification
The plant is a traditional vegetable crop of the Mediterranean basin. The cultivated variety: Cynara cardunculus L. var. scolymus L (globe artichoke) is grown for its fleshy capitula. It belongs to the family of Asteraceae 6. The leaves of Cynara scolymus were purchased from a medicinal plants farm, Faculty of Agriculture, Benha University, Egypt. The leaves were collected in September 2019.
The plant was identified by Dr. Mostafa Hamza Mohamed, Assistant professor of vegetable crops, Horticulture department, Faculty of Agriculture, Benha University, Voucher number 96647 (Figure 1).

Figure 1. Leaves of Cynara scolymus used for extraction and its identification
Preparation of plant extract
Leaves around the stems of C. scolymus were obtained, cut into smaller pieces, and dried at room temperature. The extraction was done by maceration of chopped leaves with 70% v/v (ethanol/distilled H2O). The mixture was left for 72 hr. in the refrigerator with intermittent shaking. The extract was filtered with muslin mesh, concentrated in shaking water pass at 70°C for 3 days to determine the weight of crude extract, and then stored at 4 °C until preparation for administration. It was re-constituted by dissolving 4 g in 200 ml of isosaline (0.9%) for high dose extract and dissolving 2 g in 200 ml of isosaline (0.9%) for low dose extract to give concentrations of 40 mg/ml and 20 mg/ml, respectively. The prepared extracts were used to evaluate the hepatoprotective effect of artichoke extract at high (200 mg/kg) and small (100 mg/kg) doses in rats 7. This method of extraction was repeated every week to prepare fresh extracts. Percentage of yield was determined using the formula:
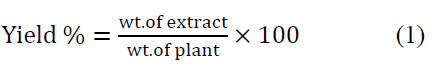
Chemicals, reagents, and Kits
Thioacetamide: It is widely used as an antipyretic and analgesic. However, the major complication reported is hepatotoxicity. It is used for experimental induction of liver injury in animal studies 8. It was obtained from Alamia company, Benha, Qalubia governorate, Egypt.
Thioacetamide powder was dissolved in saline at a concentration of 40 mg/ml. The standard hepatotoxic dose of thioacetamide is 100 mg/kg 9. A rat weighing 200 gm was injected intraperitoneally with 0.5 ml of the prepared solution (40 mg/ml), equivalent to 100 mg/kg B. Wt.
Silymarin: It is traditionally used as a raw extracted from the seeds of Silybum marianum or milk thistle and used to treat liver diseases but is currently available as a standard mixture of 4 flavonolignane isomers: silibinin, isosilibinin, silidianin and silichristin 10. Silymarin used in this study was produced by MUP (Medical Union Pharmaceuticals), Abu sultan, Ismailia, Egypt, under the commercial name Hepaticum®. It is presented as 50 mg / 5 ml suspension. Silymarin was used as such without dilution, where a rat weighing 200 g receives 2 ml of Hepaticum® suspension, equivalent to 100 mg/kg B.Wt 9.
Kits: The diagnostic kits for estimating aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT), alkaline phosphatase level (ALP), total protein, albumin, total bilirubin, and conjugated bilirubin in plasma were supplied from Centronic GmbH Company, Am Kleinfeld, Wartenberg, Germany. The diagnostic kits for estimating triglycerides, cholesterol, HDL, and LDL in plasma were supplied from BioDiagnostic Company, Dokki, Giza, Egypt. The diagnostic kits of estimating oxidative stress markers, Malondialdehyde (MDA), Glutathione peroxidase (GPx), superoxide dismutase (SOD), and Catalase (CAT) in liver tissue homogenate were also from BioDiagnostic Company, Dokki, Giza, Egypt.
Experimental animals and design
The study was performed on (42) male albino rats weighing 150-200 g, obtained from Animal house, Faculty of Veterinary Medicine, Benha University, Egypt. The animals were kept in controlled standard environmental conditions of temperature, humidity, and light/dark exposure. All experimental animals were supplied with a balanced maintenance diet, while water was provided ad libitum. Animals were housed in stainless steel wire mesh cages with bedding of groundwood chips. They were fed fresh pelleted food in a standard diet (vegetable feed containing 19 % protein). Rats were cared for at constant environmental and nutritional conditions for 15 days for acclimatization before the experiment. The research was conducted following the experimental animal care and procedure (Faculty of Veterinary Medicine, Benha University, Benha, Egypt).
Acclimatized rats were divided into seven groups; each consists of six rats. To assess the aim of the present work, groups were treated differently as follows
· Group I: Rats received no drugs (only vehicles, saline orally and intraperitoneally) and were kept as a negative control group.
· Group II: Rats were subjected to induction of liver injury by administering TAA at a dose of 100 mg per kg, intraperitoneally, twice weekly for 8 weeks; the rats were kept as a positive control group (or diseased group).
· Group III: Rats were subjected to liver injury as those of group II and treated with Silymarin (100 mg per kg, orally, daily for 8 weeks) and kept as a standard group.
· Group IV: Rats were subjected to liver injury as those of group II and treated with artichoke extract (100 mg/kg BW, orally, daily for 8 weeks) and kept as group treated with a small dose of artichoke.
· Group V: Rats were subjected to liver injury as those of group II and treated with artichoke extract (200 mg/kg BW, orally, daily for 8 weeks) and kept as group treated with a large dose of artichoke.
· Group VI: Rats received only a small dose of artichoke extract (100 mg/kg).
· Group VII: Rats received only a large dose of artichoke extract (200 mg/kg).
Samples and assessments
Blood samples: Blood for plasma was collected after 8 weeks (2 months) from the experiment's start for biochemical assessments. Samples were collected from the venous plexus located at the eye's medial canthus using heparinized capillary tubes. Each blood sample was collected in a sampling tube with an anticoagulant to avoid clotting at room temperature. Clear plasma was separated by centrifugation at 900Xg for 10 minutes and then collected in Eppendorf's tubes using automatic pipettes. Plasma samples were kept in a deep freezer (-20 °C) for analysis.
Tissue samples: On the last day of feeding and administration and after blood sampling, rats of each group were sacrificed, and liver specimens were taken and preserved in formalin (10 %) solution for histopathological examination.
