2023.08.01.15
Files > Volume 8 > Vol 8 No 1 2023
Fermentation of Agave americana L. sap produced in Cayambe – Ecuador
1 Department of Food Science and Biotechnology, Escuela Politécnica Nacional, Quito, Ecuador; [email protected]
2 Department of Food Science and Biotechnology, Escuela Politécnica Nacional, Quito, Ecuador; [email protected].
3 Department of Food Science and Biotechnology, Escuela Politécnica Nacional, Quito, Ecuador; [email protected].
4 Department of Food Science and Biotechnology, Escuela Politécnica Nacional, Quito, Ecuador; [email protected].
5 Department of Food Science and Biotechnology, Escuela Politécnica Nacional, Quito, Ecuador; [email protected].
* Correspondence: [email protected]
http://dx.doi.org/10.21931/RB/2023.08.01.15
ABSTRACT
Fermentation of agave sap, also known as exudate, has become an ancestral practice throughout Ecuadorian Andean. In Cayambe, located in this region, grows Agave americana L., which is recollected, and its sap is fermented. The agave-based fermented beverage, locally named "tzawar mishki", exhibits variable features, mainly ethanol concentration. In this work, fermentation conditions of agave sap were studied to enhance ethanol yield. Two thermal treatments for raw exudate were evaluated, pasteurization at boiling point for 30 minutes and sterilization at 121°C for 15 minutes; fermentation temperature, 30°C and room (around 18°C); and two yeast strains. Thermal pretreatments have a positive impact on reducing sugars and sucrose concentration. In the first case, an increase of 76 % and 30 % has been reported, while sucrose concentration quadrupled and doubled in pasteurized and sterilized samples, respectively. The highest ethanol concentration (63,31 g/L) and the best yield (66,21 %) were accomplished through agave sap pasteurized and fermented for 96 hours at 30°C. Negligible differences have been evidenced in ethanol and other volatile compounds content between the two yeast strains evaluated.
Keywords: agave sap, ethanol, fermentation, fermentable sugars, yeast
INTRODUCTION
Agave (Agave americana L.), also known as Penco, Cabuya or Tzawar, is an endemic multipurpose vegetable specie that likely grows in arid lands of low fertility1,2. One of its primary uses relies on the agave sap called "tzawar mishki", here in after also called exudate, produced at the plant's optimum ripening point. Cutting the flower buds and drilling a bore in the central stem of the agave promotes exudate generation3,4. The composition of agave sap includes water, sucrose, fructose, glucose, proteins, and mineral salts. Sugars represent 75% of dry matter, within which 10% are fructooligosaccharides (FOS)5. Therefore, this sap becomes a suitable raw material for fermented beverages such as Guarango in Ecuador, Pulque in Mexico, and other distilled spirits6,7. Fermentation of agave sap from Mexico has been widely investigated even for optimizing operating conditions8,9.
Although fermentation practices have spread across the Ecuadorian Andean region, few studies approach the technical aspects of Guarango production6. Agave sap fermentation carried out under non-controlled and non-aseptic conditions leads to the rise of the microbial population throughout the collection, transport, and treatment stages. Frequently, as the original microbiota of gathered exudate turns into the inoculum, it results in 24 to 96 hours of spontaneous fermentation10. Low replicability due to significant variations is reflected in ethanol concentration, volatile compounds occurrence, and ultimately different sensory attributes for the beverage. Hence, a standardized product requires controlled operating conditions highlighting temperature and yeast strain11,12.
Temperature directly influences yeast growth and, thus, the satisfactory performance of the fermentation process. Below 18°C lag phase extends, and the specific growth rate decreases. The election of a commercial yeast averts fermented product alterations and reduces the antagonistic interaction between endemic microorganisms in raw agave sap11,12. Likewise, thermal pretreatment promotes modifications in the chemical composition of agave sap, referring to sugar composition, where higher sucrose concentration is evidenced after the sterilization process13. This study aims to determine operating conditions for agave sap fermentation related to thermal pretreatment, yeast strains and temperature to achieve a standardized beverage at an optimum ethanol yield.
MATERIALS AND METHODS
Sample collection and treatment
The exudate from Agave americana L. was collected in Cayambe city, located northeast of Pichincha province, Ecuador. Samples were filtered through a strainer, stored at 4°C and transported in sterile containers before a complementary filtration with a baghouse filter. Then, two thermal treatments were conducted, sterilization at 121°C for 15 minutes and pasteurization at boiling point for 30 minutes. Raw agave sap and treated samples were frozen at -14°C for physicochemical characterization.
