2022.07.04.23
Files > Volume 7 > Vol 7 No 4 2022
NDRG1 is being investigated as a possible bladder cancer biomarker in the Iraqi population.

Noor I.A. Ibraheem1, Rawaa H. Ali2 and Mohammed B. Ismail3*
1 Department of Biochemistry, College of Medicine, University of Baghdad, Iraq. [email protected].
2Department of Biochemistry, College of Medicine, University of Baghdad, Iraq. [email protected].
3Urology department, surgical subspecialty hospital, Baghdad medical city complex.
Available from: http://dx.doi.org/10.21931/RB/2022.07.04.23
ABSTRACT
With 549,393 new cases recorded in 2018, bladder cancer is one of the most common malignancies worldwide. Urinary bladder cancer is the cause of about 3 percent of all new cancer diagnoses and 2.1 percent of all cancer deaths. This study aims to evaluate the efficiency of the N-myc downstream-regulated gene 1(NDRG1) as a biomarker for bladder cancer patients in the Iraqi population. One hundred individuals in the case-control study were enrolled and divided into two groups. The first group included 50 patients diagnosed with a bladder mass and investigated by undergoing cystoscopy examination for transurethral resection of bladder tumor (TURB). The second group included 50 healthy individuals who had normal bladder tissue. The results of the present study showed the highest level of (NDRG1) among cases with statically significant association (p=0.001). The ROC curve demonstrated that the protein level of (NDRG1) could distinguish disease patients from healthy individuals with a sensitivity of 96% and a specificity of 92%. Serum (NDRG1) protein is an efficient and noninvasive tumor marker for diagnosing bladder cancer.
Keywords: N-myc downstream-regulated gene 1 (NDRG1), non-muscle-invasive bladder cancer (NMIBC), transurethral resection of bladder tumor (TURB).
INTRODUCTION
The primary purpose of the bladder is to store urine because it is a hollow muscular organ that lies in the anterior section of the pelvic cavity and has a spherical shape when it is filled with pee1. Bladder cancer can develop from epithelial (urothelium) cells that line the inner surface of the bladder2. Or from nonepithelial (mesenchymal) cells that differentiate between developing smooth muscles of the bladder3. However, 90 to 95 percent of bladder cancer cases are urothelial carcinomas, with transitional cell carcinomas accounting for most of these cases and being considered the most common type of bladder cancer4. The four members of the N-myc downstream-regulated gene (NDRG) protein family, NDRG1, NDRG2, NDRG3, and NDRG4, share 57-65 percent of the same amino acids. Except for the C-terminal 5-amino acid residues, which are totally maintained in all four NDRG proteins, the sequence variations amongst family members are primarily found in the N- and C-terminal regions. Three 10-aa (GTRSRSHTSE) tandem repeats can be found in the C-terminal part of NDRG1, which are lacking in the other family members and are thought to be essential for the protein's function5. A 43-KDa protein of 394 amino acids called N-myc downstream-regulated gene 1 (NDRG1), also known as Cap43, RTP, Rit42, Drg1, or PROXY-166. It was first cloned and identified in 1997 and has been associated with several diseases, including esophageal squamous cell carcinoma, prostate cancer, and pancreatic cancer7.
Multiple biological processes, including cell division, growth, stress reactions, differentiation, and immunology, are influenced by NDRG18-9. The NDRG1 protein is generally cytoplasmic in origin at the cellular level. But this localization can differ between various cell types. For example, breast and intestinal epithelia express a membrane-associated protein, while prostate epithelial cells demonstrate nuclear localization of NDRG1. Additionally, mitochondrial localization is seen in specific cell types10-11. These results collectively show that NDRG1 acts in a tissue-specific way12. In breast, colorectal cancer, and glioma, NDRG1 has been tested as a prognostic marker, and many studies have revealed a link between NDRG1 expression levels and survival. While in other types of cancer, such as hepatocellular carcinoma, cervical cancer, and bladder cancer, NDRG1 is upregulated. These contradictory outcomes for NDRG1 expression show that this protein operates differently in different types of cancer depending on the tissue13. The high levels of NDRG1 typically indicate that cells have been exposed to elements that can cause DNA damage, oxidative stress, hypoxia, and heavy metal toxicity. NDRG1 regulates cellular proliferation, differentiation, angiogenesis, tumor growth, apoptosis, and metastasis by responding to those stimuli and heavy metal and hypoxia sensing14. Bladder cancer is intimately associated with smoking and is mainly brought on by chemical compounds that result in hypoxia and DNA damage. According to several studies, bladder cancer patients with high HIF-1 expression have a bad prognosis. HIF-1 is an indication of hypoxia and is extensively expressed in bladder cancer. Additionally, research suggests that NDRG1 is elevated by HIF-1, so its protein level may contribute to the development of bladder cancer and serve as a possible biomarker for the disease9.