Other specimens from the liver were taken in sterile tubes for homogenate preparation. Liver tissues were rapidly removed, washed in ice-cooled saline, dried, weighed, and kept in buffer saline, then homogenized by an electric homogenizer. Then the homogenate was centrifuged in a cooling centrifuge for excluding debris from the clear supernatant. The clear supernatant was used for the assessment of oxidative stress markers 11.
Assessments: The estimation of some biochemical parameters such as enzymes' activities in blood, tissues, and body fluids plays a major role in assessing drug safety 12. AST, ALT, GGT, and ALP activities in plasma were determined spectrophotometrically by specific kits as mentioned above according to methods described by 13, 14, 15, 16, respectively, following the instructions of the manufacturer.
Total protein, albumin, total bilirubin, conjugated bilirubin, TAGs, Cholesterol, HDL, and LDL, were determined spectrophotometrically using specific kits according to principles of 17, 18, 19, 19, 20, 21, 22, 23, respectively, following the instructions of the manufacturer.
Three parameters in plasma were calculated mathematically, namely, globulins, unconjugated bilirubin, and VLDL. The total globulin fractions were determined by subtracting the albumin value from the total protein, according to 24. Unconjugated bilirubin level was determined by subtracting the conjugated value from the total bilirubin, according to 25. VLDL concentration was calculated by dividing the triglyceride value by 5 to be the value in mg/dL according to 26.
Oxidative and antioxidative parameters in liver homogenate were determined spectrophotometrically using specific kits according to 27 (MDA), 28 (GPx), 29 (Catalase), and 30 (SOD), following the instructions of the manufacturer. Histopathological examination was done according to 31.
Data management and statistical analysis
Data are expressed as mean ± standard error of the mean of 6 samples/group. The one-way analysis of variance (ANOVA) followed by Tukey's post hoc test was performed on the data for intergroup comparisons. Database management and statistical analysis were performed using GraphPad Prism software v. 6. The significance level was set at a 0.05 probability value.
RESULTS AND DISCUSSION
Liver disease of drug origin is common as many drugs have variable hepatic adverse effects that are, if not controlled, may cause a potential health hazard and enormous economic impact on health care expenditures. Numerous efforts and studies are, thus, have been conducted to establish reliable and early biomarkers to predict liver pathologies caused by drugs, especially the newly introduced and the chronically used ones. Classical informative candidates included serum aminotransferase markers that had been available for 30 years. Afterward, additional candidates have been used to evaluate hepato-safety of drugs like newer liver enzymes, total protein, lipid profile, and oxidation markers 32.
Pharmacological approaches to protect the liver need liver disease models to be the playground of the protection trial. The hepatotoxicity of TAA has been known since 1948. In rats, single doses cause centrilobular necrosis accompanied by increases in plasma transaminases and bilirubin. To elicit these effects, TAA requires oxidative bioactivation by CYP450 oxidative enzymes, leading first to its S-oxide and then to its chemically reactive S, S-dioxide, which ultimately modifies amino acids, lipids, and proteins of hepatocytes 33,34. Because of its relative specificity and availability, many researchers, including us, have used TAA as a model of experimental liver injury upon thinking about and evaluating various liver protection and treatment remedies.
As the pathogenesis of TAA depends mainly on oxidative stress on hepatocytes; so, antioxidants are considered as a therapeutic target for hepatoprotection. Silymarin is now an available standardized extract of the milk thistle (Silybum marianum) that exerts membrane-stabilizing and antioxidant activities, promotes hepatocyte regeneration; furthermore, it reduces the inflammatory reaction and inhibits the fibrogenesis in the liver 35,36.
There are many attempts made to find a natural substance like Silymarin or better for treating liver diseases. This study aimed to study the hepatoprotective effect of hydroethanolic Cynara scolymus leaves extract (CSE) on TAA-induced liver injury. CSE was selected as it is well growing in Egypt, and it has been reported elsewhere to have good antioxidant properties that have also been investigated here.
According to our extraction procedure described in the methodology section, the plant part's yield was 10.5 % that was used in the present investigation that revealed the findings presented and discussed below.
Liver function parameters
TAA elevated enzymatic markers (AST, ALT, GGT, ALP), as shown in Table 1 that were restored by co-administration of CSE in a dose-dependent manner, 85%, 94%, 86%, 86%, respectively, when standardized against Silymarin. This restoration potential of CSE could be attributed to its antioxidant effect that was proven in the present study (discussed later). Our data might be consistent with those of 37 who approved the hepatoprotective effects of CSE (0.5 g/kg/day) on carbon tetrachloride-induced liver injury (1 ml/kg) in broiler chickens. The present study's results may also be consistent with those of 38 who showed that artichoke extract (0.4, 0.8, and 1.6 g/kg body weight) decreased elevated liver enzymes in acute alcohol-induced liver injury (12 mL/kg body weight) in mice. On the other hand, our results may be inconsistent with those of 39 who reported the ineffectiveness of different Cynara scolymus preparations (1 and 2 g/kg) on liver injury induced by CCL4 (2.5 ml/kg, per os) in rats. Again, our data might be inconsistent with those of 40 who found that artichoke leave extracts (for 12 weeks with 3200 mg per day) seemed not to be adequate to improve liver enzyme levels in patients with chronic hepatitis C. This discrepancy between our results and the above mentioned two studies may be attributed to environmental and methodological differences, namely, different liver injury models, different doses of artichoke extract, different extraction procedures, and different course of diseases and species.
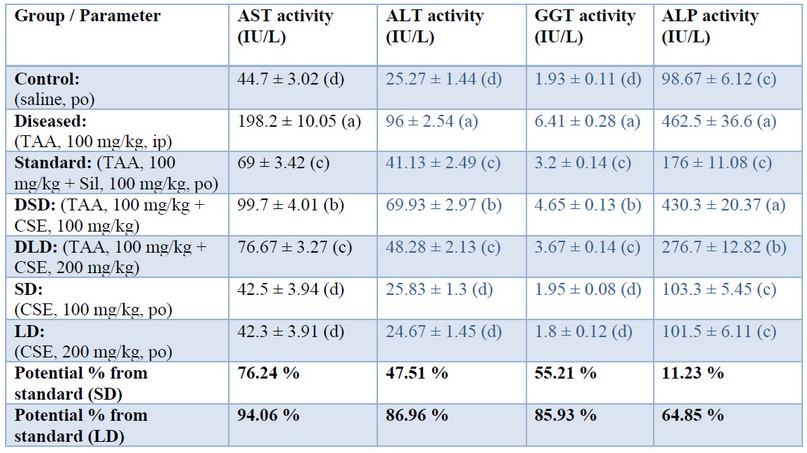
Table 1. Effects of Cynara scolumus extract (CSE) on liver enzyme activities (IU/L) in plasma of thioacetamide-intoxicated rats
The values in the same column with different letters are significantly different.