Physicochemical analysis
For untreated and thermally treated agave sap and fermented samples drawn every 24 hours, the concentration of total sugars, reducing sugars, soluble solids, glucose, fructose, sucrose, ethanol, and optical density (600 nm) was determined. In the fermentation case, the concentration of glucose, fructose, sucrose, and pH was measured only in the final product.
For total sugars, 10 μL of 80% wt. The phenolic solution was blended with 200 μL of the sample, then 1000 μL of sulphuric acid 96% was added with agitation and left for 10 minutes at room temperature. Later, the temperature rise to 25 °C for 10 minutes and absorbance was measured at 490 nm14. For reducing sugars, 250 μL of 3,5-dinitrosalicylic acid (DNS) was blended with 500 μL of sample and placed in a water bath at boiling point for 15 minutes. Then, 900 μL of distilled water was added to 100 μL of the solution to cool down, and absorbance was measured at 540 nm15.
Soluble solids content and pH were determined by AOAC 932.14 (C) and AOAC 981.12, respectively. Glucose, fructose, and sucrose concentration were quantified through a commercial enzymatic kit (Sucrose/D-Fructose/D-Glucose Assay Kit–Megazyme). Likely, ethanol concentration by means of a commercial enzymatic kit (Ethanol Assay Kit – Megazyme, AOAC Method 2017.07).
Yeast strains
Two Saccharomyces cerevisiae strains were used. A commercial fructophilic yeast for agave fermentation (DistillaMax® TQ – LALLEMAND), and a commercial baker's yeast (LEVAPAN).
Fermentation
Laboratory fermentations were performed in tubes holding 40 mL of agave exudate thermally treated, which were inoculated with pre-activated yeast, as specified by providers, at an initial OD600nm of 0,5. Fermentation tubes were covered with a cork stopper coupled with an air trap. Experiments were performed at 30 °C and room temperature (approximately 18 °C) without agitation. A total of eight treatments were conducted and identified, as shown in Table 1.
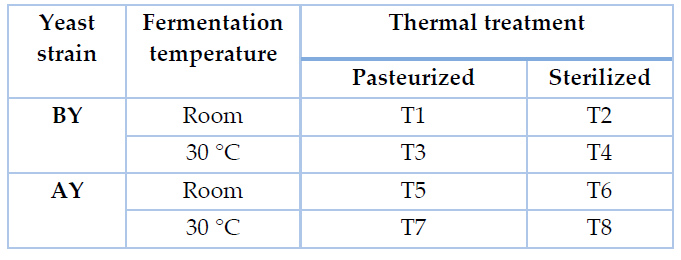
Table 1. Experimental design for laboratory fermentations
Volatile compounds determination
Methanol, n-propanol, ethyl acetate, and furfural were determined by GC using Agilent 7890A equipment fit in with a flame ionization detector (FID), Elite-volatiles column of 30 m length, 0,32 mm inner diameter, and 1,8 μm film thickness. Automatic injections of 0,5 μL were conducted with a 75:1 split at 220 °C. The column temperature was kept at 45 °C for 14 minutes. Helium was managed as the carrier gas at 6,5 mL/min. The FID detector was operated at 260 °C.
Statistical analysis
Analysis of variance (ANOVA) was managed by the software Statgraphics Centurion 19. Multiple range Fisher test was conducted for comparisons within individual assays. Mean differences were considered significant at p ≤ 0,05.
RESULTS AND DISCUSSION
Thermal pretreatment effect
The physicochemical evaluation of raw and thermally treated agave sap is shown in Table 2. Measured and theoretical ethanol concentration and the final to initial volume ratio after thermal treatment are also displayed.