MATERIALS AND METHODS
All patients in this case-control study were recruited from Ghazi Hariri Hospital for surgical specialties in Baghdad from December 2021 to June 2022. One hundred individuals were enrolled in this study and divided into two groups. The first group included 50 patients ( 46 males, 4 females) with an age range of (16-79) years. Nearly thirty-five patients smoked cigarettes for more than 5 years and were defined as smokers. All patients were first diagnosed with a bladder mass, investigated by transurethral resection of bladder tumor (TURBT), and sent to the histopathological examination for biopsy of bladder lesion. The second group included 50 healthy individuals ( 46 males, 4 females) with normal bladder tissue and no previous history of other renal systemic diseases. All the patients and the healthy individuals participating in this study were informed, and their consent was obtained before any action related to this study took place. In addition, approval has been taken by the Ethical Committee of the College of Medicine/ University of Baghdad.
•Inclusion criteria:
Patients who were represented with single superficial bladder mass.
•Exclusion criteria:
Patients with health disorders that make them unfit for general anesthesia.
RESULTS
The results of the present study showed the highest level of NDRG1 among cases with statically significant association (p=0.001). And regarding biopsy, the result showed the highest positive among cases while the highest negative among controls with statically significant association (p=0.001). as shown in (figure 1) and (table1):
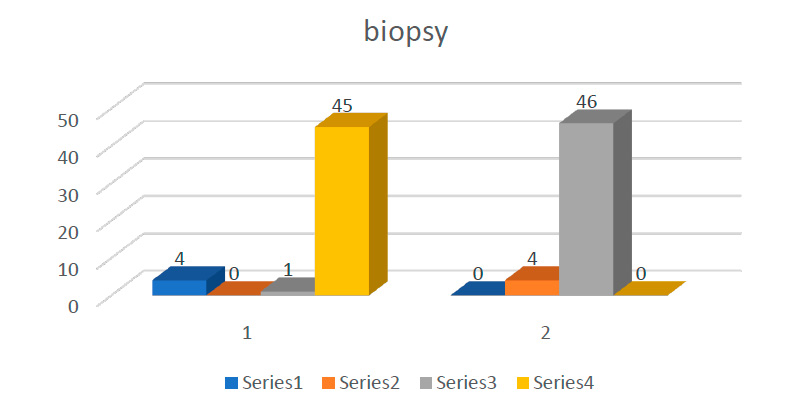
Figure 1. Distribution of study groups with biopsy
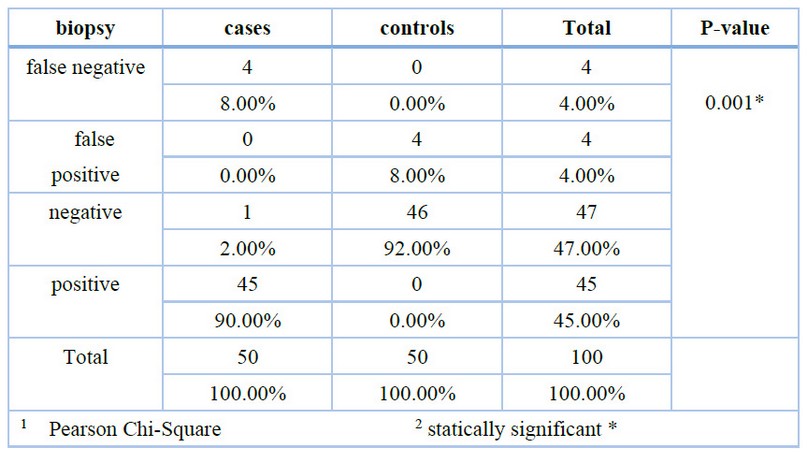
Table 1. Correlation of study groups with biopsy.