In the present study, TAA decreased total protein, albumin, and globulins as shown in table (2) that were restored by co-administration of CSE in a dose-dependent manner; being approximately (83%, 85%, 84%), respectively, when standardized against Silymarin. Decreased amounts of either albumin and globulins result in hypoproteinemia. Restoring liver function, even partly, may restore the levels of various protein fractions to their average level as exhibited by CSE in the present study. This restoration potential of artichoke could be attributed to its hepatoprotective effect based on its protection against oxidative radicals of TAA. These results of our study may be consistent with those of 41 who reported that pre-treatment of rats with artichoke leaves aqueous extract (200, 400 mg/kg for 6 weeks) protected against the dramatic decrease in albumin, globulin, and total protein upon subcutaneous injection of CCl4 (2 ml/kg diluted 1:1 by liquid paraffin). Also, the results are consistent with those of 42 who showed the prophylactic role of CSE (500 mg/kg) on doxorubicin-induced toxicity (10 mg/kg) intraperitoneally in rats indicated by a significant increase of both serum albumin and total protein in the dox-CS groups, as compared to the doxorubicin group.
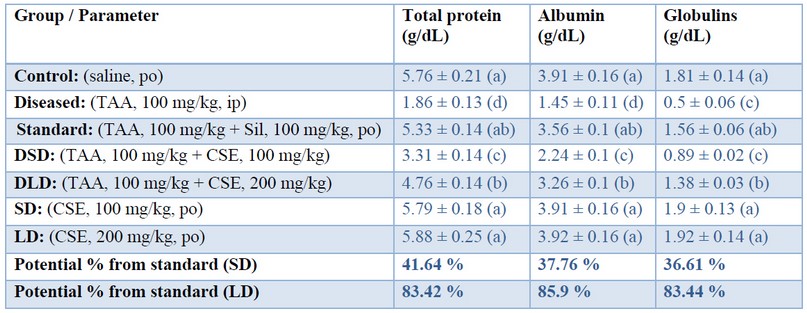
Table 2. Effects of Cynara scolumus extract (CSE) on total protein, albumin and globulins levels (g/dL) in plasma of thioacetamide-intoxicated rats
The values in the same column with different letters are significantly different.In the present study, TAA significantly increased total, conjugated and unconjugated bilirubin as shown in table (3) that were restored by co-administration of artichoke extract in a dose-dependent manner, being approximately (98 %, 77%, and 91%), respectively, when standardized against Silymarin. The restorative effect of CSE could be attributed to the maintained liver function of conjugation and albumin synthesis. Our results may be consistent with those of 43 who reported that the artichoke extracts prepared from either outer part or edible part of the plant (1.5 g/kg daily, for 2 months) significantly decreased serum bilirubin in the TAA hepatocarcinogenesis model (400 mg/kg, intraperitoneally, once weekly, for 3 months); and attributed this effect to the high phenolic content of artichoke.
Regarding the lipid profile in the present study, TAA has significantly increased total cholesterol, TAGs, and the risky lipoproteins (LDL, VLDL), but decreased the beneficial lipoprotein (HDL) as shown in table (4); such abnormalities have been protected by co-administration of CSE in a dose-dependent manner; being (97%, 97%, 81%, 86%, 86%) when standardized against Silymarin. This restoration potential of CSE could be attributed to its hypocholesterolemic and antihyperlipidemic effects that is proven in the present study based on its antioxidative and hepatoprotective properties. Moreover, CSE might decrease lipogenic enzyme activities in the liver and decrease lipid concentration in blood 44 . Our data may be consistent with those of 45 who reported antihyperlipidemic activity of artichoke leaves extract (200 mg/kg and 400 mg/kg during 8 weeks) against high fat diet-induced obesity in rats. In contrast, the data may disagree with those of Rodriguez et al. (2002), who found that repeated (twice a day for 7 consecutive days) oral administration of aqueous artichoke leaf extract (100, 200, and 400 mg/kg) to rats produced no significant differences in cholesterol, TG and phospholipids. The data are also not in agreement with 46 who reported insufficiency of cynarin, the active ingredient of artichoke (250 mg before meals either once or 3 times daily) as a therapeutic regimen in patients with familial type 2 hyperlipoproteinemia. The mean serum cholesterol and triglyceride concentrations in patients with Type II hyperlipoproteinemia had not significantly changed within 3 months, neither with 250 mg nor 750 mg Cynarin daily. These differences may be explained based on methodological and environmental differences, namely, different models of liver injury, different doses of artichoke extract, different extraction procedure, course of diseases and species.
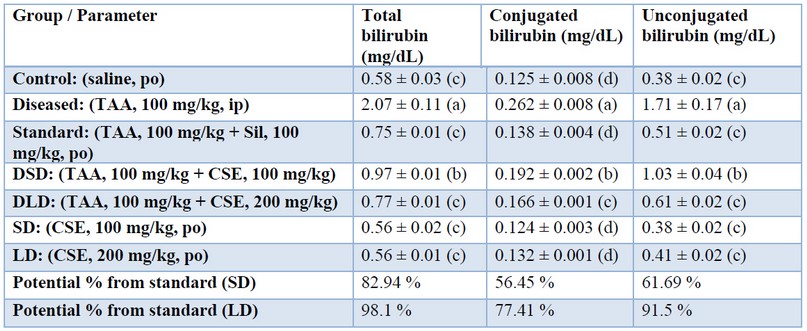
Table 3. Effects of Cynara scolumus extract (CSE) on total bilirubin, conjugated bilirubin, and unconjugated bilirubin levels (mg/dL) in plasma of thioacetamide-intoxicated rats
The values in the same column with different letters are significantly different.
Table 4. Effects of Cynara scolumus extract (CSE) on lipid parameters (mg/dL) in plasma of thioacetamide-intoxicated rats.