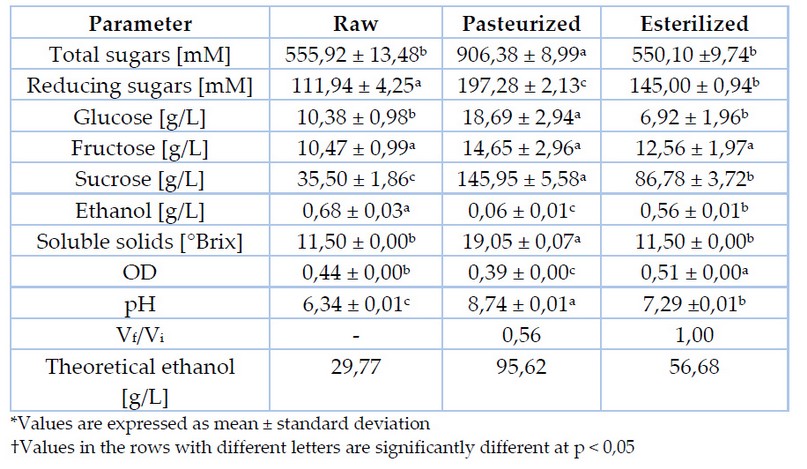
Table 2. Physicochemical composition of raw and thermally treated agave sap
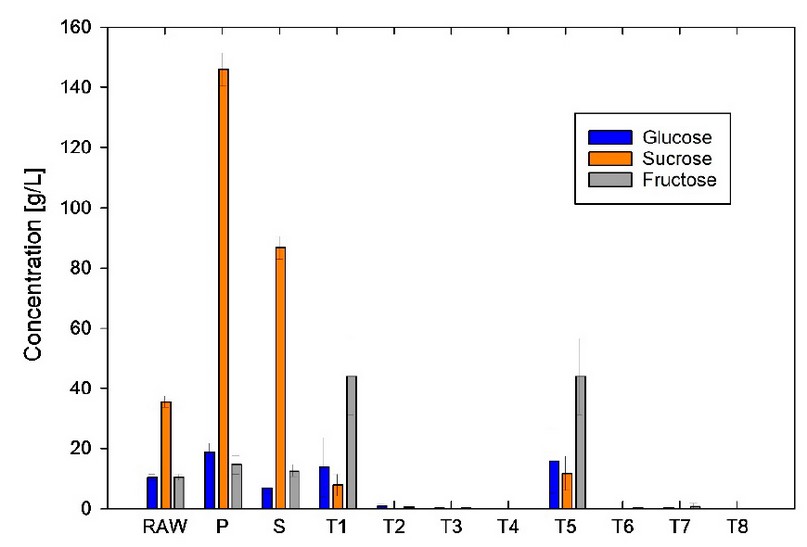
Figure 1. Glucose, fructose, and sucrose concentration for raw, pasteurized (P), sterilized (S) agave sap and for final fermented products (T1 – T8)
After thermal treatments, results reveal a modification in sugar concentration, particularly in reducing sugars and sucrose. Figure 1 and Table 2 exhibited a slight variation in glucose concentration and a non-significant difference in fructose concentration when applied thermal treatments were. On the other hand, pasteurization increases sucrose concentration four times, while sterilization doubles it. This trend is primarily due to a high concentration of inulin and another fructooligosaccharide (FOS), usually part of raw agave sap. As thermal treatments are implemented, these carbohydrates could hydrolyze to monosaccharide or another oligosaccharide of lesser polymerization degree4,16,17.
A noted increase in sucrose concentration from 1,66 g/L to 41,39 g/L was demonstrated when Agave salmiana exudate was heated up at 121°C for 15 minutes. The hydrolysis of fructans such as inulin with β- (2-1) bonds and levan with β- (2-6) is a likely cause13. Nevertheless, since the wide variety of oligosaccharides form different agave species, it is not feasible to grant inulin as the main hydrolyzable polysaccharide in agave18. Likewise, an increment of reducing sugars up to 46% has been reported after thermal treatments13, which exceeds the increase of 30% reached in this work by means of sterilization. This variation could respond to the polymerization degree in sugars forming the samples. Moreover, fructans' structure and concentration are shifting since they mainly depend on environmental conditions and the plant's age4,17.
A higher increase in total sugar concentration is evidenced after pasteurization due to the thermal hydrolysis of fructans and FOS forming agave sap. Also, water evaporation produced over 30 minutes of thermal treatment raises soluble solids in the sample. An increment of reducing sugars up to 63% has been stated when Agave americana L. sap is pasteurized at 80 °C for 30 minutes19. Primary outcomes are considerably lower than the 76% achieved in the present work.
The physicochemical composition of agave sap is also influenced by environmental factors as well as earlier fermentation processes in plant hollows as a result of multiple recollections throughout the day as common practice13. This statement has been validated since sugar composition strongly changes throughout the day; thus, fructan content at first recollection is significantly higher than 7 hours later20. Such variability in the initial composition of agave sap encourages standardization and industrialization employing dilution or concentration after thermal pretreatment.