The current study showed a positive relationship between the mean of NDRG1 and age through increased level of NDRG1 with increased generation of more than 60 years with statical significance (p=0.045). In addition to that, we see the same result among patients with smoking and hematuria with a statical difference (p=0.001). as shown in (figure2) and (table2):

Figure 2. Distribution of study sample according to NDRG1 mean.

Table2. Distribution of study sample according to NDRG1 mean.
Next, the receiver operating characteristic (ROC) curve based on the ELISA results was plotted to evaluate the potential of NDRG1 as a noninvasive biomarker for diagnosing disease. The ROC curve demonstrated that the protein level of NDRG1 could distinguish disease patients from healthy controls. The optimum cut-off value for the diagnosis of disease patients was 294.94 ng/mg. The area under the curve (AUC) of NDRG1 expression to diagnose disease was 0.942 (95% CI, 0.890–0.995), with a sensitivity of 96% and a specificity of 92%. as shown in (figure3) and (table3):
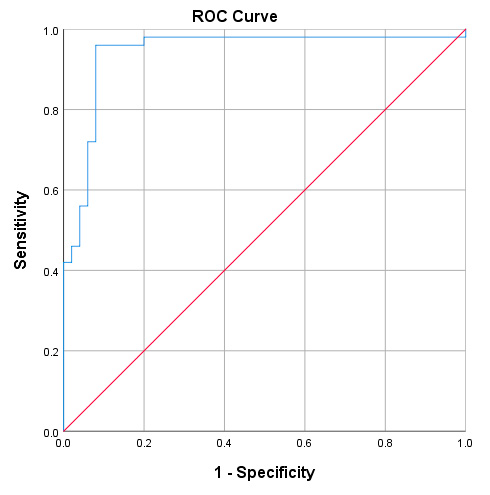
Figure 3. The receiver operating characteristic (ROC) curve.

Table3. Area under the curve. Test Result Variable(s): NDRG1
DISCUSSION
Bladder cancer represents a spectrum of illnesses, from chronically recurrent, noninvasive tumors that can be controlled to aggressive, advanced-stage diseases requiring invasive and multimodal treatment15. Regarding tumor suppression, metastatic suppression, and oncogenesis, N-myc downstream-regulated gene 1 (NDRG1) is essential for understanding cancer biology. NDRG1, typically found in human epithelial cells, regulates the biological processes of several cancer cells, including bladder cancer16. Depending on our results, we can recognize that the levels of NDRG1 protein were the highest among the bladder cancer patients group with a statically significant association (p=0.001). With a sensitivity of 96% and a specificity of 92% and these results are in agreement with the results of other studies such as Aiwei Li et al. They revealed that the expression level of NDRG1 mRNA and protein is considerably increased in bladder cancer tissues with an association for NDRG1 protein (P=0.002) and they were an independent prognostic factor. In NMIBC patients, high NDRG1 expression was linked to poor overall survival and a higher likelihood of developing MIBC. And they discovered that NDRG1, which has a sensitivity of 84.6 percent and a specificity of 86.7 percent, maybe a potential biomarker for the diagnosis of bladder cancer9. One hundred nineteen bladder cancer patients and 65 patients with non-cancerous bladder diseases were included in the study. According to Sun, Y.J., et al., the expression of NDRG1/Cr was significantly higher in bladder cancer patients than in non-cancerous bladder disease patients (P=0.009), with a sensitivity of 63.8 percent and a specificity of 73.4 percent17. Using ELISA, it was discovered that NDRG1 could be identified in the serum of bladder cancer patients as a differentiating marker18. If smoking is one of the causes that lead to hypoxia and is strongly related to bladder cancer, and as is represented in our results, there is a positive relationship between the mean of NDRG1 and smoking with a statical difference (p=0.001). This ensures Cangul, H.'s study offers convincing proof that HIF-1-dependent and –independent pathways regulate the expression of the NDRG1 gene at both the protein and RNA levels19-20. In addition, hematuria showed a positive relationship with NDRG1 in the current study with statically significant (p=0.001). These results are consistent with other studies: In the cohort of more than 2,000 patients who experienced asymptomatic microscopic hematuria, Gonzalez, N.A. et al. revealed that 25 patients (1.2%) had bladder cancer discovered by cystoscopy; these cases were noninvasive and were found in patients older than 50 years21. According to Samson, P. et al., only 6 of the 1049 patients who experienced asymptomatic microscopic hematuria, with a mean age of 77 years, and the youngest patient diagnosed at 59 years, had bladder cancer22. As shown in the above studies, we have only disagreed on the mean and prevalence of hematuria among patients with bladder cancer, which is higher in our study. That may be due to our small sampling group compared to the cohorts born in previous studies. Despite all the positive aspects of NDRG1 as a biomarker for bladder cancer mentioned above, there is a need for further investigations and research in the future which makes it not suitable for routine work yet.