The values in the same column with different letters are significantly different.Oxidative stress parameters
In the present study, TAA significantly increased MDA from one hand and decreased GPx, SOD, and CAT from the other hand, as shown in figure (2); such abnormalities have been restored to a large extent by co-administration of CSE in a dose-dependent manner; being approximately (96%, 96%, 86%, 81%) when standardized against Silymarin. This restoration potential of artichoke could be attributed to its antioxidant effect proven in the present study; however, active principles in the extract responsible for such effect need to be investigated.
The antioxidant effect recorded in the present study may be consistent with 47 who reported a protective effect of the artichoke leaf extract (300 mg/kg BW) against cadmium (100 mg/L)-induced oxidative stress in rats. Artichoke leaf extract significantly improved the antioxidant system and hepatorenal function with a significant decline in MDA and elevation of GSH, GPX, SOD, and catalase. Moreover, our data are consistent with those of 48 who reported hepato-curative effects of aqueous artichoke leaf extract (1.5 g/kg orally for 14 days) on hepatic damage and oxidative stress generated by alpha-amanitine (3 mg/kg single dose ip) in male rats.
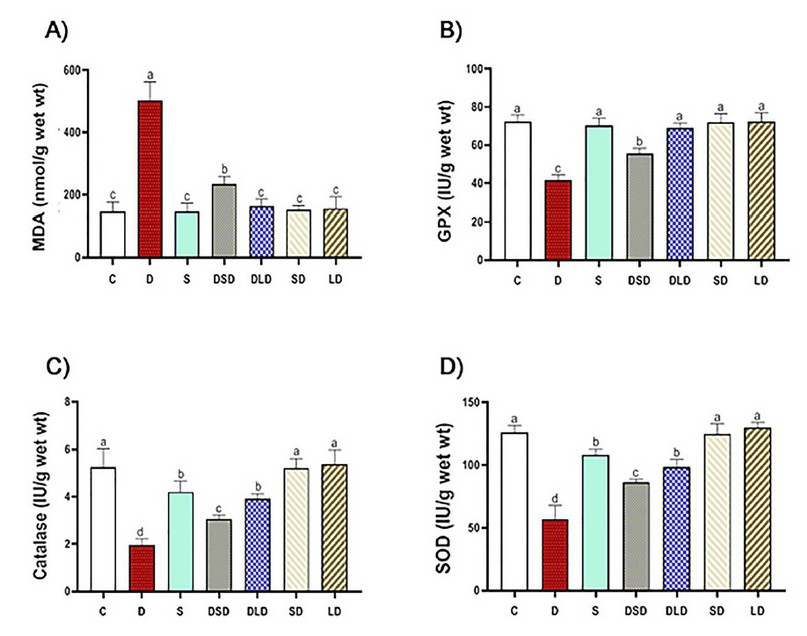
Figure 2. Effects of Cynara scolymus extract (CSE) on oxidative and antioxidative parameters in liver homogenate of thioacetamide-intoxicated rats: Bar-graph showing the effects of CSE on thioacetamide-induced hepatic oxidative stress evaluated by MDA (Malonyl-di-aldehyde, nmol/g wet wt, A), GPx (Glutathione peroxidase, IU/g wet wt, B), Catalase (IU/g wet wt, C) and SOD (Superoxide dismutase, IU/g wet wt, D) in different treatment groups. C (Control: saline, po); D (Diseased: thioacetamide, 100 mg/kg, ip), S (Standard: diseased rats treated with Silymarin, 100 mg/kg, po), DSD (diseased rats treated with a small dose of CSE, 100 mg/kg, po); DLD (diseased rats treated with a large dose of CSE, 200 mg/kg, po); SD (normal rats treated with a small dose of CSE); LD (normal rats treated with a large dose of CSE). The results represent the mean ± SE of 6 rats/group. The bars with different letters are significantly different at P ≤ 0.05 (Tukey's after ANOVA).
However, our data may be inconsistent with those of 39 who reported non-efficacy of different Cynara scolymus preparations (1 and 2 g/kg) on CCl4-induced liver injury in rats. Pre-treatment with either of those extracts or chlorogenic acid did not reduce significantly the production of MDA. This difference may happen because of environmental and methodological variations, namely, liver injury models, doses and types of artichoke extracts, and practical courses.
Pathological findings in the present study come parallel to biochemical data. Macroscopically, no gross changes were detected in these rats' examined livers, which received only the vehicles of drugs used (normal saline orally and parenterally). The liver showed standard, smooth, soft, and shiny brown surfaces, with no irregularities on the surface. Livers dissected from rats treated with TAA (100 mg/Kg, IP, twice weekly for 8 weeks) showed hepatic enlargement with multiple nodular irregularities, pale red color, rough surface. Livers dissected from rats treated with silymarin (100 mg/Kg, PO, daily for 8 weeks) and thioacetamide showed apparent recovery signs as red-brown color, smooth and shiny brown surface, with no or much fewer irregularities. Livers dissected from rats treated with low dose (100 mg/kg, orally) of CSE for 8 weeks and TAA showed hepatic enlargement with multiple nodular irregularities, pink color, rough surface. Livers obtained from rats of group 5, treated with CSE high dose (200 mg/kg, orally) for 8 weeks along with TAA, revealed good restoration of the hepatic architecture with almost normalized shape (smooth with no irregularities). Livers dissected from rats treated with either low or large CSE doses (100 or 200 mg/Kg, PO, daily, respectively) for 8 weeks showed normal liver with deep red color, soft, smooth, with no irregularities on the surface (Fig. 3 a-f).