The relevance of thermal pretreatments relies on fructans hydrolysis to get free sugars and initial microbiota reduction. Therefore, inoculation with commercial yeast strains is feasible after pasteurization or sterilization of agave sap. Native microbiota of Agave americana sap mainly comprises yeast (particularly Saccharomyces and Kluyveromyces species), lactic acid bacteria (LAB), and exopolysaccharides (EPS) producer bacteria14,21. This microbiota combination advocates much more complex fermented products with significant variability within batches referred to as ethanol concentration and organoleptic features. In this context, thermal treatments likely reduce heterogeneity in the final outcome of fermentation.
Fermentation
For each set of fermentation conditions, pH decreases to approximately 5 despite temperature and yeast strain. Concerning fermentation kinetics, Figure 2A reveals that at 30 °C stationary phase was achieved after about 48 hours, while maximum OD was evidenced after 96 hours at room temperature. At 18 °C fermentation process slows down regardless of yeast strain whenever it is a commercial one22.
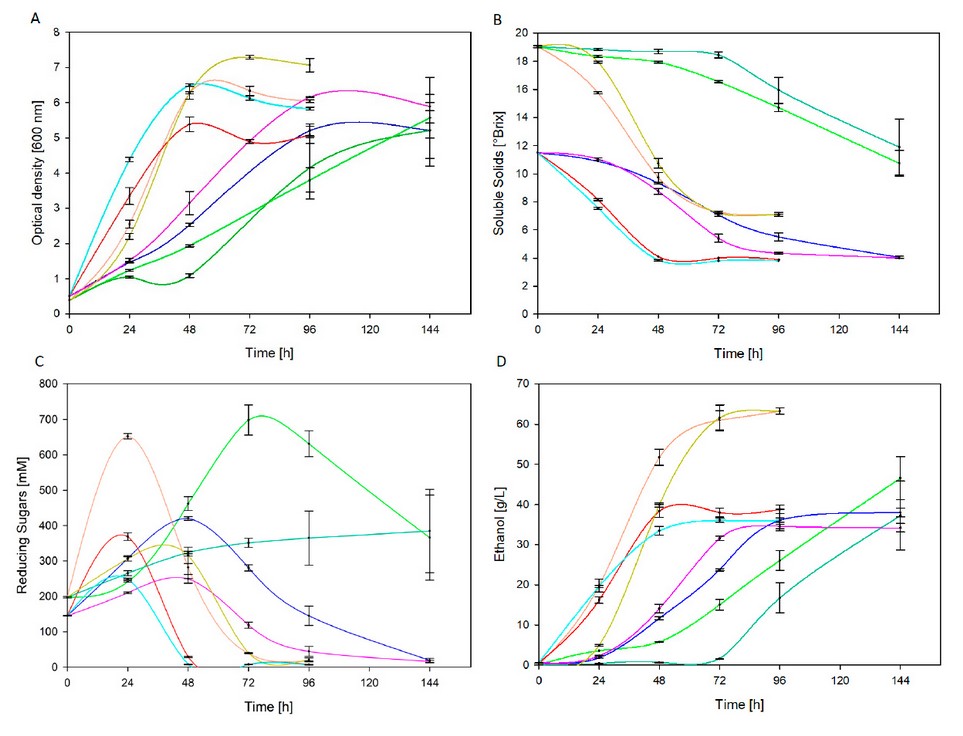
Figure 2. Fermentation kinetics of thermally treated agave sap at 30°C and room temperature related to optical density (A), soluble sugars (B), reducing sugars (C) and ethanol (D)
Despite the fact that more than 90% of total sugars were consumed in all treatments (except T1 and T5), the consumption rate was significantly different. Figure 2B denotes a maximum reduction of soluble solids at 30 °C after 48 hours and 96 hours for sterilized and pasteurized agave sap, respectively. Contrastingly, at room temperature, the maximum reduction was accomplished after 96 hours for sterilized agave sap; instead, uncompleted sugars consumption was registered after 144 hours for pasteurized agave sap.
According to Figure 2C, similar profiles can be distinguished for all treatments, excluding T5. In the early stages reducing sugars were accumulated until a maximum concentration due to invertase (β – D -fructosidase) action, which is released by yeast in peri plasmatic space and ultimately hydrolyses sucrose into glucose and fructose23. This is consistent with results in Figure 1, where pasteurized and sterilized agave sap exhibit higher sucrose concentrations than glucose and fructose before fermentation. At high sucrose concentrations, a significant increase of invertase activity is registered along with a temporary rise of reducing sugar concentration until yeast24,25 metabolizes monosaccharides. Such behavior is also depicted in Figure 2C, where a maximum peak is evidenced. Then, decreasing sugars are metabolized in the cell interior through glycolysis and pyruvate converts to ethanol under anaerobic conditions26.