CONCLUSIONS
Serum NDRG1 protein is an efficient and noninvasive tumor marker for the diagnosis of bladder cancer. Its levels were found in the patient's group to be significantly higher than in the healthy individual's group. The NDRG1 protein was tested by ELISA technique for the quantitative detection of NDRG1 protein by determining its concentration in serum. It is readily available and causes no patient discomfort or health complication.
.
Funding: This research received no external funding.
Institutional Review Board Statement: The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the College of the Medicine/ University of Baghdad. (protocol code 1573 and date of approval 2021/11/23).
Informed Consent Statement: Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest: The authors declare no conflict of interest.
REFERENCES
1. Delancey, J., Gosling, J., Creed, K., Dixon, J., Delmas, V., Landon, D., Norton, P. Gross Anatomy and Cell Biology of the Lower Urinary Tract. Committee. 2002;1. P:20.
2. Sanli, O., Dobruch, J., Knowles, A.M., Burger, M., Alemozaffar, M., Nielsen, E. M., Lotan, Y. Bladder cancer. Nature Reviews Disease Primers. 2017; 13:3:17022.
3. Pokrywczynska, M., Jundzill, A., Warda, K., Buchholz, L., Rasmus, M., Adamowicz, J., et al. Does the Mesenchymal Stem Cell Source Influence Smooth Muscle Regeneration in Tissue-Engineered Urinary Bladders?. STAGE Publishing. 2017; 26(11):1780-1791.
4. Jassim, O.R., Hamood, Q.H. and Naif, H.H. Role of Magnetic Resonance Imaging Protocols in Detecting and Staging of the Urinary Bladder Carcinoma in Comparison with Histopathological Findings. Annals of the Romanian Society for Cell Biology. 2021; 25(6):4045 – 4058.
5. Le, N., Hufford, M.T., Park, S.J., Brewste, M.R. Differential expression and hypoxia-mediated regulation of the N-myc downstream regulated gene family. The Federation of American Societies for Experimental Biology Journal. 2021; 35(11):e21961..
6. Nishigaki, A., Tsubokura, H., Ishida, M., Hashimoto, Y., Yoshida, A., Hisamatsu, Y., Nakao-T. T., Murata, H., Okada, H. NDRG1 is expressed in human granulosa cells: An implicative role of NDRG1 in the ovary. . Reproductive Medicine and Biology. 2022; 21:e12437.
7. Li, N., Shi, H., Hou, P., Gao, L., Shi, Y., Mi, W., Zhang, G., Wang, N., Dai, W., Wei, L., Shi, Y., Guo, S. The Influence of NDRG1 Single Nucleotide Polymorphisms on Glioma Risk and Prognosis in Chinese Han Population. Cellular and Molecular Neurobiology. 2021; 42(6):1949-1964.
8. Melotte, V., Qu, X., Ongenaert, M., van Criekinge, W., de Bruïne, A. P., Baldwin, H. S., & van Engeland, M. The N-myc downstream regulated gene (NDRG) family: diverse functions, multiple applications. The Federation of American Societies for Experimental Biology Journal. 2010; 24(11), 4153–4166.