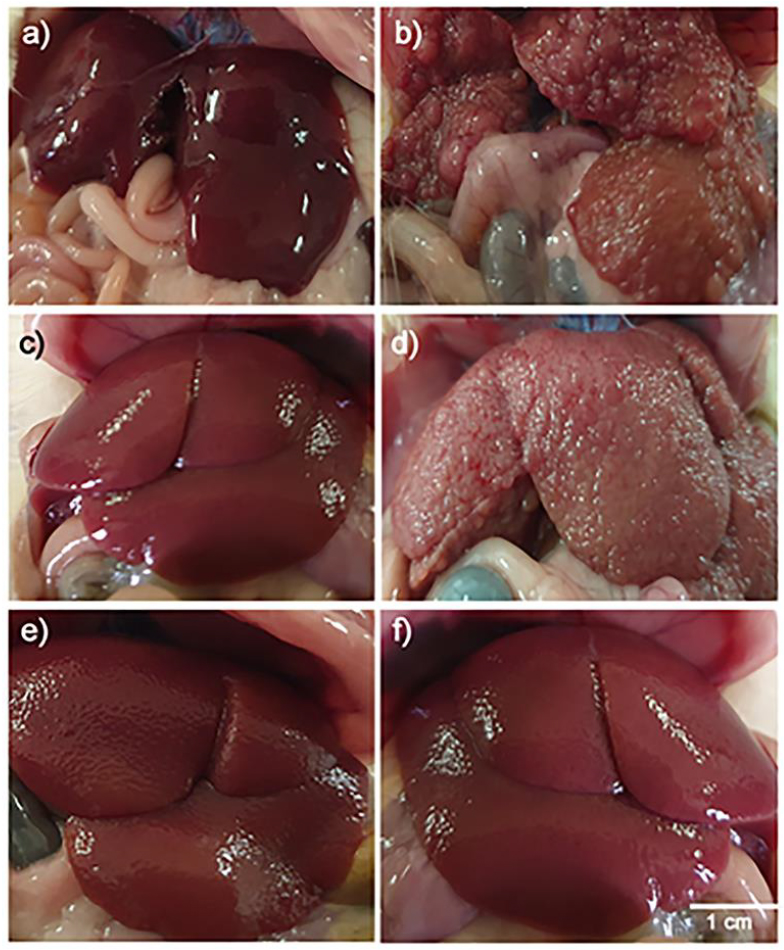
Figure 3. Macroscopic picture of livers obtained from rats of control (a), TAA-treated (b), TAA + Silymarin treated (c), TAA + CSE SD treated (d), TAA + CSE LD treated (e), CSE LD (f), showing (a) typical obvious signs of red-brown color, smooth and shiny brown surface, with no irregularities on the surface, (b) hepatic enlargement with multiple nodular irregularities, pale red color and rough surface, (c) signs of recovery as red color, smooth and shiny brown surface, with no irregularities, (d) hepatic enlargement with multiple nodular irregularities, pale color, rough surface, and edges, (e) good restoration of the hepatic architecture like normal appearance (smooth, soft, with no irregularities on the surface), (f) normal liver with deep red color, soft, smooth, with no irregularities on the surface.
Microscopically, the standard histological structure was detected in the examined livers of these rats as the liver showed standard histological criteria of blood vessels, bile ducts, and hepatic cords (Fig. 4a).
The microscopical examination of livers obtained from thioacetamide-treated rats revealed extensive congestion and dilatation of central and portal veins, dilatating hepatic blood sinusoids, and activation of Kupffer cells in association with perivascular fibrous connective tissue proliferation admixed with few mononuclear leukocytes. Fibrin thrombi were occasionally seen in portal veins. Obvious hydropic degeneration and fatty changes characterized by swollen, pale, vacuolated cytoplasm were commonly demonstrated in the hepatocytes (Fig. 4b). The proliferation of fibrous connective tissue was frequently seen around the central vein and in portal areas with occasional bridging from the central vein to central vein (Fig. 4c) and from the central vein to adjacent portal areas. The portal areas were moderately expanded by fibrous connective tissue proliferation with few mononuclear leukocytic cellular infiltration, prominent lymphocytes with mild hyperplasia of the biliary epithelium peri-ductal fibrous connective tissue proliferation (Fig. 4d). Multifocally, there were strands of fibrous connective tissue infiltrated by mononuclear inflammatory cells and replacing the hepatic parenchyma with a distinct nodular pattern with the formation of pseudo-lobulation; the hepatic parenchyma was also noticed as well as fibrous connective tissue proliferation was also demonstrated adjacent to degenerated and necrotic hepatocytes (Fig. 4e). Multifocally and randomly, there were small focal areas of lytic necrosis characterized by replacing the hepatic parenchyma with eosinophilic cellular and karyorrhectic debris, admixed with fibrin, and fewer lymphocytes (Fig. 4f). Occasionally, large areas of lytic necrosis, with the vacant space's replacement by erythrocytes and few inflammatory cells, were demonstrated.
Meanwhile, the histopathological examination of the liver of rats treated with Silymarin for 8 weeks revealed diffuse degeneration of the hepatocytes, characterized by marked enlargement of the cells by multiple variably sized discrete empty vacuoles that distend the cell cytoplasm were observed. The liver's prominent microscopical findings were a noticeable reduction in the intensity and spreading of fibrous tissue proliferation that appeared as thin strands in between hepatic lobules in most examined sections was detected (Fig. 5a). Multifocally, mild periportal fibrous connective tissue proliferation effacing, separating, and surrounding the adjacent hepatic parenchyma (Fig. 5b).