The highest ethanol concentration reached was 63,31 g/L at 30°C with pasteurized agave sap through treatments T3 and T7 with yeast strain BY and AY, respectively. ANOVA analysis reveals that thermal pretreatment, fermentation temperature and interaction among them significantly influence (p ≤ 0,05) ethanol concentration, similar to yeast strain (p > 0,05). In turn, ethanol concentration achieved in fermentations from sterilized agave sap is not significantly different.
Figure 2D shows that fermentations conducted at 30 °C (T4 and T8) reached maximum ethanol concentration after 48 hours, while a similar concentration is attained at room temperature (T2 and T6) in twice as long. Furthermore, in treatments T1 and T5, sugars are partially metabolized as depicted in Figure 1, which advocates longer fermentations to achieve comparable ethanol concentration. Likewise, outcomes at present work overcome the final ethanol concentration of 28,67 g/L achieved after 72 hours by Kluveromyces marxianus strain fermentation of a 22 °Brix (60 % Agave americana sap and 40 % panela) must27. Similarly, ethanol concentration by fermentation of sterilized agave sap corresponds to that achieved afterward in the optimization of the fermentation process of Agave salmiana sap. At 28 °C and 105 g/L initial sugar concentration, a maximum ethanol concentration of 36,63 g/L was attained8.
Ethanol yield and volatile compounds
Final ethanol concentration and yield for each set of conditions throughout fermentation are indicated in Table 3.
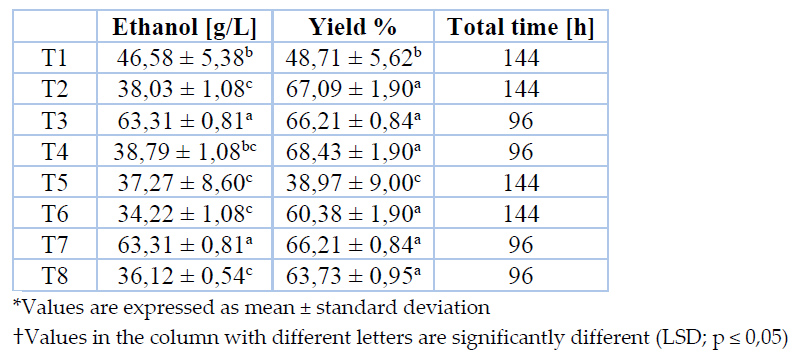
Table 3. Ethanol yields and concentration in the final fermented product
Ethanol yields in different treatments are equivalent to 65 % and 66% achieved through the evaluation of two Saccharomyces cerevisiae strains for agave sap fermentation with an initial sugar concentration of 220 g/L. Superior ethanol yields, up to 81%, are reported by Kluveromyces marxianus; thus, this yeast seems more suitable than Saccharomyces cerevisiae for agave sap fermentation28.
ANOVA analysis denotes that yeast strain, thermal pretreatment, fermentation temperature, and the interaction among two last significantly affect (p ≤ 0,05) ethanol yield. Even though no significant differences are identified between treatments (except T1 and T5), fermentations conducted at 30 °C fulfilled the maximum concentration in half of the time of analogous fermentations carried out at room temperature.
According to ethanol concentration in the final fermented product, treatments T3 and T7 have been selected as the optimum ones. In this sense, to accomplish superior productivity, it is proposed to pasteurize agave sap at boiling point for 30 minutes to implement a fermentation process at 30 °C employing any commercial yeast strain evaluated in this work. Table 4 depicts the concentration of volatile compounds identified in fermented products from optimum treatments.
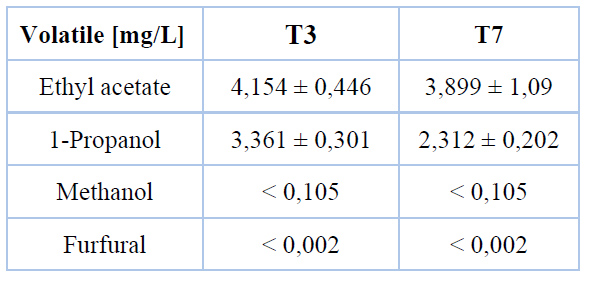
Table 4. Concentration of volatile compounds in the final fermented product
Slight differences are evidenced in the volatile compounds profile when commercial Saccharomyces cerevisiae strains are employed. One of the major volatile compounds appearing in agave beverages is ethyl acetate, which builds up throughout fermentation by the esterification of acetyl CoA29. The concentration reached goes with 5 mg/L achieved in tequila must fermentation at 35 °C with Saccharomyces cerevisiae isolated strains29 but differs significantly from 270 mg/L reported for commercial pulque30. Such divergence responds to the fermentation process, which is mainly spontaneous for non-thermal treated agave sap12. The generation of ethyl acetate strongly depends on microbiota variety and density, where non-Saccharomyces strains exhibit an excessive production that negatively impacts the aroma profile of the beverage12,31.