9. Li, A., Zhu, X., Wang, C., Yang, S., Qiao, Y., Qiao, R., & Zhang, J. Upregulation of NDRG1 predicts poor outcome and facilitates disease progression by influencing the EMT process in bladder cancer. Scientific Reports. 2019; 9(1):5166.
10. Chekmarev, J., Azad, G. M., and Richardson, R. D. The Oncogenic Signaling Disruptor, NDRG1: Molecular and Cellular Mechanisms of Activity. Cells. 2021; 10(9):2382.
11. Wei, M., Zhang, Y., Yang, X., Ma, P., Li, Y., Wu, Y., et al. Claudin-2 promotes colorectal cancer growth and metastasis by suppressing NDRG1 transcription. Clinical and Translational Medicine. 2021; 11(12):e667.
12. Kovacevic, Z. and Richardson, D,R. The metastasis suppressor, Ndrg-1: a new ally in the fight against cancer. Carcinogenesis. 2006; 27 (12):2355–2366.
13. Abdul-Lateif, K. M., & Abdulateef, S. M. The effect of injecting hatching eggs with different concentrations of biotin on the quality and physiological characteristics of the hatched chicks. Iraqi Journal of Veterinary Sciences. 2012, 26, 391-397
14. Nishigaki, A., Tsubokura, H., Ishida, M., Hashimoto, Y., Yoshida, A., Hisamatsu, Y., Nakao-T. T., Murata, H., Okada, H. NDRG1 is expressed in human granulosa cells: An implicative role of NDRG1 in the ovary. Reproductive Medicine and Biology. 2022; 21:e12437.
15. Lenis, A. T., Lec, P. M., Chamie, K., & MSHS, M. Bladder Cancer. JAMA. 2020; 324(19):1980-1991.
16. Tsui, K.-H., Hou, P.-C., Chang, S.-K., Lin, Y.-H., Feng, T.-H., Chen, C.-C., Shin, Y.-S., and Juang, H.-H. Metallothionein 3 Is a Hypoxia-Upregulated Oncogene Enhancing Cell Invasion and Tumorigenesis in Human Bladder Carcinoma Cells. International Journal of Molecular Sciences. 2019; 20, 980.
17. Sun, Y.J., Li, A.W., Zhang, Y.C., Dai, J.-H., Zhu. X. Evaluation of Urine NDRG1 as Noninvasive Biomarker for Bladder Cancer Diagnosis. Europe PMC. 2022; 1;67(3).
18. Wang, D., Tian, X. & Jiang, Y. NDRG1/Cap43 overexpression in tumor tissues and serum from lung cancer patients. Journal of cancer research and clinical oncology. 2012; 138(11):1813-20.
19. Cangul, H. Hypoxia upregulates the expression of the NDRG1 gene leading to its overexpression in various human cancers. BMC genetics. 2004; 2;5:27.
20. Zhang, Z.-Y., Zhang, S.-L., Chen, H.-L., Mao, Y.-Q., Li, Z.-M., Kong, C.-Y., Han, B.,et al. The up-regulation of NDRG1 by HIF counteracts the cancer promoting effect of HIF in VHL-deficient clear cell renal cell carcinoma. 2020; 53(7):e12853.
21. Gonzalez, N.A., Lipsky, J.M., Li, G., Rutman, P.M., Cooper, L.K., Weiner, M.D., and Anderson, B.C. The Prevalence of Bladder Cancer During Cystoscopy for Asymptomatic Microscopic Hematuria. Urology. 2019; 126: 34−38.
22. Samson, P., Waingankar, N., Shah, P., Friedman, D., Kavoussi, L., & Han, J. Predictors of genitourinary malignancy in patients with asymptomatic microscopic hematuria. Urologic Oncology: Seminars and Original Investigations. 2018; 36(1), 10.e1–10.e6.
Received: July 20, 2022 / Accepted: October 15, 2022 / Published:15 November 2022
Citation: Ibraheem N I A, Ali R H, Ismail M B. NDRG1 is being investigated as a possible bladder cancer biomarker in the Iraqi population.Revis Bionatura 2022;7(4) 23. http://dx.doi.org/10.21931/RB/2022.07.04.23