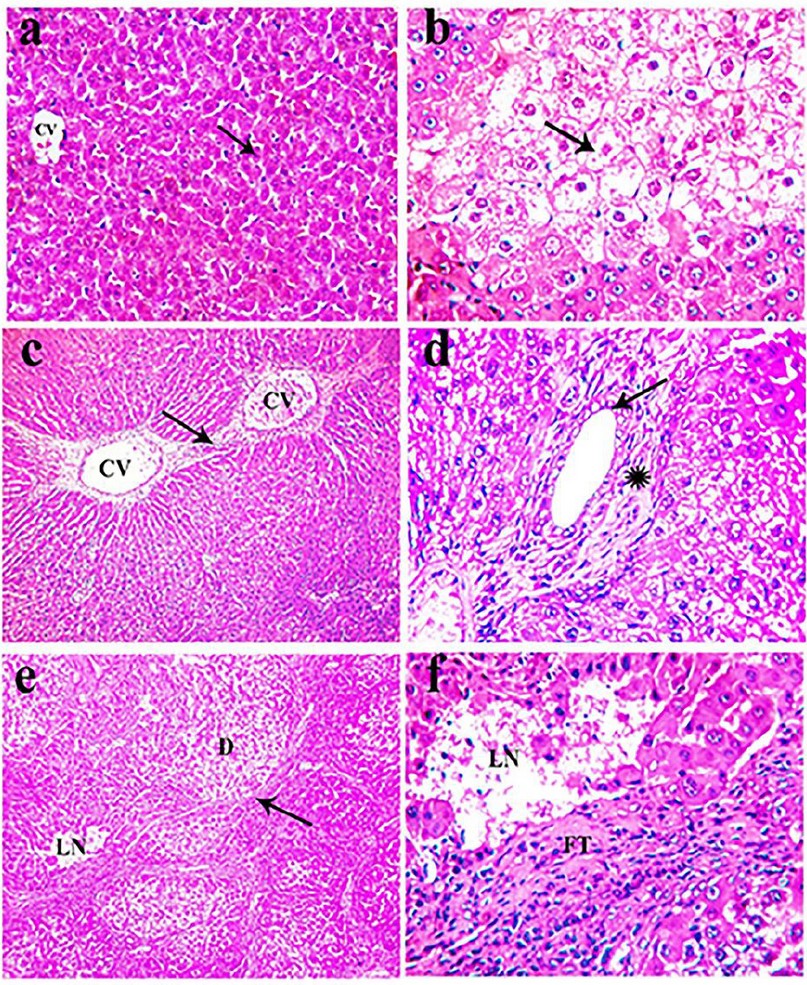
Figure 4. H&E stained section of liver obtained from rats of the control group (a) and intoxicated group that received 100 mg/kg TAA, twice a week for 8 weeks (b-f), showing: (a) standard histological criteria of blood vessels (CV) and hepatocyte (arrow, x 200), (b) extensive hydropic degeneration (arrow) of hepatocytes characterized by swollen, pale, vacuolated cytoplasm (arrow, x400), (c) fibrous connective tissue bridging (arrow) from the central vein (CV) to the central vein (CV, x100), (d) mild hyperplasia of the lining epithelium of bile duct (arrow) with peri-ductal fibrous connective tissue proliferation (asterisk, x400), (e) strands of fibrous connective tissue proliferation (arrow) in the hepatic parenchyma forming pseudo-lobulation adjacent to degenerated hepatocytes (D) and necrotic tissue (LN, x 100), (f) discrete foci lytic necrosis (LN) with fibrous connective tissue proliferation (FT) in the hepatic parenchyma (x 400)
However, the microscopical examination of the liver of animals treated for 8 weeks with a low dose of CSE showed less prominent histopathological changes when compared with the group treated with TAA for the same period. Mild vacuolar and hydropic degeneration in the hepatocytes with the expansion of the portal areas with edema admixed with a few inflammatory cells, mainly lymphocytes and fewer macrophages. Furthermore, the bile ducts were ecstatic and revealed mild periductal fibrosis infiltrated by few mononuclear inflammatory cells (Fig. 5c). Multifocally, there were variably small-sized discrete strands of fibrous connective tissue proliferation in the hepatic parenchyma, mainly adjacent to the degenerated hepatocytes (Fig. 5d). The proliferated fibrous tissue was infiltrated and rimmed by mononuclear inflammatory cells. Mild periportal fibrous connective tissue proliferation (Fig. 5e).
Concerning liver obtained from rats of group 5 treated with TAA and a high dose of CSE for 8 weeks revealed good restoration of the hepatic parenchymal cells as mild congestion of the central vein and hepatic sinusoid. Mild degenerative changes in the hepatocytes with Kuepfer cells' activation as the hepatocytes have small intracytoplasmic vacuoles were demonstrated. Meanwhile, the portal area nearly showed typical histological structures except for a few mononuclear leukocytic cellular infiltration. However, a marked reduction in the intensity and spreading of fibrous tissue proliferation appeared as thin strands of the fibrous connective tissue between hepatic lobules were noticed in most examined sections (Fig. 5f). Liver specimens from rats of 6 and 7 treated only with CSE revealed normal-like findings (not shown).
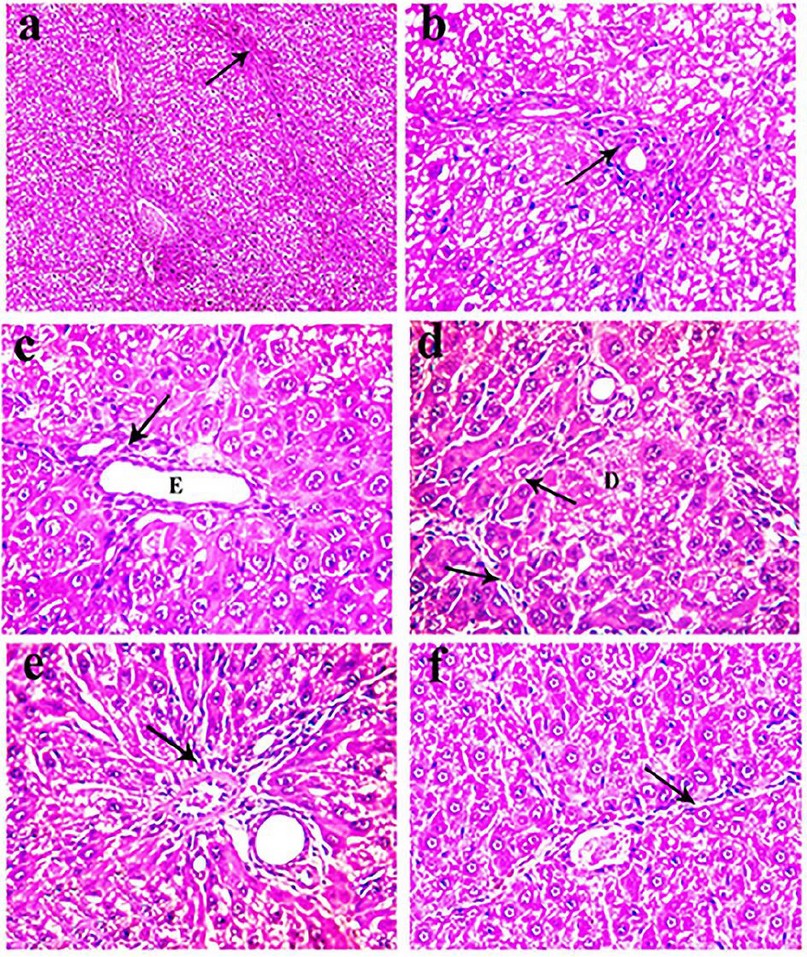
Figure 5. H&E stained sections of livers obtained from rats of group 3 (a-b), group 4 (c-e), and group 5 (f), showing (a) fine strands of fibrous connective tissue in the hepatic parenchyma (arrow, x 100), (b) mild peri-portal fibrous connective tissue proliferation (arrow, x400), (c) ectatic bile duct (E) with mild periductal fibrosis infiltrated by few mononuclear inflammatory cells (arrow, x400), (d) fine strands of fibrous connective tissue (arrow) adjacent to the degenerated hepatocytes (D, x400), (e) mild peri-portal fibrous connective tissue proliferation (arrow, x400), (f) thin strands of the fibrous connective tissue in the hepatic parenchyma (arrow, x400).