1-Propanol, as higher alcohol, promotes floral and fruity aromas as well as flavor release in the beverage31. The concentration of this alcohol in commercial pulque has been detected to be lower than 10 mg/L30 while could be up to 15 mg/L for agave must fermentation29. 1-Propanol can be generated through the α-ketobutyrate and subsequent decarboxylation or amino acid synthesis from sugars enzymatically managed29,32. The first is likely the pathway since significant reducing sugar consumption has been reported during fermentation, even though the path conducted by Saccharomyces strains is unspecified.
Methanol is the product of pectin demethylation at high temperatures and low pH during agave pineapple cooking 33. As thermally treated agave sap has been fermented, like in commercial pulque, very low methanol concentration has been detected. Referring to furfural, it is part of all agave-based beverages since agave heads and sap are rich in inulin and FOS, which thermally hydrolyzed produce this aromatic aldehyde34. Agave sap recollected from 8 to 10 years old species heating up to boiling point under moderate monitoring of pH, and soluble solids content exhibits 0,051 mg/L furfural concentration35. In contrast, sap from 4 to 5 old Agave salmiana boiled and stored for two weeks at 25°C and 85 % of relative humidity evinces 0,004 mg/L34. In this sense, undetected concentration has been found in the fermented product, most probably because of Agave americana L. age during harvest.
CONCLUSIONS
Thermal pretreatments in agave sap positively impact reducing sugar concentration involving glucose, fructose, and sucrose.
The fermentation of thermally treated agave sap conducted at 30°C speeds up sugars to ethanol conversion compared to the fermentation process at room temperature.
Fermentation of pasteurized agave sap at 30°C (T3 and T7) achieved an ethanol concentration of 63,31 g/L, which significantly differs from the maximum concentration reported for sterilized agave sap at the same fermentation conditions.
Similar ethanol concentration was measured in sterilized agave sap samples. However, fermentation of these samples at 30°C reached the maximum ethanol concentration in half the time (48 hours) of those fermented at room temperature (96 hours).
Author Contributions: Francisco Munive: laboratory experiments performance and original draft writing. María Páez: writing review and editing. Cristina Romero Granja: funding acquisition and project administration. Neyda Espín: language review. Mary Casa-Villegas: experiments design and data analysis and interpretation.
Funding: Authors thank the financial support of Cooperación Técnica Alemana (GIZ).
Acknowledgments: The authors want to acknowledge the administrative and technical support of Vanesa Naranjo.
Conflicts of Interest: The authors declare no conflict of interest.
REFERENCES
1. Chen, Y., Chen, X., Hu, F., Yang, H., Yue, L., Trigiano, R., y Cheng, Z. Micropropagation of Agave Americana. HortScience, 2014, 49(3), 320–327.
2. Santos-Zea, L., Leal-Díaz, A., Cortés-Ceballos, E., y Gutiérrez- Uribe, J. Agave (Agave spp.) and its Traditional Products as a Source of Bioactive Compounds. Current Bioactive Compounds, 2012, 8(3), 218–231.
3. Molina-Guerrero, J. A., Botello-Álvarez, J. E., Estrada-Baltazar, A., Navarrete-Bolaños, J. L., Jiménez-Islas, H., Cárdenas-Manríquez, M., & Rico-Martínez, R. Compuestos volátiles en el mezcal. Revista Mexicana de Ingeniería Química, 2007, 6(1), 41-50.
4. Velázquez-Martínez, J., González-Cervantes, R., Hernández-Gallegos, M., Campos, R., Jiménez, A., y Arenas, M. Prebiotic potential of Agave angustifolia haw fructans with different degrees of polymerization. Molecules, 2014, 19(8), 12660–12675.
5. Ortiz, R., Pourcelly, G., Doco, T., Williams, P., Dormer, M., y Belleville, M. Analysis of the Main Components of the Aguamiel Produced by the Maguey-Pulquero (Agave mapisaga) throughout the Harvest Period. Journal of Agricultural and Food Chemistry, 2008, 56(10), 3682–3687.
6. De La Torre, L., Cummins, I., y Logan-Hines, E. Agave americana and Furcraea andina: Key Species to Andean Cultures in Ecuador. Botanical Sciences, 2018, 96(2), 246–266.
7. Paniagua-Zambrana, N., Bussmann, R., y Romero, C. Agave americana L. Agavaceae. Ethnobotany of the Andes. Ethnobotany of Mountain Regions. Springer, Cham. 2020, 1–6.
8. De León-Rodríguez, A., Escalante-Minakata, P., Barba de la Rosa, A., y Blaschek, H. Optimization of fermentation conditions for the production of the mezcal from Agave salmiana using response surface methodology. Chemical Engineering and Processing: Process Intensification, 2008, 47(1), 76–82.
9. Flores, J., Gschaedler, A., Amaya-Delgado, L., Herrera-López, E., Arellano, M., y Arrizon, J. Simultaneous saccharification and fermentation of Agave tequilana fructans by Kluyveromyces marxianus yeasts for bioethanol and tequila production. Bioresource Technology, 2013, 146, 267–273.
10. Valadez-Blanco, R., Bravo-Villa, G., Santos-Sánchez, N., Velasco-Almendarez, S., y Montville, T. The Artisanal Production of Pulque, a Traditional Beverage of the Mexican Highlands. Probiotics and Antimicrobial Proteins, 2012, 4(2), 140–144.
11. Escalante, A., López-Soto, D., Velázquez-Gutiérrez, J., Giles-Gómez, M., Bolívar, F., y López-Munguía, A. Pulque, a Traditional Mexican Alcoholic Fermented Beverage: Historical, Microbiological, and Technical Aspects. Frontiers in Microbiology, 2016, 7, 1026.
12. Lappe-Oliveras, P., Moreno-Terrazas, R., Arrizón-Gaviño, J., Herrera-Suárez, T., García-Mendoza, A., & Gschaedler-Mathis, A. Yeasts associated with the production of Mexican alcoholic nondistilled and distilled Agave beverages. FEMS yeast research, 2008, 8(7), 1037-1052.
13. Muñiz-Márquez, D., Contreras, J., Rodríguez, R., Mussatto, S., Wong-Paz, J., Teixeira, J., y Aguilar, C. Influence of thermal effect on sugars composition of Mexican Agave syrup. CyTA - Journal of Food, 2015, 13(4), 1–6.
14. Albalasmeh, A. A., Berhe, A. A., & Ghezzehei, T. A. A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. Carbohydrate polymers, 2013, 97(2), 253–261.
15. Zhang, P., Hong, J., y Ye, X. Cellulase assays. Methods in molecular biology, 2009, 581, 213–231.
16. Garcia-Soto, M., Jiménez-Islas, H., Navarrete-Bolaños, J., Rico-Martinez, R., Miranda-López, R., y Botello-Álvarez, J. Kinetic study of the thermal hydrolysis of Agave salmiana for mezcal production. Journal of Agricultural and Food Chemistry, 2011, 59(13), 7333–7340.
17. Michel, C., Gallegos, G., Maldonado, E., y Aguilar, N. Effect of temperature and pH environment on the hydrolysis of maguey fructans to obtain fructose syrup. Revista Mexicana de Ingeniería Química, 2015, 20(3), 615–622.
18. Waleckx, E., Gschaedler, A., Colonna-Ceccaldi, B., y Monsan, P. Hydrolysis of fructans from Agave tequilana Weber var. azul during the cooking step in a traditional tequila elaboration process. Food Chemistry, 2008, 108(1), 40–48.
19. Chagua, P., Malpartida, R., y Ruíz, A. Tiempo de pasteurización y su respuesta en las características químicas y de capacidad antioxidante de aguamiel de Agave americana L. Revista de Investigaciones Altoandinas - Journal of High Andean Research, 2020, 22(1), 45–57.
20. Peralta-García, I., González-Muñoz, F., Rodríguez-Alegría, E., Sánchez-Flores, A., y López-Munguía, A. Evolution of Fructans in Aguamiel (Agave Sap) During the Plant Production Lifetime. Frontiers in Nutrition, 2020, 7, 175.
21. Villarreal-Morales, S., Montañez-Saenz, J., Aguilar-González, C., y Rodriguez-Herrera, R. Metagenomics of Traditional Beverages. Advances in Biotechnology for Food Industry, 2018, 14, 301–326.