This study's above-mentioned pathological findings may be partially consistent with those of 43 who found that TAA (400 mg/kg, IP)-induced multilobular liver cirrhosis with the trabecular arrangement of hepatocytes with dilatation of hepatoportal blood vessel revealing criteria of malignancy. However, these criteria were regressed by artichoke extract (1.5 g/kg for 2 months), and the liver showed cirrhosis with focal vacuolation of the hepatocytes, dilatation of hepatoportal blood vessel with regression of the trabecular arrangement of the hepatocytes. Our study's pathological findings may also be partially consistent with those of 49 who reported that di-ethyl-nitrosamine (100 mg/kg) affected the liver cells through the occurrence of hepatic cellular degeneration and necrosis. Simultaneously, treatment with artichoke heads or leaves (0.5, 1 g) for 25 days led to significant amelioration of such changes in the liver's histological architecture. Moreover, our results are partially consistent with those of 38 who reported the protective effects of ethanolic extracts from artichoke (0.4, 0.8, and 1.6 g/kg body weight) by gavage once daily, an edible herbal medicine, against acute alcohol-induced liver injury (12 mL/kg body weight) orally in mice for 10 days. Histopathological examination showed that artichoke attenuated degeneration, inflammatory infiltration and necrosis of hepatocytes.
CONCLUSIONS
It could be concluded that Artichoke extract has a significant hepatoprotective potential indicated by: i) It reduced elevated levels of enzymatic markers (AST, ALT, GGT & ALP); ii) It increased the levels of total protein, albumin, globulins and decreased levels of bilirubin; iii) It decreased high TAGs, cholesterol & risky lipoproteins (LDL & VLDL) while increased the level of beneficial lipoprotein (HDL), iv) It increased GPx, SOD, CAT, while decreased MDA in thioacetamide-induced liver injury rat model.
Overall, a large dose of artichoke could be a useful adjuvant in the prevention of liver injury. We highly recommend using artichoke extract as a supplement drug with the treatment of liver injury. However, the active constituents and molecular bases of the artichoke effect need further investigation.
Acknowledgments
Not applicable.
Declaration
Conflict of interest: There is no actual or potential conflict of interest in relation to this article.
REFERENCES
1. Schuppan D, Afdhal NHJTL. Liver cirrhosis. 2008;371(9615):838-851.
2. Zimmerman HJ. Drug-induced liver disease. Clinics in liver disease 2000;4(1):73-96.
3. Sgro C, Clinard F, Ouazir K, et al. Incidence of drug‐induced hepatic injuries: a French population‐based study. Hepatology 2002;36(2):451-455.
4. Pérez-García F, Adzet T, Cañigueral S. Activity of artichoke leaf extract on reactive oxygen species in human leukocytes. Free Radical Research 2000;33(5):661-665.
5. Salem MB, Affes H, Ksouda K, et al. Pharmacological studies of artichoke leaf extract and their health benefits. 2015;70(4):441-453.
6. Giorgi D, Pandozy G, Farina A, et al. Karyotype of globe artichoke (Cynara cardunculus var. scolymus): preliminary studies to define its chromosome morphology. VIII International Symposium on Artichoke, Cardoon and their Wild Relatives 9832012:133-138.
7. Zhu X, Zhang H, Lo R. Phenolic compounds from the leaf extract of artichoke (Cynara scolymus L.) and their antimicrobial activities. Journal of Agricultural and Food Chemistry 2004;52(24):7272-7278.
8. Bernacchi A, De Castro C, de Toranzo E, et al. Pyrazole prevention of CC14-induced ultrastructural changes in rat liver. British journal of experimental pathology 1980;61(5):505.
9. Yan-Yu X, Yun-mei S, Zhi-peng C, Qi-neng P. Preparation of silymarin proliposome: a new way to increase oral bioavailability of Silymarin in beagle dogs. International journal of pharmaceutics 2006;319(1-2):162-168.
10. Gillessen A, Schmidt HH-J. Silymarin as supportive treatment in liver diseases: A narrative review. Advances in Therapy 2020:1-23.
11. Del Maestro R, McDonald W. Oxidative enzymes in tissue homogenates. Handbook of methods for oxygen radical research 1985:291-296.
12. Gheith I, El-Mahmoudy A, Elmajdoub A, Awidat S. Pharmacovigilance of tilmicosin in mice. Acta Scientiae Veterinariae 2015;43:1-10.
13. Reitman S, Frankel S. Colorimetric methods for aspartate and alanine aminotransferase. Am J Clin Pathol 1957;28:55-60.
14. Casillas E, Sundquist J, Ames W. Optimization of assay conditions for, and the selected tissue distribution of, alanine aminotransferase and aspartate aminotransferase of English sole, Parophrys vetulus Girard. Journal of Fish Biology 1982;21(2):197-204.
15. Sato K, Tsukamasa Y, Imai C, Ohtsuki K, Shimizu Y, Kawabata M. Improved method for identification and determination of. epsilon.-(. gamma.-glutamyl) lysine cross-link in protein using proteolytic digestion and derivatization with phenyl isothiocyanate followed by high-performance liquid chromatography separation. Journal of Agricultural and Food Chemistry 1992;40(5):806-810.
16. Haussament T. Quantitative determination of serum alkaline phosphatase. Clin Chem Acta 1977;35:271-273.
17. Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. Journal of biological chemistry 1949;177(2):751-766.
18. Doumas BT, Peters Jr T. Origins of dye-binding methods for measuring serum albumin. Clinical Chemistry 2009;55(3):583-584.
19. Coolidge TBJJoBC. Chemistry of the van den Bergh reaction. 1940;132(1):119-127.
20. Fossati P, Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clinical chemistry 1982;28(10):2077-2080.
21. Richmond W. Enzymatic determination of total serum cholesterol. Clin Chem 1973;20:470-475.
22. Lopes-Virella MF, Stone P, Ellis S, Colwell JA. Cholesterol determination in high-density lipoproteins separated by three different methods. Clinical chemistry 1977;23(5):882-884.