22. Nuñez, M., Salazar, E., Páez, J., Rodríguez, R., y Soto, N. Caracterización fisiológica de dos levaduras nativas en cultivo puro y mixto usando fermentaciones de jugo de agave. Ciencia e Investigación Agraria, 2019, 46(1), 1–11.
23. Walker, G., y Stewart, G. Saccharomyces cerevisiae in the Production of Fermented Beverages. Beverages, 2016, 2(4), 30.
24. Islam, M., y Lampen, J. Invertase secretion and sucrose fermentation by Saccharomyces cerevisiae protoplasts. Biochimica et Biophysica Acta, 1962, 58(2), 294–302.
25. Marques, W., Raghavendran, V., Stambuk, B., y Gombert, A. Sucrose and Saccharomyces cerevisiae: a relationship most sweet. FEMS Yeast Research, 2016, 16(1), 107.
26. Batista, A., Miletti, L., y Stambuk, B. Sucrose fermentation by Saccharomyces cerevisiae lacking hexose transport. Journal of Molecular Microbiology and Biotechnology, 2004, 8(1), 26–33.
27. Archila, M. A., Ventura-Canseco, C., Ruiz-Valdiviezo, V. M., Gutiérrez-Miceli, F. A., Ruiz-Cabrera, M. A., Lara-Hidalgo, C., & Grajales-Lagunes, A. Agave americana honey fermentation by Kluyveromyces marxianus strain for "comiteco" production, a spirit from Mexican southeast. Revista Mexicana de Ingeniería Química, 2017, 16(3), 771-779.
28. López-Alvarez, A., Díaz-Pérez, A., Sosa-Aguirre, C., Macías-Rodríguez, L., y Campos-García, J. Ethanol yield and volatile compound content in fermentation of agave must by Kluyveromyces marxianus UMPe-1 comparing with Saccharomyces cerevisiae baker's yeast used in tequila production. Journal of Bioscience and Bioengineering, 2012, 113(5), 614–618.
29. Arellano, M., Pelayo, C., Ramírez, J., y Rodriguez, I. Characterization of kinetic parameters and the formation of volatile compounds during the tequila fermentation by wild yeasts isolated from agave juice. Journal of Industrial Microbiology and Biotechnology, 2008, 35(8), 835-841.
30. Léon-Rodríguez, D., Escalante-Minakata, P., Jiménez-García, M. I., Ordoñez-Acevedo, L. G., Flores Flores, J. L., y Barba de la Rosa, A. P. Characterization of volatile compounds from ethnic agave alcoholic beverages by gas chromatography-mass spectrometry. Food Technology and Biotechnology, 2008, 46(4), 448-455.
31. Gamero, A., Dijkstra, A., Smit, B., y de Jong, C. Aromatic potential of diverse non-conventional yeast species for winemaking and brewing. Fermentation, 2020, 6(2), 50.
32. Guzmán, A. M. V., López, M. G., y Chávez-Servia, J. L. Chemical composition and volatile compounds in the artisanal fermentation of mezcal in Oaxaca, Mexico. African Journal of Biotechnology, 2012, 11(78), 14344-14353.
33. Díaz-Montaño, D. M., Délia, M. L., Estarrón-Espinosa, M., y Strehaiano, P. Fermentative capability and aroma compound production by yeast strains isolated from Agave tequilana Weber juice. Enzyme and Microbial Technology, 2008, 42(7), 608-616.
34. Santos-Zea, L., Leal-Díaz, A. M., Jacobo-Velázquez, D. A., Rodríguez-Rodríguez, J., García-Lara, S., y Gutiérrez-Uribe, J. A. (2016). Characterization of concentrated agave saps and storage effects on browning, antioxidant capacity and amino acid content. Journal of Food Composition and Analysis, 45, 113-120.
35. Maldonado-Guevara, B. I., Martín del Campo, S. T., y Cardador-Martínez, A. (2018). Production process effect on Mexican agave syrups quality: A preliminary study. Journal of Food Research, 7(3), 50-57.
Received: September 26, 2022 / Accepted: October 15, 2022 / Published:15 February 2023
Citation: Munive, F.; Páez, M.; Romero Granja, C. ; Espín, N. ; Casa-Villegas M. Fermentation of Agave americana L. sap produced in Cayambe – Ecuador. Fermentation of Agave americana L. sap produced in Cayambe – Ecuador. Revis Bionatura 2023;8 (1)15. http://dx.doi.org/10.21931/RB/2023.08.01.15