23. Wieland H, Seidel D. A simple specific method for precipitation of low density lipoproteins. Journal of Lipid Research 1983;24(7):904-909.
24. Pool JG, Robinson J. Assay of plasma antihaemophilic globulin (AHG). British journal of haematology 1959;5(1):17-23.
25. Walter M, Gerade H. Determination of the" Total" bilirubin and its conjugated" Direct" fraction. Microchem J 1970;15:231-240.
26. Wilson PW, Abbott RD, Garrison RJ, Castelli WP. Estimation of very-low-density lipoprotein cholesterol from data on triglyceride concentration in plasma. Clinical chemistry 1981;27(12):2008-2010.
27. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical biochemistry 1979;95(2):351-358.
28. Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. The Journal of laboratory and clinical medicine 1967;70(1):158-169.
29. Aebi H. Catalase in vitro Methods Enzymol 105: 121–126. Find this article online 1984.
30. Nishikimi M, Roa N, Yogi K. Determination of superoxide dismutase in tissue homogenate. Biochem Bioph Res Common 1972;46:849-854.
31. Banchroft D, Cook C, Stirling R, Turner D. Manual of histopathological techniques and their diagnostic application. Chuchill Livingston. Edinburg; 1996.
32. Wang K, Zhang S, Marzolf B, et al. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proceedings of the National Academy of Sciences 2009;106(11):4402-4407.
33. Hajovsky H, Hu G, Koen Y, et al. Metabolism and toxicity of thioacetamide and thioacetamide S-oxide in rat hepatocytes. Chemical research in toxicology 2012;25(9):1955-1963.
34. Singh G, Bhatia D. Chemicals/drugs-hepatotoxicants: an overview. Der Pharma Chem 2015;7:164-8.
35. Feher J, Lengyel G. Silymarin in the prevention and treatment of liver diseases and primary liver cancer. Current pharmaceutical biotechnology 2012;13(1):210-217.
36. Freitag AF, Cardia GFE, da Rocha BA, et al. Hepatoprotective effect of Silymarin (Silybum marianum) on hepatotoxicity induced by acetaminophen in spontaneously hypertensive rats. Evidence-Based Complementary and Alternative Medicine 2015;2015.
37. Nateghi R, Samadi F, Ganji F, Zerehdaran S. Hepatoprotective effects of Cynara scolymus L. extract on CCl4 induced liver injury in broiler chickens. International Journal of AgriScience 2013;3(9):678-688.
38. Tang X, Wei R, Deng A, Lei T. Protective effects of ethanolic extracts from artichoke, an edible herbal medicine, against acute alcohol-induced liver injury in mice. Nutrients 2017;9(9):1000.
39. Speroni E, Cervellati R, Govoni P, Guizzardi S, Renzulli C, Guerra M. Efficacy of different Cynara scolymus preparations on liver complaints. Journal of ethnopharmacology 2003;86(2-3):203-211.
40. Huber R, Müller M, Naumann J, Schenk T, Lüdtke R. Artichoke leave extract for chronic hepatitis C–a pilot study. Phytomedicine 2009;16(9):801-804.
41. Shalaby M, Hammoda A. Hepatoprotective effect of artichoke leaves aqueous extract in ccl4 intoxicated rats. World Journal of Pharmacy and Pharmaceutical Sciences 2014;4(1):138-154.
42. Mustafa HN, El Awdan SA, Hegazy GA, Jaleel GAA. Prophylactic role of coenzyme Q10 and Cynara scolymus L on doxorubicin-induced toxicity in rats: Biochemical and immunohistochemical study. Indian journal of pharmacology 2015;47(6):649.
43. El-Mesallamy AM, Abdel-Hamid N, Lamis Srour SA. Identification of Polyphenolic Compounds and Hepatoprotective Activity of Artichoke (Cynara Scolymus L.) Edible Part Extracts in Rats. Egyptian Journal of Chemistry 2020;63(6):2273-2285.
44. El-Mahmoudy AM, Abdel-Fattah FA, Abd El-Mageid AD, Gheith IM. Effect of the growth promotant mannan-oligosaccharide on the lipogram and organ function profile in hyperlipidemic albino rats. Am J Phytomed Clin Ther 2014;2:334-347.
45. Ben Salem M, Ksouda K, Dhouibi R, et al. LC-MS/MS analysis and hepatoprotective activity of artichoke (Cynara Scolymus L.) leaves extract against high fat diet-induced obesity in rats. BioMed research international 2019;2019.
46. Heckers H, Dittmar K, Schmahl F, Huth K. Inefficiency of cynarin as therapeutic regimen in familial type II hyperlipoproteinaemia. Atherosclerosis 1977;26(2):249-253.
47. El-Boshy M, Ashshi A, Gaith M, et al. Studies on the protective effect of the artichoke (Cynara scolymus) leaf extract against cadmium toxicity-induced oxidative stress, hepatorenal damage, and immunosuppressive and hematological disorders in rats. Environmental Science and Pollution Research 2017;24(13):12372-12383.
48. Kaymaz MB, Kandemir FM, Pamukcu E, Eröksüz Y, Özdemir N. Effects of aqueous artichoke (Cynara scolymus) leaf extract on hepatic damage generated by alpha-amanitine. Kafkas Univ Vet Fak Derg 2017;23(1):155-160.
49. Metwally N, Kholeif T, Ghanem K, Farrag A, Ammar N, Abdel-Hamid A. The protective effects of fish oil and artichoke on hepatocellular carcinoma in rats. European review for medical and pharmacological sciences 2011;15(12):1429-1444.
Received: 15 January 2021
Accepted: 16 February 2021
Deena El-Deberky1, Manar Rizk1, Faten Elsayd1, Aziza Amin2, Abubakr El-Mahmoudy1*
1Department of Pharmacology, Faculty of Vet. Medicine, Benha University, 13736 Qalioubeya, EGYPT;
2Department of Pathology, Faculty of Vet. Medicine, Benha University, 13736 Qalioubeya, EGYPT.
Corresponding author:
*Abubakr El-Mahmoudy,
Professor of Pharmacology, Benha University.
13736 Qalioubeya, EGYPT.
Facsimile: +20-13-2460640
E-mail: [email protected]